Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 10
Pediatric acute kidney injury: prevalence, impact and management challenges
Authors Ciccia E, Devarajan P
Received 20 December 2016
Accepted for publication 28 February 2017
Published 29 March 2017 Volume 2017:10 Pages 77—84
DOI https://doi.org/10.2147/IJNRD.S103785
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Eileen Ciccia, Prasad Devarajan
Division of Nephrology and Hypertension, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA
Abstract: The incidence of pediatric acute kidney injury (AKI) is increasing globally, as are the associated morbidities and mortality. A recent standardization of the definition of AKI has allowed for a more accurate assessment of the epidemiology of pediatric AKI. Recent advances in leveraging electronic medical health record systems have allowed for real-time risk stratification and prevention of pediatric AKI in the hospital setting. Newly developed and validated clinical scores have improved our ability to predict AKI and provide a rational context for biomarker utilization in hospitalized children. Novel non-invasive diagnostic and predictive biomarkers have been launched globally to improve our ability to diagnose and predict AKI and its adverse outcomes as well as recovery. This review summarizes the most current literature, focusing on the epidemiology, management, and early diagnostic strategies in pediatric AKI.
Keywords: acute kidney injury, acute renal failure, children, epidemiology, biomarkers, neutrophil gelatinase-associated lipocalin
Introduction
Acute kidney injury (AKI) is described as a spectrum of abruptly compromised renal functions that result in impaired balance of fluid, electrolytes, and waste products. It is recognized as an increasingly common cause of morbidity and mortality in children. This review summarizes the most impactful current literature, focusing on the definition, epidemiology, impact and management, and early diagnostic strategies of AKI in the pediatric clinical and research communities.
Definition
Several definitions have been used, most notably the Risk, Injury, Failure, Loss of kidney function, End-stage renal disease (RIFLE) criteria, the subsequent pediatric RIFLE (pRIFLE) score, and the Acute Kidney Injury Network (AKIN) criteria. Each of these definitions defined and staged kidney injury slightly differently, which made comparison studies and standardized recommendations regarding management more difficult.1
A standardized definition of AKI was proposed by the Kidney Disease: Improving Global Outcomes (KDIGO) AKI working group in 2012 and has been validated in pediatric populations subsequently.2–4 This definition identifies and stages AKI based on changes in serum creatinine from baseline or urine output as detailed in Table 1. Baseline creatinine is defined as the lowest serum creatinine value in the previous 3 months, estimating the baseline glomerular filtration rate (GFR) using the original Schwartz equation.5 Where no previous serum creatinine measures are available, it is recommended to use a presumed baseline of 120 mL/min/1.73 m2.6 In the future, we may see these definitions further expanded to include criteria using serum cystatin C or urinary biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL), both of which have recently been shown to represent earlier markers of AKI and recovery than serum creatinine.7,8
  | Table 1 KDIGO staging of AKI3 Abbreviations: KDIGO, Kidney Disease: Improving Global Outcomes; AKI, acute kidney injury; GFR, glomerular filtration rate. |
Epidemiology
Given the historical discrepancy of definitions for AKI in the pediatric population, it has been difficult to establish consistent measures of incidence and prevalence trends over time. Additionally, most investigations had previously been limited to small single-center retrospective studies. Assessment of pediatric AKI epidemiology will be easier with the adoption of standardized definitions using the KDIGO AKI staging criteria in multiple centers worldwide. However, there will still be ongoing limitations in capturing AKI cases, for example, most data continue to focus exclusively on inpatients rather than outpatient populations. Therefore, further studies on specific populations at increased risk, for example, children with chronic kidney disease, nephrotic syndrome, etc., are warranted.9
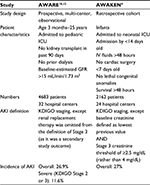
Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology (AWARE) – critically ill children
The AWARE study began in 2014 as a prospective observational study of AKI incidence, outcomes, and risk factor stratification in critically ill children aged 3 months through 25 years admitted to intensive care settings in 32 hospitals throughout Asia, Australia, Europe, and North America.10 As part of the assessment of AKI risk, the renal angina index (RAI), which will be described in more detail later in this review, was validated in this international population.11 Data continue to be published from this extensive database. Epidemiologic analysis published in November 2016 found that the overall incidence of AKI in the 4683 critically ill children evaluated was 26.9%, and the incidence of severe AKI (KDIGO Stage 2 or 3) was 11.6%. Diagnosis of severe AKI conferred an increased risk of mortality by an adjusted odds ratio of 1.77 (95% CI, 1.17–2.68), with a mortality rate of 11% versus 2.5% (P<0.001) in patients without severe AKI.12 The benefit of including multiple criteria (both serum creatinine and urine output) by which to increase sensitivity was also confirmed, as 67.2% of the patients found to have AKI by oliguria would have been missed if using serum creatinine alone. Strikingly, a significant increase in mortality was also observed (7.8% versus 2.9%, P=0.02) when severe AKI threshold (KDIGO Stage 2 or 3) was achieved due to oliguria compared to creatinine.12
AWAKEN – neonates
In 2014, an interdisciplinary 24-center collaboration developed a large retrospective cohort (the Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates [AWAKEN]) with goals to better understand the physiology of fluid and electrolyte balance in neonates, understand the risk factors for AKI in this population, and validate the KDIGO AKI definition in neonates.4 Given that maternal serum creatinine is transmitted across the placental barrier and is cleared at varying rates based on the infant gestational age, the KDIGO definition was altered such that baseline creatinine was instead the lowest serum creatinine level in each infant. In addition, the serum creatinine threshold for Stage 3 AKI was determined to be ≥2.5 mg/dL rather than the KDIGO threshold of 4 mg/dL. Preliminary data from the AWAKEN study have revealed an AKI rate of 27% among neonates, remarkably similar to the AWARE results. Table 2 lists a concise comparison of these landmark pediatric studies.
Management and prevention
Despite the ever-expanding body of knowledge surrounding AKI in the pediatric population, management at this time is still chiefly limited to reducing risk factors and providing supportive care. There is still a lack of curative treatment. At this time, validated strategies available in the current literature primarily focus on injury prevention and mitigation. Table 3 lists a summary of the strategies detailed in the following.
Nephrotoxic Injury Negated by Just-in-time Action (NINJA) – reducing nephrotoxic AKI
The NINJA project piloted in 2011 at the Cincinnati Children’s Hospital Medical Center (CCHMC) and now in use at >15 children’s hospitals throughout the US has provided a translatable framework for near real-time risk stratification and prevention of AKI in the hospital setting. Under this paradigm, a hospital pharmacist who rounds with the inpatient care team receives a daily report (via automated data mining of the hospital’s electronic health record) of the patients receiving more than three nephrotoxic medications concurrently or at least 3 consecutive days of intravenous aminoglycoside therapy. This information is then utilized during team rounds so that daily monitoring of serum creatinine levels can be optimized and nephrotoxin exposure minimized as clinically appropriate.13,14
Since its introduction at the Cincinnati Children’s Hospital, the NINJA project has shown significant and lasting effects in decreasing the incidence of pediatric AKI in quality improvement assessments. Overall, there was a decrease by 38% in nephrotoxic medication exposure rates with AKI rates decreased by 64%. Importantly, despite these decreases in nephrotoxin exposures (including several antimicrobials), no negative unintended consequences of persistent bacterial or fungal infections were noted compared with baseline rates.13
RAI – clinical AKI scoring
The RAI is another paradigm that has been proposed as a means to better predict and intervene in pediatric AKI.15 In 2014, it was validated in a two-center, four-cohort study, which utilized patient demographic data along with clinical and laboratory indicators of renal injury at the time of admission to the pediatric intensive care unit (ICU) to predict those patients at high risk of developing severe AKI within 72 hours.11 This prediction model allows for discriminating acquisition of AKI biomarkers in appropriate patients such that the positive predictive value of these tests can be augmented, in much the same way that discretionary use of troponin I in a patient with coronary angina symptomatology allows for improved patient care and outcomes. More concretely, utilization of this risk stratification model to guide management of patient care in real time during the critical early period of ICU admission improves outcomes and decreases incidence of severe AKI.
The results of this cohort study showed that across three of the four cohort groups, the incidence of AKI on Day 3 of admission was significantly higher in the group assessed to having renal angina (RAI score of ≥8). More helpfully, an RAI score of >8 had a significant negative predictive value of >95% in all three validation cohorts. Therefore, calculating an RAI score at pediatric ICU admission can be expected to provide context for which patients the novel AKI biomarkers may be judiciously obtained.11
Challenges of dialysis in children
Even as there have been rapid advancements in developing novel means of predicting and ameliorating pediatric AKI, there remain significant challenges to its treatment and management, especially when renal replacement therapy is required. While peritoneal dialysis is the modality of choice, particularly for neonates, it is unfortunately not always available or effective, such as the case in patients with previous abdominal surgeries or infections or in those patients requiring clearance and fluid removal more rapidly than the exchange across the peritoneal membrane allows. Dialysis via hemocatheter poses its own set of difficulties. Access remains a major challenge, given the small size of pediatric vessels. Also unique to the pediatric population, the large relative total extracorporeal volume used in conventional renal replacement modalities requires exposure to blood products for blood priming in the smallest patients. Likewise, the standard volume-based ultrafiltration measurements used on these machines are less accurate than weight-based measurements and have increased risk of inadvertent clinically significant fluid gains or losses in small patients.
CARPEDIEM – dialysis for neonates
To address some of the limitations of using conventional continuous renal replacement therapy (CRRT) in neonatal patients, a 5-year prospective study was designed to develop and implement use of a miniaturized Cardio-Renal Pediatric Dialysis Emergency Machine (CARPEDIEM).16 This device has an extracorporeal priming volume of <30 mL and accurate ultrafiltration to within 1 g and has been used effectively in an infant as small as 2.9 kg in weight. This technology, and other advancements like it, will continue to expand the therapies and interventions available to the smallest of patients affected with AKI.
Novel diagnostic and predictive biomarkers
Pediatric AKI is largely asymptomatic and often occurs in the wake of other underlying conditions such as cardiac surgical procedures, critical illness, and nephrotoxin use. Making the diagnosis in the estimated 5% of all hospitalized children and ~30% of critically ill neonates and children who suffer from AKI and its consequences currently depends on serial measurements of functional biomarkers such as serum creatinine. This approach is flawed due to several reasons.17–19 First, non-renal factors such as age, gender, diet, muscle mass, and medications can influence serum creatinine concentration independent of changes in kidney structure or function. Serum creatinine in a neonate is merely reflective of maternal creatinine for approximately the first 10 days of life. GFR is normally low in infants, and physiological maturation of renal function occurs until ~2 years of age. Children with chronic conditions such as congenital heart disease display low muscle mass, resulting in falsely lowered serum creatinine. Second, a previously healthy kidney displays a significant functional reserve, such that >50% of kidney function can be lost due to an acute damaging insult without any change in serum creatinine. This is especially true in the pediatric population, where the lack of chronic comorbid conditions generally results in pristine kidneys with maximal functional reserve. Third, a rise in serum creatinine concentration accompanies any condition that leads to transient renal hypoperfusion. These episodes of “pre-renal azotemia” are common in children with gastrointestinal illness and must be differentiated from the more ominous forms of “intrinsic AKI” with accompanying structural damage. Fourth, there is typically a lag period of hours to days after an acute injurious event before serum creatinine rises. During this time, structural damage is known to occur to the kidney tubules. Accumulated experimental data have identified several interventions that can prevent and/or treat AKI during this “sub-clinical” phase, before the serum creatinine rises. The paucity of an early biomarker of structural AKI has hampered our ability to translate these promising interventions to human AKI in a timely manner. The search for biomarkers for the early diagnosis of AKI and its outcomes is an area of intense contemporary research that has yielded several promising candidates. A select few that are especially pertinent to pediatric AKI are summarized in the following and in Table 4.
NGAL
The most extensively studied and promising biomarker in pediatric AKI is NGAL. Preclinical gene expression analyses reported in >150 distinct studies performed in AKI models from several species ranging from rodents to humans have consistently revealed the NGAL gene to be one of the most dramatically upregulated genes in the kidney soon after an ischemic or a nephrotoxic insult.20,21 The NGAL protein is also highly induced in regenerating and recovering kidney tubule cells.22 NGAL binds iron; chelation of toxic iron is an important mechanism that protects the kidney tubules from worsening injury. Thus, the biologic role of NGAL in AKI is one of enhanced tubule cell proliferation and recovery.23 The induced NGAL protein is rapidly secreted into both the urine and the plasma in animal models of AKI, and a decade of translational studies in human beings have now established NGAL as a biomarker to predict AKI and its adverse outcomes independent of serum creatinine.
Cardiac surgery-associated acute kidney injury (CS-AKI) is a common cause of AKI in children, with a reported incidence of 20–60%.24 However, the increase in serum creatinine typically occurs only 1–3 days after cardiopulmonary bypass (CPB). As first highlighted by Mishra et al,25 a dramatic increase in both urine and plasma NGAL is detected within 2–6 hours of CPB in children destined for AKI, with a predictive area under the receiver operating characteristic curve (AUC) of >0.9. These findings have now been confirmed in >7500 patients,26–28 with measurements obtained within 4–6 hours after initiation of CPB, yielding an overall predictive pooled AUC of 0.86. The predictive performance for CS-AKI was similar for both urine and plasma NGAL. Subgroup analyses revealed that NGAL displays the highest predictive accuracy for CS-AKI in children (AUC 0.89 versus 0.83 in adults) and in subjects without pre-existing renal insufficiency (AUC 0.87 versus 0.81 with pre-existing renal insufficiency).
Critical illness, including sepsis, is the most common cause of AKI worldwide, with a reported incidence of 30–50%. The ability of NGAL to predict AKI in this heterogeneous population has been confirmed in >8500 critically ill patients,27 with measurements obtained within 6 hours of clinical presentation yielding an overall predictive AUC of 0.8.11 In a recent meta-analysis28 specifically examining the value of NGAL for the prediction of AKI in patients with sepsis, the specificity of plasma NGAL for predicting AKI was inferior to that of urine NGAL in sepsis, although the sensitivities and pooled AUCs were similar and promisingly high (0.8–0.9).
Contrast-induced acute kidney injury (CI-AKI) is a common cause of AKI, although less common in children compared to adults. However, the increase in serum creatinine occurs only 1–3 days after contrast administration. As first described by Hirsch et al,29 both urine and plasma NGAL concentrations increase within 2–6 hours of contrast administration, with a predictive AUC of >0.9. A recent meta-analysis30 of 1520 patients has confirmed the high diagnostic accuracy of NGAL in the early detection of CI-AKI, with measurements obtained 2–24 hours after contrast administration yielding a pooled AUC of 0.93.
An increase in serum creatinine occurs in both true structural (intrinsic) AKI and functional volume-responsive pre-renal azotemia or even in chronic kidney disease. It is critical to make these distinctions in the acute setting, since the medical management of each is different and mismanagement is deleterious.31 Nickolas et al32 first showed that measurement of urinary NGAL at the time of initial patient encounter can differentiate those who subsequently develop intrinsic AKI from those who would follow a more benign course of pre-renal azotemia, with an AUC of 0.95. These findings have now been confirmed in studies involving >2000 patients,33–35 confirming the diagnostic accuracy of NGAL in the early prediction of intrinsic AKI, with AUCs in the 0.81–0.87 range.
AKI with structural nephron damage portends a number of adverse outcomes, including worsening severity, increased length of hospital stay, and death. Initial single-center studies of cardiac surgical patients by Bennett et al36 and Dent et al37 identified the correlation of early NGAL measurements in the urine and plasma, respectively, with severity and duration of AKI, length of stay, dialysis requirement, and death. In a meta-analysis of 2000 patients with predominantly cardiorenal syndrome,26 early NGAL measurements predicted dialysis and death with a pooled AUC of 0.78 and 0.75, respectively.
NGAL identifies structural kidney injury in the absence of an increase in serum creatinine. In a multicenter pooled analysis of 10 prospective studies involving >2300 patients with predominantly cardiorenal syndrome, ~ 20% of patients displayed increased NGAL concentrations but no increase in serum creatinine.38 This previously undetectable condition (termed subclinical AKI) was associated with an almost threefold increased risk of mortality or dialysis requirement and a doubling of median length of hospital stay. This “added value” of NGAL measurements for the prediction of AKI and its adverse consequences over and above clinical and functional scores has now been repeatedly demonstrated in several large studies involving cardiac surgical patients.39–41
NGAL is protease resistant and remarkably stable in urine and blood. Short-term storage of samples at 4°C for up to 24 hours and long-term storage at −80°C for up to 5 years result in no clinically significant loss in the NGAL signal.42 There are currently three clinical analytic platforms for NGAL measurement in patient samples, with results available within 15–30 minutes. These include a point-of-care immunoassay for plasma NGAL (Alere Triage® NGAL test), a urine immunoassay developed for an exclusive platform (ARCHITECT; Abbott Diagnostics), and a particle-enhanced turbidimetric immunoassay for urine and plasma NGAL that can be run on a large variety of standard automated clinical chemistry analyzers (NGAL TestTM; BioPorto Diagnostics). All of these three tests are CE (Conformité Européene [European Conformity]) marked and launched for clinical diagnostic use worldwide, but are currently pending US Food and Drug Administration (FDA) approval for diagnostic use in the USA.
Kidney injury molecule-1 (KIM-1)
Preclinical studies have also identified the KIM-1 gene to be induced in the proximal tubule cells of ischemic rat kidneys.21 In experimental AKI, KIM-1 protein regulates phagocytosis of damaged cells and thereby limits injury.43 An extracellular domain of KIM-1 can be detected by enzyme linked immuno-sorbent assays and is useful as a urinary biomarker in patients with AKI. In a study of 40 children undergoing CPB, urinary KIM-1 levels were markedly increased in the children who developed AKI, with an AUC of 0.83 at 12 hours post-CPB for predicting AKI, indicating that it is a delayed biomarker of AKI compared to NGAL.44 Subsequent pediatric single-center studies have yielded conflicting results. A prospective multi-center study of 311 children undergoing cardiac surgery confirmed the delay in upregulation of urinary KIM-1 in AKI patients and showed that KIM-1 was not significantly associated with AKI after adjusting for other injury biomarkers.45 Elucidation of the role of KIM-1 as an AKI biomarker awaits additional confirmatory studies. No clinical laboratory assays for urine KIM-1 are currently available.
Interleukin-18 (IL-18)
Animal studies have shown that IL-18 is induced in the proximal tubule and detectable in the urine following ischemic AKI. IL-18 represents a proinflammatory cytokine that might worsen the degree of AKI. In children undergoing CPB, urinary IL-18 is an early, predictive biomarker, with levels increased at 6-hour post-CPB and an AUC of 0.75 for predicting AKI.46 Subsequent pediatric studies have confirmed that urine IL-18 obtained 6- to 12-hour post-CPB moderately predicts AKI, with AUCs in the 0.72–0.82 range.39–41 Clinical assays for IL-18 measurement are not available at the present time.
Liver-type fatty acid-binding protein (L-FABP)
L-FABP is an anti-oxidant, renoprotective molecule induced in the proximal tubule early after experimental AKI. In children undergoing CPB, urinary L-FABP increases within 4 hours of initiating CPB in those destined for AKI, with a predictive AUC of 0.81.47 Follow-up single-center studies in children39–41 indicated that urine L-FABP levels obtained 6 hours post-CPB are predictive of AKI, with AUCs in the 0.75–0.78 range. However, a prospective multi-center study of 311 children undergoing cardiac surgery indicated that the increase in L-FABP was not significantly associated with AKI after adjusting for other injury biomarkers.45 Confirmation of the role of L-FABP as a potential AKI biomarker awaits additional confirmatory studies, and no clinical laboratory assays are currently available.
Markers of cell-cycle arrest
Targeted proteomic approached have identified markers of cell-cycle arrest, such as tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7), to be induced in tubule cells after AKI. It is postulated that the resultant cell-cycle arrest limits proliferation of damaged tubule cells. The product of these TIMP-2 and IGFBP7 can be measured in the urine using a point-of-care kit that has been approved by the FDA. In a small study of children undergoing CPB, the urinary TIMP-1/IGFBP7 product was increased at 4 hours post-CPB in children who developed AKI, with an AUC of 0.85 for predicting AKI.47 The corresponding AUC for urinary NGAL was reported to be comparably good at 0.87. Although promising, additional studies are required to elucidate cell-cycle markers as AKI biomarkers in children.
Conclusion
The investigations into how best to define, predict, diagnose, assess, prevent, and manage the care of pediatric patients with AKI are ongoing. Just as rapid progress over the past few years has brought the advances and interventions highlighted here, it can be hoped that the field continues to expand and allow for improved treatment and prevention strategies in the future.
Acknowledgment
PD has received funding from the National Institutes of Health (grant number P50 DK096418).
Disclosure
PD is a co-inventor on patents (7776824 and 7977110) related to NGAL as a biomarker of kidney injury and declares licensing agreements with BioPorto Diagnostics and Abbott Diagnostics. EC reports no conflict of interest in this work.
References
Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–561. | ||
Selewski DT, Cornell TT, Heung M, et al. Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med. 2014;40(10):1481–1488. | ||
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Working Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138. | ||
Jetton JG, Guillet R, Askenazi DJ, et al. Assessment of worldwide acute kidney injury epidemiology in neonates: design of a retrospective cohort study. Front Pediatr. 2016;4:68. | ||
Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58(2):259–263. | ||
Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3(4):948–954. | ||
Lau L, Al-Ismaili Z, Harel-Sterling M, et al. Serum cystatin C for acute kidney injury evaluation in children treated with aminoglycosides. Pediatr Nephrol. 2017;32(1):163–171. | ||
Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10(1):147–155. | ||
Rheault MN, Zhang L, Selewski DT, et al. AKI in children hospitalized with nephrotic syndrome. Clin J Am Soc Nephrol. 2015;10(12):2110–2118. | ||
Basu RK, Kaddourah A, Terrell T, et al. Assessment of worldwide acute kidney injury, renal angina and epidemiology in critically ill children (AWARE): study protocol for a prospective observational study. BMC Nephrol. 2015;16:24. | ||
Basu R, Zappitelli M, Brunner L, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(3):659–667. | ||
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(1):11–20. | ||
Goldstein SL, Mottes T, Simpson K, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016;90(1):212–221. | ||
Goldstein SL, Kirkendall E, Nguyen H, et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. 2013;132(13):e756–e767. | ||
Goldstein SL, Chawla LS. Renal angina. Clin J Am Soc Nephrol. 2010;5(5):943–949. | ||
Ronco DC, Garzotto F, Brendolan A, et al. Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturised machine (CARPEDIEM). Lancet. 2014;383(9931):1807–1813. | ||
Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4(2):265–280. | ||
Devarajan P. Neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology (Carlton). 2010;15(4):419–428. | ||
Devarajan P. Pediatric acute kidney injury: different from acute renal failure but how and why. Curr Pediatr Rep. 2013;1(1):34–40. | ||
Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63(5):1714–1724. | ||
Devarajan P. Genomic and proteomic characterization of acute kidney injury. Nephron. 2015;131(2):85–91. | ||
Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–2543. | ||
Mishra J, Mori K, Ma Q, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15(12):3073–3082. | ||
Jefferies JL, Devarajan P. Early detection of acute kidney injury after pediatric cardiac surgery. Prog Pediatr Cardiol. 2016;41:9–16. | ||
Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. | ||
Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024. | ||
Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. 2014;51(pt 3):335–351. | ||
Zhou F, Luo Q, Wang L, Han L. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: a meta-analysis. Eur J Cardiothorac Surg. 2016;49(3):746–755. | ||
Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22(12):2089–2095. | ||
Wang K, Duan CY, Wu J, et al. Predictive value of neutrophil gelatinase-associated lipocalin for contrast-induced acute kidney injury after cardiac catheterization: a meta-analysis. Can J Cardiol. 2016;32(8):1033.e19–1033.e29. | ||
Devarajan P. NGAL for the detection of acute kidney injury in the emergency room. Biomark Med. 2014;8(2):217–219. | ||
Nickolas TL, O’Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148(11):810–819. | ||
Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59(3):246–255. | ||
Singer E, Elger A, Elitok S, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80(4):405–414. | ||
Soto K, Papoila AL, Coelho S, et al. Plasma NGAL for the diagnosis of AKI in patients admitted from the emergency department setting. Clin J Am Soc Nephrol. 2013;8(12):2053–2063. | ||
Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3(3):665–673. | ||
Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11(6):R127. | ||
Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57(17):1752–1761. | ||
Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58(22):2301–2309. | ||
Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22(9):1737–1747. | ||
Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22(9):1748–1757. | ||
Schuh MP, Nehus E, Ma Q, et al. Long-term stability of urinary biomarkers of acute kidney injury in children. Am J Kidney Dis. 2016;67(1):56–61. | ||
Yang L, Brooks CR, Xiao S, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 2015;125(4):1620–1636. | ||
Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73(7):863–869. | ||
Parikh CR, Thiessen-Philbrook H, Garg AX, et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8(7):1079–1088. | ||
Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70(1):199–203. | ||
Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73(4):465–472. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.