Back to Journals » OncoTargets and Therapy » Volume 12
PD-1 inhibitors dependent CD8+ T cells inhibit mouse colon cancer cell metastasis
Authors Gao CE, Zhang M, Song Q, Dong J
Received 25 January 2019
Accepted for publication 31 May 2019
Published 28 August 2019 Volume 2019:12 Pages 6961—6971
DOI https://doi.org/10.2147/OTT.S202941
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Chang E Gao,1,2 Ming Zhang,3 Qian Song,4 Jian Dong2
1The First Affiliated Hospital of Kunming Medical University, Department of Medical Oncology, Kunming, Yunnan 650031, People’s Republic of China; 2Yunnan Cancer Hospital & the Third Affiliated Hospital of Kunming Medical University & Yunnan Cancer Center, Department of Medical Oncology, Kunming, Yunnan 650018, People’s Republic of China; 3Yunnan Cancer Hospital & the Third Affiliated Hospital of Kunming Medical University & Yunnan Cancer Center, Cancer Research Institute, Kunming, Yunnan 650018, People’s Republic of China; 4Yunnan Cancer Hospital & the Third Affiliated Hospital of Kunming Medical University & Yunnan Cancer Center, Department of Radiation Oncology, Kunming, Yunnan 650018, People’s Republic of China
Correspondence: Jian Dong
Yunnan Cancer Hospital & the Third Affiliated Hospital of Kunming Medical University & Yunnan Cancer Center, Department of Medical Oncology, NO. 519 Kunzhou Road, Kunming, Yunnan 650018, People’s Republic of China
Tel +86 871 6818 5656
Email [email protected]
Background: Colon cancer is a common digestive tract malignancy which ranks as the third leading cause of cancer death worldwide. A current focus of anti-cancer research is harnessing the patient’s own immune system for therapy. Programmed cell death protein 1 (PD-1), an immune suppressor, is upregulated in various activated immune cells, such as T cells, and in viral infections and tumors.
Purpose: The objective of this study was to investigate the function of PD-1 inhibitor on the metastasisi of mouse colon cancer cells.
Patients and methods: In the present study, we established an in situ colon cancer mouse model using the CT26 cell line. Hematoxylin-eosin (HE) staining was performed to detect colon cancer cell metastasis. The levels of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-12 (IL-12) in serum and mesenteric lymph nodes (MLNs) were detected by Enzyme-linked immunosorbent assay (ELISA). CD44high, CD62Llow, memory T cells, CD4+, FoxP3+, regulatory T cells, and IFN-γ and TNF-α levels in MLNs and spleen were detected by flow cytometry (FCM).
Results: We found that anti-PD-1 therapy inhibited colon cancer cells metastasis to the small intestine, liver, and lung, and lengthened the survival time of mice. However, the depletion of CD8 suppressed the activity of anti-PD-1 antibodies. In response to anti-PD-1 immunotherapy, the levels of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-12 (IL-12) in serum and mesenteric lymph nodes (MLNs) were significantly increased, while IL-6, IL-17, and transforming growth factor-β (TGF-β) were decreased. CD8 depletion had the opposite effect. In addition, anti-PD-1 treatment significantly increased CD44high, CD62Llow, memory T cells, decreased CD4+, FoxP3+, regulatory T cells, and increased IFN-γ and TNF-α levels in MLNs and spleen. Furthermore, anti-PD-1 treatment cannot exert these roles when CD8 is depleted.
Conclusion: These results suggest that PD-1 inhibitors rely on CD8+ T cells to exert anti-tumor immunity in colon cancer.
Keywords: programmed death 1 (PD-1), CD8 depletion, metastasis, IFN-γ, TNF-α, colon cancer
Introduction
Colon cancer is a common digestive tract malignancy and ranks as the third leading cause of cancer death worldwide.1,2 The highest incidence of colon cancer is in patients 40–50 years of age, with males 2–3 times as likely as females to be diagnosed.1 Over one million new colon cancer cases are diagnosed each year, with approximately 600,000 patients dying of colon cancer.1 In China, especially in underdeveloped areas, the incidence of colon cancer is rapidly increasing, dictating a strong need for effective treatment regiments.3
The primary treatment for colon cancer is surgery supplemented by chemotherapy, immunotherapy, and traditional Chinese medicine.4–6 Despite continued advances in therapy, colon cancer remains a huge threat due to its high rates of recurrence and metastasis. Many anti-cancer immunotherapies are currently being investigated, but tumors escape from the host immune response remain a major obstacle to this treatment modality.7,8
Antagonist antibodies to programmed cell death protein-1 (PD-1)/programmed cell death protein ligand-1 (PD-L1) signaling are currently used in the treatment of some human cancers.13 PD-1, an immune suppressor, is activated by binding to its ligand PD-L1. Previous studies have reported upregulation of PD-1 expression in various activated immune cells in response to viral infections and tumors.9,10 PD-1/PD-L1 signaling can antagonize tumors via down-modulating natural killer (NK)-cells cytotoxicity.11,12 Interruption of PD-1/PD-L1 signaling leads to improved clinical responses in several cancers.13–16 PD-1 regulates anti-tumor immune responses and is significantly lower in the PD-L1-positive tumor regions of non-small cell lung cancer.17
Numerous studies have shown the prognostic value of lymphocyte infiltration in colon cancer. In particular, infiltrating CD8+ cytotoxic T cells contribute to improved survival rates.18–22 These cells can directly bind to antigen through major histocompatibility complex (MHC)-I and have the function of killing target cells. Targeted therapy of PD-1 in human ovarian cancer has been shown to improve the anti-tumor function of NY-ESO-1-specific CD8+ T cells.23 However, the role of PD-1 in CD8-related colon cancer cell metastasis is less well understood.
Multiple apoptotic signaling pathways, such as pathways mediated by interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β), participate in cancer progression, which are important to understanding the role of PD-1 and CD8 in colon cancer metastasis. IFN-γ, a potent immunomodulatory cytokine, is secreted by adaptive and innate immune cells, such as T-cells and NK cells.24 IFN-γ can regulate a variety of effects including anti-proliferative, anti-cancer, and adaptive immune responses, and it is reported to induce apoptosis and suppress the progression of the cell cycle.24,25 TNF-α is a major pro-inflammatory cytokine mainly secreted by macrophages and tumor cells, and it regulates cell apoptosis and survival in cancer.26,27 TGF-β signaling regulates various cellular responses and plays a critical role in the development and carcinogenesis.28,29 Interleukins (IL) mediate the interaction between leukocytes or immune cells and play important roles in transmitting information, activating and regulating immune cells, mediating the activation of T or B cells, proliferation and differentiation, and also inflammatory responses.30–32
In this study, we established an in situ colon cancer mouse model using the CT26 cell line. We found that anti-PD-1 therapy inhibited colon cancer cells metastasis to in the small intestine, liver, and lung. This lengthened the survival time of mice and changed the levels of tumor immunity-associated cytokines in serum and mesenteric lymph nodes (MLNs). In addition, after anti-PD-1 treatment, increased CD44high CD62Llow memory T cells, decreased CD4+ FoxP3+ regulatory T cells, and increased IFN-γ and TNF-α levels were observed in MLNs and spleen. Furthermore, anti-PD-1 treatment cannot exert these effects when CD8 is depleted. These results suggest that PD-1 inhibitors rely on CD8+ T-cells to exert anti-tumor immunity in colon cancer.
Materials and methods
Model establishment
Mouse colon cancer cell line CT26, purchased from the Type Culture Collection of the Chinese Academy of Science (Shanghai, China), was used to establish an in situ colon cancer mouse model using 48 6- to 8-week-old female BALB/c mice. The mice were fasted for 8 hrs before operating and then anesthetized with 0.5% sodium pentobarbital (intraperitoneal injection, 35–50 mg/kg). After routine disinfection of the skin, an incision of about 1 cm was cut in the right lower abdomen of the mouse and the abdominal wall muscle was separated from the peritoneum. The peritoneum was lifted using ophthalmic forceps, cut by an ophthalmic scissor, and the mouse cecum was isolated using ophthalmic forceps. Matrigel gel was mixed with the donor cell suspension (5×107/mL) at 1:1 (20 μL) and inoculated into the serosal layer of the mouse colon. After inoculation, the colon of the mouse was returned to the enterocoelia. Subsequently, the peritoneum, abdominal muscles, and skin were sutured layer by layer, followed by skin disinfection with 75% alcohol. After the operation, no active bleeding was observed, and the mice were observed until they were completely awake. The entire surgical procedure was completed within 2 hrs of preparing the single cell suspension. After surgery, no infection or death occurred in the mice, and obvious tumor metastasis occurred only in the model group. The experiments conducted in this study were approved by the Animal Ethics Committee of Yunnan Cancer Hospital (Kunming, Yunnan, China) and followed the Laboratory Animal Management and Welfare.
Experimental grouping
After 1 week, 36 mice were injected with 250 μg of CD8-depleting antibodies (Clone 53.6.7, BioXcell, West Lebanon, New Hampshire, USA) through tail veins once a day for 2 days. After that, 24 mice were received with 10 mg/kg anti-mouse PD-1 antibody (RMP1–14, rat IgG2b, BioXcell) by tail vein injections once every 2 days for 30 days. During this period, CD8-depleting antibodies were injected once on the 5th and 8th days, and then every 7 days until the mouse died or the experiment ended. The remaining in situ colon cancer mice were injected with equal PBS through tail veins and used as a control. All mice were divided into four groups as follows: tumor (control, n=12), tumor+CD8-depleting antibodies (n=12), tumor+CD8-depleting antibodies+anti-mouse PD-1 antibody (n=12), and tumor+anti-mouse PD-1 antibody (n=12).
Preparation of MLNs and lymphocytes from MLNs
Mice were injected intraperitoneally with 5 mL serum-free RPMI-1640 medium. After gently pressing the abdomen of the mice, the liquid in the abdominal cavity was aspirated and centrifuged for 5 mins at 1,500 r/min. The supernatant of the liquid was used for the subsequent ELISA assay. Next, the cell pellet was resuspended with 1 mL serum-free RPMI-1640 medium. Four milliliters of mouse lymphocyte separation solution (Catalog no. P9000, Solarbio) was added to a 15-mL centrifuge tube and then 1 mL of cell suspension was added to the lymphocyte separation solution. After centrifugation at 1,500 rpm for 5 mins in a horizontal centrifuge, the second layer of gray-white cells of the stratified liquid was taken and washed twice with PBS for flow detection.
Preparation of lymphocytes from spleen
After cervical dislocation, the mice were soaked in 75% ethyl alcohol. Under aseptic conditions, the spleen of the mouse was taken out and fascia removed. The cleaned spleen was placed in Hanks’ solution in a 2-mL centrifuge tube and chopped. A 200-mesh nylon mesh was placed on a six-well plate and ground with a syringe head, and Hanks’ solution was continuously added during the process. The spleen cell suspension was collected and centrifuged at 1,500 rpm for 10 mins, and the cells were resuspended in 5 mL of serum-free RPMI1640 medium. A total of 4 ml of mouse lymphocyte separation solution (Catalog no. P9000, Solarbio) was added to a 15-mL centrifuge tube and then 1 mL of cell suspension was added to the lymphocyte separation solution. After centrifugation at 1,500 rpm for 5 mins in a horizontal centrifuge, the second layer of gray-white cells of the stratified liquid was taken and washed twice with PBS for flow detection.
Hematoxylin–eosin (HE) staining
Six mice in each group were treated continuously in the above manner until the mice died, and the liver, lung, and intestines were collected. After embedding and fixing, the tissues were cut into 4–7 µm sections, which were then baked for 30 mins in an oven at 65°C. Later, the sections were immersed in xylene I and xylene II (Shanghai Sinopharm) for 15 mins, sequentially immersed in 100%, 95%, 85%, and 75% ethanol for 5 mins, and rinsed for 10 mins with tap water. Thereafter, the sections were stained in hematoxylin solution for 5 mins, followed by color separation for several seconds in ammonia water. Following 15 mins of rinsing with running water, the sections were dehydrated for 10 mins sequentially in 70% and 90% alcohol, and then stained in eosin solution for 1–2 mins, followed by alcohol dehydration. The tissue sections were imaged on a microscope (ECLIPSE Ni, NIKON) and then analyzed using IMS image analysis system (DS-Ri2, NIKON).
Enzyme-linked immunosorbent assay (ELISA)
After continuous treatment in the above manner, the mice were killed and the blood and MLNs were collected. After 1 hr, the blood was centrifuged at 3,000 rpm for 10 mins, and then the serum in the supernatant was obtained. The levels of tumor immune-related cytokines in serum and MLNs were detected by ELISA (X-Y Biotechnology, Shanghai, China) according to the manufacturer’s instructions.
Flow cytometry (FCM) detection
After death, MLNs and spleen were collected for FCM detection. Lymphocytes were isolated from MLNs and spleen, and then overnight incubated with PerCP-conjugated Rat Anti-CD8 antibody (catalog no.553,036, BD Biosciences, Franklin Lakes, NJ, USA), FITC-conjugated Mouse Anti-Human CD44 antibody (catalog no.560,977, BD Biosciences), and PE-conjugated Mouse Anti-Human CD62L antibody (catalog no.555,544, BD Biosciences) at 4°C. After centrifugation for 10 mins at 3,000 rpm to remove the antibodies, the samples were washed 3 times with PBS and resuspended in 200 µL PBS. Likewise, 100 µL of cell suspension was further incubated with antibodies against CD3 (catalog no.12–0031082, PE-conjugated, Thermo Fisher Scientific, eBioscienceTM, Waltham, MA, USA) and CD4 (catalog no.12–0041-82, PE-conjugated, Thermo Fisher Scientific, eBioscience™) or CD8 (catalog no.12–0081-81, PE-conjugated, Thermo Fisher Scientific, eBioscience™) and CD45 (catalog no.11–0451-82, FITC-conjugated, Thermo Fisher Scientific, eBioscience™) at 4°C in the dark for 1–2 hrs. Following fixation in 1×FIX buffer at 4 °C for 30 mins, the samples were centrifuged and then resuspended in 0.5 mL PBS (containing 0.1% Triton X-100) at 4°C for 30 mins in the dark. Finally, samples were incubated with antibodies against PD-1 (catalog no.12–9985-82, PE, Thermo Fisher Scientific, eBioscienceTM), FoxP3 (catalog no.12–5773-82, PE, Thermo Fisher Scientific, eBioscience™), IFN-γ (catalog no.11–7311-82, FITC, Thermo Fisher Scientific, eBioscience™), and TNF-α (catalog no.12–7321-82, PE, Thermo Fisher Scientific, eBioscience™). After another centrifugation step, the samples were resuspended in 200 µL PBS. Subsequently, a flow cytometer was used to study the ratio of CD44+ CD62L− in CD8+ cells, CD4+ FoxP3+ cells in CD3+ positive cells, and CD8+ IFN-γ+ TNF-α+ cells in CD45+ positive cells were evaluated using the BD AccuriTM C6 Software (Version 1.0.264.21, BD Biosciences, USA).
Statistical analysis
Statistical significance was analyzed using the software of GraphPad prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). All results were shown as mean±SD with 3 repeated experiments. The significance among three or more comparisons was analyzed by one-way analysis of variance (ANOVA) posted with Tukey’s multiple comparison. P-value<0.05 indicated statistical significance.
Results
PD-1 expression was significantly elevated in CD8+ cells in the colon cancer mouse model
After establishment of the colon cancer mouse model, the expression of PD-1 was analyzed by FCM. As shown in Figure 1, PD-1 expression was significantly elevated in CD8+ cells of the MLNs and spleen of colon cancer mice, suggesting that PD-1 may contribute to colon cancer progression.
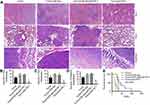
Anti-PD-1 treatment, depending on CD8+ cells, inhibited colon cancer cells metastasize to the intestine, liver, and lung, and lengthened the survival time of mice
After treatment of colon cancer positive mice with CD8-depleting and anti-PD-1 antibodies, liver, lung, and intestinal tissues were collected. HE staining of the liver, lung, and intestines found that anti-PD-1 treatment significantly inhibited the metastasis of colon cancer cells to liver, lung, and intestines. This inhibitory effect was decreased by CD8 depletion (Figure 2A), as evidenced by the increase in the number of metastases to the liver, lung, and intestine (Figure 2B-D). In addition, the survival time of Tumor/anti-PD-1 mice was significantly shortened after CD8 depletion (Figure 2E). These findings indicate that anti-PD-1 therapy could inhibit colon cancer cell metastasis and lengthen the survival time of mice, which may be dependent on CD8+ cells.
Anti-PD-1, depending on CD8+ cells, regulated the levels of tumor immune-related cytokines in serum and MLNs of colon cancer mice
Colon cancer mice were treated with CD8-depleting and anti-PD-1 antibodies, and then serum and MLNs were collected to detect the cytokines expression. ELISA assays found that in response to anti-PD-1 antibody, the levels of IFN-γ (Figure 3A), TNF-α (Figure 3B), and IL-12 (Figure 3C) in serum were significantly increased, and IL-6 (Figure 3D), IL-17 (Figure 3E), and TGF-β (Figure 3F) were decreased, whereas CD8 depletion had the opposite effect. Anti-PD-1 treatment in CD8 depleted-mice had no significant effect on the expression of these cytokines. In addition, there was a similar tendency with the expression of IFN-γ, TNF-α, IL-12, IL-6, IL-17, and TGF-β in MLNs to serum (Figure 4A-F). Taken together, we inferred that anti-PD-1 treatment, depending on CD8+ cells, regulated the expression of tumor immune-related cytokines in the serum and MLNs of mice with colon cancer.
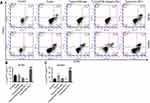
Anti-PD-1 treatment, depending on CD8+ cells, increased CD44high CD62llow memory T cell in MLNs and spleen of colon cancer mice
Further, after CD8 depletion and anti-PD-1 treatment of colon cancer mice, FCM detection of the cell proportion of CD44 and CD62L in CD8+ cells in MLNs and spleen found that the number of CD44high CD62Llow memory T cell in CD8+ cells in MLNs (Figure 5A and B) and spleen (Figure 5A and C) was significantly increased by anti-PD-1 treatment, while CD8 depletion strongly reversed the effect of anti-PD-1. Furthermore, anti-PD-1 antibody cannot exert this effect when CD8 is depleted. Therefore, we conjecture that CD8 depletion reduced the numbers of CD8+ memory T cells in the MLNs and spleen of colon cancer mice. Depending on CD8+ cell expression, anti-PD-1 treatment could increase memory T cell in MLNs and spleen of colon cancer mice.
Anti-PD-1 treatment, depending on CD8+ cells, decreased CD4+ Foxp3+ regulatory T cell in MLNs and spleen of colon cancer mice
After CD8 depletion and anti-PD-1 treatment of colon cancer mice, FCM was conducted to detect the cell proportion of CD4+ and FoxP3+ in CD3+ cells in MLNs and spleen. CD3 is present on the surface of peripheral blood T cells and forms a complex with the T cell antigen receptor (TCR) to transmit the antigen signal to the cell. CD4, the main surface marker of helper T cell (Th), binds to the MHC-II molecule and stabilizes the binding of the TCR to the antigen peptide-MHC molecule complex, helping to initiate signal transduction. The winged-helix/forkhead transcription factor, FoxP3, is critical in T regulatory cells.33,34 In our study, as shown in Figure 6, we found that the percentages of CD4+ FoxP3+ regulatory T cell in both MLNs (Figure 6A and B) and spleen (Figure 6A and C) were significantly decreased by anti-PD-1 treatment, which was potently counteracted by CD8 depletion. Moreover, the increase in CD4+ FoxP3+ regulatory T cell in MLNs and spleen after CD8 depletion in colon cancer mice was not reversed by anti-PD-1. These indicated that CD8 depletion increased the number of regulatory T cell in CD3+ cells in MLNs and spleen of colon cancer mice, and anti-PD-1 could decrease regulatory T cell in the MLNs and spleen of colon cancer mice via CD8 cells.
Anti-PD-1 treatment, depending on CD8+ cells, increased CD8+ IFN-γ+ TNF-α+ in MLNs and spleen of colon cancer mice
After CD8 depletion or treatment with anti-PD-1 in colon cancer mice, FCM detected the proportion of CD8+ cells secreting IFN-γ and TNF-α. In response to anti-PD-1 treatment, the percentages of CD8+ IFN-γ+ TNF-α+ cells in both MLNs (Figure 7A and B) and spleen (Figure 7A and C) were significantly increased, while CD8 depletion potently counteracted the effect of anti-PD-1 treatment. In addition, anti-PD-1 could not exert this effect when CD8 was depleted in the colon cancer mice. It has been reported that IFN-γ and TNF-α are cytokines produced by lymphocytes (CD4+ and CD8+) that regulate apoptosis, survival, and anti-cancer effects.35 These results show that CD8 depletion reduced the secretion of IFN-γ and TNF-α by CD8+ cells in the MLNs and spleen of colon cancer mice. Depending on the presence or absence of CD8+ cells, anti-PD-1 treatment could increase the secretion of IFN-γ and TNF-α in the MLNs and spleen of colon cancer mice.
Discussion
Cumulative reports have shown that inhibition of PD-1 contributes to the treatment of various cancers. For example, patients with colon cancer, renal cell cancer, or melanoma are partially or completely responsive to immunotherapy with anti-PD-1 antibodies.36 An anti-PD-1 immune checkpoint inhibitor, nivolumab, has shown clinically meaningful activity in the treatment of advanced, refractory, squamous non-small cell lung cancer.37 Further, it has been reported that blockade of PD-1 can overcome immune resistance and has anti-tumor activity.16 In this study, we established an in situ colon cancer mouse model with the CT26 cell line. HE staining revealed that anti-PD-1 treatment, depending on CD8+ cells, inhibits colon cancer cells metastasis to the intestines, liver, and lung, which lengthened the survival time of colon cancer mice. This suggests that the CD8+ cell-dependent inhibition of PD-1 may be a viable treatment for colon cancer.
Further, the underlying mechanisms of anti-PD-1 regulation of colon cancer cell metastasis were investigated. It is reported that IFN-γ plays a role in anti-tumor immunity and is critical in suppressing the early development of cancer. IFN-γ is often associated with TNF-α, a cytokine that controls infection and cancer,38,39 and IL-12 mainly acts on T cells and NK cells, inducing cytotoxic activity of CTL and NK cells and promoting the secretion of cytokines such as IFN-γ and TNF-α. These defined functions agree with our findings that the levels of IFN-γ, TNF-α, and IL-12 in serum and MLNs were significantly increased after treatment with anti-PD-1 antibody. TGF-β is important for the maintenance of the peripheral T regulatory cells.40,41 IL-6 can effectively promote TNF-induced cachexia, and IL-17, secreted by CD4+ T cell, is the main effector of Th17 cells which can secrete IL-17A, IL-17F, IL-6, and TNF-a, and effectively mediate the inflammatory response. Consistent with these, decreased IL-6, IL-17, and TGF-β were found in serum and MLNs after anti-PD-1 antibody treatment. However, CD8 depletion potently counteracted the effect of anti-PD-1 treatment, suggesting the involvement of these tumor-related cytokines in the regulation of colon cancer by PD-1. Moreover, anti-PD-1 treatment, depending on CD8+ cells, significantly increased CD44high CD62Llow memory T cell and decreased CD4+ FoxP3+ regulatory T cell in MLNs and spleen, as well as increased secretion of IFN-γ and TNF-α. These results are consistent with the previous reports that CD4+ FoxP3+ regulatory T cell can suppress immune disorders.42 Based on these findings, it can be inferred that a PD-1 inhibitor, depending on CD8+ cells, inhibits colon cancer cell metastasis, possibly through the modulation of memory T cell and regulatory T cell via apoptotic signaling by IFN-γ, TNF-α, and TGF-β.
In summary, the present study demonstrates the CD8+ cell-dependent inhibitory effect of anti-PD-1 treatment on the metastasis of colon cancer cells. This activity may be due to the modulation of memory and regulatory T cells via apoptotic signaling by IFN-γ, TNF-α, and TGF-β. Therefore, PD-1 is an exciting potential target for the remission or even cure of colon cancer.
Acknowledgment
This work was funded by Yunnan Applied Basic Research Projects (Grant 2016FC007), the joint special funds for the Department of Science & Technology of Yunnan Province-Kunming Medical University (Grant 2017FE468), and Key Laboratory of Translational Medicine for Cell Therapy of Yunnan Province (Grant 2015DG034).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2010;60(5):277–300. doi:10.3322/caac.20073
2. Ung L, Lam KY, Morris DL, Chua TC. Tissue-based biomarkers predicting outcomes in metastatic colorectal cancer: a review. Clin Transl Oncol. 2014;16(5):425–435. doi:10.1007/s12094-013-1154-6
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Sostres C, Gargallo CJ, Lanas A. Aspirin, cyclooxygenase inhibition and colorectal cancer. World J Gastrointest Pharmacol Ther. 2014;5(1):40–49. doi:10.4292/wjgpt.v5.i1.40
5. Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359(9325):2224–2229. doi:10.1016/S0140-6736(02)09290-5
6. André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. doi:10.1200/JCO.2008.20.6771
7. Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv324–328rv324. doi:10.1126/scitranslmed.aad7118
8. Turcotte S, Gros A, Hogan K, et al. Phenotype and function of T cells infiltrating visceral metastases from gastrointestinal cancers and melanoma: implications for adoptive cell transfer therapy. J Immunol. 2013. doi:10.4049/jimmunol.1300538
9. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi:10.1084/jem.20100643
10. Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen–specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi:10.1084/jem.20100637
11. Benson DM, Bakan CE, Mishra A, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel, monoclonal anti-PD-1 antibody. Blood. 2010. doi:10.1182/blood-2010-02-271874
12. Ndhlovu LC, Lopez-Vergès S, Barbour JD, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012. doi:10.1182/blood-2011-11-392951
13. Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19(5):1021–1034. doi:10.1158/1078-0432.CCR-12-2063
14. Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (Anti–PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi:10.1056/NEJMoa1305133
15. Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med E. 2012;366(26):2455–2465. doi:10.1056/NEJMoa1200694
16. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 Antibody in Cancer. N Engl J Med. 2012;366(26):2443–2454. doi:10.1056/NEJMoa1200690
17. Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. doi:10.1158/1078-0432.CCR-04-0428
18. Di Giorgio A, Botti C, Tocchi A, Mingazzini P, Flammia M. The influence of tumor lymphocytic infiltration on long term survival of surgically treated colorectal cancer patients. Int Surg. 1992;77(4):256–260.
19. Jass JR, Love SB, Northover JMA. A new prognostic classification of rectal canceR. Lancet. 1987;329(8545):1303–1306. doi:10.1016/S0140-6736(87)90552-6
20. Guidoboni M, Gafà R, Viel A, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159(1):297–304. doi:10.1016/S0002-9440(10)61695-1
21. Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–3494.
22. Diederichsen ACP, Hjelmborg J, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52(7):423–428. doi:10.1007/s00262-003-0388-5
23. Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107(17):7875–7880. doi:10.1073/pnas.1003345107
24. Gattoni A, Parlato A, Vangieri B, Bresciani M, Derna R. Interferon-gamma: biologic functions and HCV therapy (type I/II) (1 of 2 parts). Clin Ter. 2006;157(4):377–386.
25. Stark GR, Kerr IM, Williams BRG, Silverman RH, Schreiber RD. How cells respond to interferons. AnnuRev Biochem. 1998;67(1):227–264. doi:10.1146/annurev.biochem.67.1.227
26. Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-α as a tumour promoter. Eur J Cancer. 2006;42(6):745–750. doi:10.1016/j.ejca.2006.01.012
27. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361. doi:10.1038/nrc2628
28. Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700.
29. Derynck R, Zhang YE. Smad-dependent and smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577. doi:10.1038/nature02006
30. Diouf I, Fievet N, Doucouré S, et al. IL-12 producing monocytes and IFN-γ and TNF-α producing T-lymphocytes are increased in placentas infected by plasmodium falciparum. J Reprod Immunol. 2007;74(1):152–162. doi:10.1016/j.jri.2006.10.001
31. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17–producing human T helper cells. Nat Immunol. 2007;8:942. doi:10.1038/ni1496
32. Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363. doi:10.1038/ni1537
33. Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22(1):531–562. doi:10.1146/annurev.immunol.21.120601.141122
34. Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212(1):8–27. doi:10.1111/j.0105-2896.2006.00427.x
35. Yoo HJ, Byun H-J, Kim B-R, Lee KH, Park S-Y, Rho SB. DAPk1 inhibits NF-κB activation through TNF-α and INF-γ-induced apoptosis. Cell Signal. 2012;24(7):1471–1477. doi:10.1016/j.cellsig.2012.03.010
36. Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19(2):462–468. doi:10.1158/1078-0432.CCR-12-2625
37. Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (checkMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi:10.1016/S1470-2045(15)70054-9
38. Willimsky G, Czéh M, Loddenkemper C, et al. Immunogenicity of premalignant lesions is the primary cause of general cytotoxic T lymphocyte unresponsiveness. J Exp Med. 2008;205(7):1687–1700. doi:10.1084/jem.20072016
39. Shankaran V, Ikeda H, Bruce AT, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107. doi:10.1038/35074122
40. Huber S, Schramm C, Lehr HA, et al. Cutting edge: TGF-β signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T Cells. J Immunol. 2004;173(11):6526–6531. doi:10.4049/jimmunol.173.11.6526
41. Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26(5):579–591. doi:10.1016/j.immuni.2007.03.014
42. Kwon H-K, Lee C-G, So J-S, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci. 2010;107(5):2159–2164. doi:10.1073/pnas.0904055107
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.