Back to Journals » Vascular Health and Risk Management » Volume 18
PCSK9 Inhibitors in the Management of Cardiovascular Risk: A Practical Guidance
Authors Jia X, Al Rifai M , Saeed A, Ballantyne CM, Virani SS
Received 5 May 2022
Accepted for publication 13 July 2022
Published 20 July 2022 Volume 2022:18 Pages 555—566
DOI https://doi.org/10.2147/VHRM.S275739
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Xiaoming Jia,1 Mahmoud Al Rifai,1 Anum Saeed,2 Christie M Ballantyne,1 Salim S Virani1,3
1Department of Medicine, Baylor College of Medicine, Houston, TX, USA; 2Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA, USA; 3Department of Medicine, Michael E. DeBakey Veterans Affairs Medical Center, Houston, TX, USA
Correspondence: Salim S Virani, Michael E. DeBakey Veterans Affairs Medical Center, 2002 Holcombe Boulevard, Houston, TX, 77030, USA, Tel +1 713-440-4410, Email [email protected]
Abstract: Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are potent medications in the toolkit for treatment of atherosclerotic cardiovascular disease. These agents have been well studied in clinical trials supporting their efficacy in dramatically reducing low-density lipoprotein cholesterol (LDL-C) and impact on cardiovascular outcomes. Since the approval of commercial use for PCSK9 inhibitors in 2015, we have also gained significant experience in the use of these therapeutics in the real-world setting. In this article, we review current guideline recommendations, clinical trial evidence on efficacy and safety as well as data on cost-effectiveness, prescription and adherence. We focus primarily on the monoclonal antibody class of PCSK9 inhibitors in this review while also touching on other types of therapeutics that are under development.
Keywords: PCSK9 inhibitor, CVD prevention, lipids
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are potent medications that lower low-density lipoprotein (LDL) cholesterol (LDL-C) for the treatment of atherosclerotic cardiovascular disease (ASCVD). In humans, PCSK9 regulates the recycling of LDL receptors (LDL-R), with inhibition of PCSK9 leading to increased LDL receptors present at the cell surface for binding and removal of circulating LDL particles.1,2 From a historic perspective, observations that gain of function mutations in PCSK9 were a cause for familial hypercholesterolemia while loss of function mutations lower LDL-C led to the recognition of PCSK9 as a biologic target for cholesterol lowering.3–6 Thus, PCSK9 inhibitors were probably one of the first cholesterol-lowering medications to be conceived directly on the concept of targeting select genes that act as drivers of ASCVD.
As of the writing of this review, there are two commercially available monoclonal antibody (mAb) agents, alirocumab and evolocumab, each with supportive cardiovascular outcome trial data.7,8 These medications are given either every 2 weeks or monthly via subcutaneous injection. PCSK9 inhibitors have largely been used in the secondary prevention of ASCVD but use in patients with genetic hypercholesterolemia disorders with very high LDL-C levels is also indicated.9,10 A small interfering ribonucleic acid (siRNA) therapeutic targeting PCSK9, inclisiran, has also become commercially available, although the cardiovascular outcome trial for this agent is ongoing. There are also several protein, RNA and DNA-based technologies still in the research pipeline.11,12 In this clinically oriented review, we focus primarily on mAb-based PCSK9 inhibitors given the large quantity of available data and real-world experience in their use. However, we dedicate a short section later in this article to discuss inclisiran as well as other novel PCSK9 inhibitors that are in development.
Effect PCSK9 Inhibition on Lipid Parameters and Biomarkers
PCSK9 inhibitors are potent agents for the lowering of LDL-C. In phase 3 trials, evolocumab and alirocumab have been shown to reduce LDL-C from baseline, ranging from 36% to 62% at 24 weeks when added to high intensity or maximally tolerated statin therapy.13–16 PCSK9 inhibitors, as a monotherapy, have been shown to reduce LDL-C by 47–57%.17,18 Among patients with significant statin associated muscle side effects not able to tolerate statin therapy, PCSK9 inhibition reduced LDL-C by 45–56%.19–22 PCSK9 inhibitors also reduce levels of apolipoprotein B (apoB), non-high-density lipoprotein cholesterol (non-HDL-C), lipoprotein (a), and triglycerides (TG) as well as increase of HDL-C and apolipoprotein A1 (apoA1).15 For instance, in the ODYSSEY LONG-TERM phase 3 trial, alirocumab reduced apoB by 54.0%, non-HDL-C by up to 52.3% and TG by 17.3%, as well as increased HDL-C by 4.6% and apoA1 by 2.9%.14
PCSK9 inhibitors have also been shown to reduce levels of lipoprotein (a) (Lp(a)), although the mechanism is not completely understood. Significant reductions in Lp(a) have not been observed with statins or ezetimibe. Data from clinical trials found percent reduction of Lp(a) mostly in the range of 20–36% from baseline with PCSK9 inhibitor treatment.23–25 Lp(a) has been shown in Mendelian randomization and genome-wide association studies to represent a likely causal risk factor for ASCVD.26,27 Analysis from cardiovascular outcomes trials of both alirocumab and evolocumab have shown that individuals with elevated Lp(a) levels have the highest risk for major adverse cardiovascular events and derive the most benefit from PCSK9 inhibitor therapy.24 Moreover, reduction in Lp(a) while on PCSK9 inhibitor was associated with reduction in adverse events independent of LDL-C reduction.28,29 The evidence supporting efficacy of PCSK9 inhibitors in patients with elevated Lp(a) levels and high ASCVD risk is important given the dearth of effective therapies that directly target Lp(a) lowering for ASCVD risk reduction. Moreover, recent analysis from the FOURIER and ODYSSEY-OUTCOMES trials found that treatment with PCSK9 inhibitors was associated with decreased risk for venous thromboembolism (VTE) and found that Lp(a) reduction may be a mediator for this effect.30 Relatedly, alirocumab have been shown in animal models to reduce factor VIII levels which may be another potential mechanism by which PCSK9 inhibition may reduce risk for VTEs.31
PCKS9 inhibitors have not been shown to significantly affect inflammatory markers. In the FOURIER and ODYSSEY-OUTCOMES trials, treatment with a PCSK9 inhibitor did not significantly alter levels of high-sensitivity C-reactive protein (hs-CRP). However, elevated hs-CRP identified higher risk individuals who derive greater absolute risk reduction from the use of PCSK9 inhibitors.8,32,33
Use of PCSK9 Inhibitors in High-Risk Secondary Prevention
PCSK9 inhibitors are primarily used in secondary prevention settings among patients with very high risk of ASCVD events. The efficacy in PCSK9 inhibition for secondary ASCVD prevention is supported by two large cardiovascular outcomes trials – FOURIER for evolocumab and ODYSSEY-OUTCOMES for alirocumab7,8 (Table 1). In the FOURIER trial, 27,564 patients with clinical ASCVD and LDL-C ≥70 mg/dL or a non–HDL-C level ≥100 mg/dL on mostly high intensity statin ± ezetimibe were randomized to evolocumab 140 mg subcutaneously every 2 weeks or 420 mg monthly versus placebo. At a median follow-up of 2.2 years, there was a 15% relative risk reduction (1.5% absolute risk reduction) in the primary composite outcome of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization in the evolocumab group compared with controls. Among individual endpoints, those randomized to evolocumab had lower rates of MI, strokes and coronary revascularization but did not have significant difference in cardiovascular deaths compared with placebo. Furthermore, evaluation of treatment with evolocumab on coronary artery plaque using intravascular ultrasound among statin-treated individuals in the GLAGOV randomized controlled trial found modest but significant reduction in percent atheroma volume (−1.0%, 95% CI: −1.8% to −0.64%). After 76 weeks of treatment, there was also a greater proportion of patients exhibiting plaque regression in the evolocumab group compared with control (64.3% vs 47.3%).34 Similarly, in the PACMAN-AMI trial, treatment with alirocumab after urgent percutaneous coronary intervention of the culprit lesion among patients with acute MI was associated with significantly greater coronary plaque regression in the non-culprit vessels as determined by intravascular ultrasound after 52 weeks.35
 |
Table 1 Cardiovascular Outcome Trials of PCSK9 Inhibitors |
The efficacy and safety of alirocumab was evaluated in the ODYSSEY-OUTCOMES randomized placebo-controlled trial, which included 18,924 patients who had an acute coronary syndrome in the preceding 1–12 months. Over a follow-up duration of 2.8 years, the primary outcome (composite of death from coronary heart disease, non-fatal MI, fatal or non-fatal ischemic stroke, or unstable angina requiring hospitalization) for alirocumab vs placebo was 9.5% vs 11.1%, respectively, resulting in a relative risk reduction of 15% (absolute risk reduction 1.6%). Among secondary outcomes, alirocumab group was found to have lower major coronary heart disease events, any cardiovascular event but not mortality.
A third PCSK9 inhibitor, bococizumab, was evaluated in the SPIRE trials, which recruited predominantly secondary prevention patients but also a subset of high-risk primary prevention patients.36 This mAb differed from evolocumab and alirocumab as it is a humanized mAb containing small regions of murine sequences. In SPIRE-1, no significant differences in the primary endpoint (non-fatal MI, non-fatal stroke, hospitalization for unstable angina requiring urgent revascularization, or CV death) were observed among randomized patients with LDL-C ≥70 mg/dl over a 7 months follow-up period. However, in SPIRE-2 which enrolled participants with a higher LDL-C of ≥100 mg/dL, there was a significant risk reduction in the primary endpoint, suggesting that clinical benefit can be achieved with treatment among a higher risk cohort over a longer period. However, development of bococizumab was discontinued by sponsors largely due to high rates of antidrug antibodies.
Since the publication of the outcomes trials, both European and North American guidelines on cholesterol management have incorporated the use of PCSK9 inhibitors in treatment algorithms (Table 2). The 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) Dyslipidemia Guidelines recommend treatment to an LDL-C goal of ≥50% from baseline and <1.4 mmol/L (<55 mg/dL) among patients with established ASCVD, with consideration for an LDL-C goal of <1.0 mmol/L (<40 mg/dL) for patients with ASCVD, who experience a second vascular event within 2 years while taking maximally tolerated statin therapy.9 With respect to therapy selection, ESC/EAC guideline recommends a stepwise approach starting with high intensity statin (maximally tolerated) with the addition of ezetimibe followed by PCSK9 inhibitor therapy in order to reach the LDL-C goals, though PCSK9 inhibitors can also be added directly to statin therapy. The average LDL-C reduction from high-intensity statin plus ezetimibe is around 65% while high-intensity statin plus PCSK9 inhibitor can reduce LDL-C by an average of 75%.
 |
Table 2 Indications for PCSK9 Inhibitor Use Based on Contemporary European and North American Cholesterol Guidelines |
The 2018 American College of Cardiology (ACC)/American Heart Association (AHA) Multi-society Guideline on the Management of Blood Cholesterol recommends the use of PCSK9 inhibitors for secondary prevention patients at very high risk of ASCVD.10 It should be noted that the definition of “very high risk” differs between the North American and European guidelines. Whereas all secondary prevention and even some primary prevention patients fall into very high risk in the European guideline, very high risk ASCVD per the North American guideline consists of those with a history of multiple major ASCVD events (recent ACS within past 12 months, history of MI, history of ischemic stroke, symptomatic PAD) or one major ASCVD event with at least 2 high-risk conditions (age ≥65 years, heterozygous familial hypercholesterolemia, history of prior coronary revascularization outside of major ASCVD events, diabetes mellitus, hypertension, chronic kidney disease, current smoking, persistently elevated LDL-C ≥100 mg/dL despite maximally tolerated statins and ezetimibe, and history of congestive heart failure). The guideline recommends this approach as “very high-risk ASCVD patients” are the ones who have the highest risk of recurrent ASCVD events and therefore, derive more absolute risk reduction from aggressive LDL-C lowering with PCSK9 inhibitor therapy.37 Moreover, the North American guidelines recommend initiation of PCSK9 inhibitors on top of high-intensity or maximally tolerated statin therapy and ezetimibe, if LDL-C remains ≥70 mg/dL or non-HDL-C remains ≥100 mg/dL.
Practical considerations when deciding choice for adjunct therapy to maximally tolerated statins among very high-risk patients include patient preference, cost/insurance coverage, degree of ASCVD risk, extent of LDL-C reduction still needed and patient adherence. For instance, the addition of ezetimibe is likely reasonable for a patient with stable chronic coronary artery disease and LDL-C of 80 mg/dL on a high-intensity statin. Meanwhile, a patient with multiple myocardial infarctions within a two-year period who has a LDL-C of 100 mg/dL on maximally tolerated statin may warrant the addition of a PCSK9 inhibitor as the second lipid lowering agent. Early measurement of lipids, especially those with acute coronary syndrome, is indicated in order to assess adherence and to initiate additional therapies to bring LDL-C levels to goal.38,39 As such, the guidelines recommend initiation of PCSK9 inhibitors among ACS patients who do not reach LDL-C goals after 4–6 weeks on maximally tolerated statin and ezetimibe therapy. The European guidelines included consideration for initiating PCSK9 inhibitor in-hospital after an ACS event if the patient is already on maximally tolerated statin plus ezetimibe prior to the event.9 The EVOPACS trial, which assessed feasibility, efficacy and safety of initiating evolocumab during hospitalization for very high-risk patients with recent ACS, demonstrated that PCSK9 inhibitor added to a high-intensity statin during the in-hospital phase of ACS was safe and resulted in a significant proportion of participants achieving target LDL-C levels.40 However, at this time, there are as yet no outcomes data to support this practice especially among participants who are not optimized or fully adherent to statins.
Ischemic Stroke and Peripheral Artery Disease
It is important to recognize that patients with a history of stroke and symptomatic peripheral artery disease (PAD) derive similar risk reduction for ASCVD events with PCSK9 inhibitor treatment compared with those with coronary heart disease. Moreover, those with multi-bed vascular disease have been shown to be at higher risk compared with individuals with only coronary heart disease and thus can derive great absolute risk reduction from treatment.41 Finally, analysis from RCTs further showed that treatment with a PCSK9 inhibitor reduces risk for ischemic stroke and limb events. In FOURIER sub-analysis, evolocumab significantly reduced all stroke (HR 0.79, 95% CI: 0.66–0.95) and ischemic stroke (HR 0.75, 95% CI: 0.62–0.92), with no difference in hemorrhagic stroke.42 This was also seen in ODYSSEY-OUTCOMES where alirocumab reduced risk for any stroke (HR 0.72, 95% CI: 0.57–0.91) and ischemic stroke (HR 0.73, 95% CI: 0.57–0.93) without increasing hemorrhagic stroke.43 The effect of PCSK9 inhibitor was similar among those with and those without a history of prior cerebrovascular disease. Meta-analysis of 16 RCTs of PCSK9 inhibitors including 39,104 patients showed that treatment with a PCSK9 inhibitor was associated with significantly lower risk for ischemic stroke (RR 0.77, 95% CI: 0.64–0.93) compared with those not treated, with no significant difference in risk of neurocognitive deficits.44
With respect to patients with PAD, a pre-specified analysis from FOURIER consisting of participants with lower limb PAD demonstrated that these patients were at higher absolute risk of both major adverse cardiovascular events (MACE) as well as major adverse limb events (MALE) with treatment using evolocumab resulting in relative risk reduction of the primary composite endpoint by 21% and MALE by 42%.45 Similar results were found for alirocumab in analysis from ODYSSEY-OUTCOMES where treatment with PCSK9 inhibitor among patients with a history of PAD resulted in significant relative risk reduction of limb events by 41% and absolute risk reduction of 8.6%.29
Studies on statin utilization have shown that LDL-C is undertreated among patients with ischemic cerebrovascular disease and even more so among those with PAD compared with patients with coronary heart disease.46,47 As such, clinicians must recognize the significant risk for adverse cardiovascular events that are associated with atherosclerotic disease within both cardiac as well as non-cardiac vascular beds, and initiate/intensify therapies accordingly. While improving appropriate statin prescription and ensuring statin adherence remains of upmost importance, practitioners should further work to avoid clinical inertia in the utilization of non-statin lipid-lowering therapies such as PCSK9 inhibitors when necessary.48–51
Familial Hypercholesterolemia and Other Primary Prevention Considerations
Patients with familial hypercholesterolemia (FH) are at high risk for ASCVD events due to prolonged lifetime exposure to high LDL-C levels, usually starting from childhood. Risks for adverse cardiovascular events are further compounded as many individuals with FH also have elevated lipoprotein (a) (Lp(a)). In the European guidelines, both FH patients with ASCVD as well as primary prevention FH patients with another major risk factor such as diabetes mellitus or advanced chronic kidney disease are categorized as very high risk with recommended LDL-C goals of ≥50% reduction from baseline and an LDL-C <1.4 mmol/L (<55 mg/dL). FH patients without ASCVD or another major risk factor are categorized as high risk with LDL-C reduction goals of ≥50% reduction of LDL-C from baseline and an LDL-C <1.8 mmol/L (<70 mg/dL). Statins remain the foundation of lipid-lowering therapy for FH patients and evidence suggests that early initiation of statins can reduce progression of atherosclerosis and lower risk for cardiovascular events in adulthood.52,53 However, not all patients can achieve adequate LDL-C lowering on statin therapy alone. Especially in those at very high ASCVD risk, the addition of adjunct therapy such as ezetimibe and PCSK9 inhibitors may be necessary. In the North American AHA/ACC Multisociety guidelines, PCSK9 inhibitors should be considered in heterozygous FH (HeFH) patients aged 30–75 years with an LDL-C level ≥2.6 mmol/L (100 mg/dL) while on maximally tolerated statins and ezetimibe. RCTs have shown that treatment with evolocumab or alirocumab can significantly reduce LDL-C in patients with HeFH without significant adverse side effects. In the RUTHERFORD trials, evolocumab was shown to reduce LDL-C by 60% at 12 weeks with continued use shown to be associated with persistent LDL-C reduction with a mean of 53.6% after 48 weeks.54–56 Similarly, data from ODYSSEY FH1 and FH2 found reduction of LDL-C within a range of 50–60% in the PCSK9 group versus placebo after 24 weeks, with effect persisting at 78 weeks.57 There are currently no trials specific for HeFH patients that assess PCSK9 inhibitor and ASCVD outcomes. The effect of evolocumab on homozygous HF (HoFH) was also assessed in the TESLA trial.58 At 12 weeks, evolocumab significantly reduced LDL-C by 30.9% vs placebo. However, some patients with HoFH may have very low to no expression of LDL-R in the liver and thus will have a lower response to PCSK9 inhibition.59
PCSK9 inhibitors are rarely needed in non-FH primary prevention patients and currently there is a lack of strong clinical evidence to support such use. Even so, extrapolating from data demonstrating continued risk reduction for ASCVD with LDL-C lowering, primary prevention patients at very high risk may benefit from PCSK9 inhibitors when LDL-C goals are not achieved via treatment with statins and ezetimibe. Both the European and North American guidelines provide recommendations for the use of PCSK9 inhibitors in these very high-risk primary prevention patients. In the European guidelines, primary prevention patients at very high risk are defined as diabetes mellitus with target organ damage or at least three major risk factors, or early onset of type 1 diabetes mellitus of long duration (>20 years); severe CKD (eGFR <30 mL/min/1.73m2); calculated SCORE ≥10% for 10-year risk of fatal CVD; or FH with another major risk factor. In the North American guidelines, adults 40–75 years with severely elevated LDL-C levels ≥5.7 mmol/L (220 mg/dL) with LDL-C of ≥3.4 mmol/L (130 mg/dL) while on maximally tolerated statins and ezetimibe should also be considered for PCSK9 inhibitor therapy.
Side Effects
Large RCTs have shown that PCSK9 inhibitors are overall safe and well-tolerated. Common side effects associated with these agents are generally mild and include injection-site reactions, nasopharyngitis, and flu-like symptoms. In RCTs, there were similar rates of myalgias between the treatment and comparator arms. Real-world data from a large registry of 15,554 individuals found that the rate of reported myalgia was 8.3%, which was comparable with rates between 3.5–7.2% that have been reported in randomized studies.60
Some earlier trials of evolocumab and alirocumab reported a low but significant increase in neurocognitive side effects with PCSK9 inhibitors leading to some concerns for reducing LDL-C to ultra-low levels.14,15,61 However, results from the large FOURIER and ODYSSEY-OUTCOMES trials did not show a significant increase in adverse events including neurocognitive deficits in the treatment group compared with placebo.7,8 Analysis from the EBBINGHAUS trial, which specifically aimed to evaluate cognitive function with PCSK9 inhibitor treatment, demonstrated no significant difference in cognitive function between those on evolocumab compared with placebo over 19 months follow-up.62 A secondary analysis from the FOURIER and EBBINGHAUS trials further showed evolocumab treatment did not significantly affect neurocognition among individuals with the APOE genotype.63 Moreover, in an RCT of 2086 patients randomized to alirocumab versus placebo, there was no significant effect of alirocumab treatment on neurocognitive function over a 96-weeks treatment period.64 A meta-analysis of 59,733 patients pooling data from RCTs on the impact of PCSK9 inhibitors on neurocognitive adverse events found no significant increase in risk of neurocognitive adverse effects with PCSK9 inhibitors.65 Meanwhile, among older adults, subgroup analysis from both the FOURIER and ODDYSSEY-OUTCOMES trials found no significant interaction for efficacy and no additional increase in safety outcomes when stratified by age.66–68
A Mendelian randomization study of PCSK9 variants showed an increased risk for diabetes (odds ratio 1.11, 95% CI: 1.04–1.19) in the setting of impaired fasting glucose, which was comparable with variants for HMGCR.69 Increased rates of mild hyperglycemia episodes but not diabetes were further observed in a large real-world, pharmacovigilance study, primarily with treatment with evolocumab.70 However, no significant signal for increased diabetes risk has been observed with RCTs of alirocumab or evolocumab.
Finally, while a great majority of patients treated with PCSK9 inhibitors show significant reduction in LDL-C, hypo-responsiveness to these agents has been reported. Proposed biologic mechanisms for “resistance” to PCSK9 inhibitors include dermatologic factors leading to impairment in systemic absorption, mutations that alter antibody binding to circulating PCSK9, development of significant titers of anti-drug antibodies against PCSK9 inhibitors, exaggerated secretion of PCSK9 or dysfunctional LDL receptor, or apoB.71 Yet the most common cause of hypo-responsiveness to medication is likely to be medication non-adherence to the PCSK9 inhibitor or concurrent lipid-lowering agents as well as improper administration of the medication.72 Thus, clinicians should take a careful history regarding medication use in patients with unexpectedly lower reduction in LDL-C who are on treatment with a PCSK9 inhibitor.
Cost and Real-World Utilization
There have been concerns with respect to the cost-effectiveness of PCSK9 inhibitors. With an initial annual cost of over 14,000 US dollars, multiple simulation studies have found that use of these agents would result in immense cost to the healthcare system and would ultimately be cost ineffective even when prescribed to very high-risk patients.73–77 Impact of high out of pocket costs to individual patients has also been studied with analysis showing high rates of unfilled prescriptions because of cost.78,79 From a prescriber perspective, large national insurance datasets have found that only 20.8% received approval on the first try and 47.2% ever received approval.78 The time and resources required to complete prior authorizations, in many cases multiple times, places a significant burden on clinical practices. Importantly, the high rates of rejections and abandonment for PCSK9 inhibitor prescriptions have led to an increased risk for ASCVD events compared with those receiving medication in the real world.80
The cost for PCSK9 inhibitors has been significantly lower in most other developed countries, around the range of 7000 US dollars. However, despite this, the uptake of PCSK9 inhibitors in other countries have been much lower compared with the US. For instance, in an analysis of international sales data from 2015–2019, 57.9% of people on PCSK9 inhibitors globally were from the US, while 30.9% were in Europe and 11.2% were in other countries.81 Starting in 2018, the manufacturers of evolocumab and alirocumab reduced the annual price to 5850 US dollars. An updated cost-effectiveness analysis for evolocumab using the new price found that at the reduced price, the addition of evolocumab to standard background therapy met accepted cost-effectiveness thresholds in very high-risk secondary prevention patients.82
With respect to adherence, because these medications are taken every 2 weeks or every month and have relatively favorable side effect profiles, theoretically PCSK9 inhibitors should have good adherence. However, real-world data have been mixed with some studies showing full adherence as high as 89% while other studies have found high discontinuation rates with subpar adherence with proportion of days covered ≥80% of 29%.83–85 Most studies on adherence for PCSK9 inhibitors are small and more large-scale studies on adherence are needed to better understand patient utilization patterns of these medications (Figure 1).
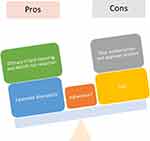 |
Figure 1 Pros and cons of PCSK9 inhibitor use in the real-world setting. |
Other Therapeutic Platforms for PCSK9 Inhibition
While this review focuses on anti-PCSK9 monoclonal antibodies, there are other classes of therapeutics targeting PCSK9. Inclisiran is a small interfering RNA (siRNA) therapeutic agent against PCSK9 inhibitor that has become available. These medications are administered subcutaneously and only require maintenance dosing every 6 months. In phase 3 trials, inclisiran demonstrated a significant LDL-C reduction of 49.9–52.3% at 510 days when compared with placebo among patients with ASCVD or ASCVD risk factors and elevated LDL-C despite receiving maximally tolerated statin therapy.86 Inclisiran has also been shown to significantly decrease LDL-C by 47.9% when compared with placebo after 510 days follow-up in patients with HeFH, elevated LDL-C and on maximally tolerated statins with or without ezetimibe.87 At the time of writing, there is no RCT data available on the efficacy of inclisiran in ASCVD risk reduction although the cardiovascular outcomes trial is ongoing.
Another PCSK9 inhibitor under development are the orally bioavailable macrocyclic peptides against PCSK9. These small molecules differ from current mAb based therapies as they are administered orally.88 Phase 1 data on MK-0616, an orally administered PCSK9 inhibitor, found significant reduction in LDL-C without serious adverse safety events. Gene editing of PCSK9 with CRISPR base editing technologies is also under development and has shown durable LDL-C lowering in non-human primates.89,90
Conclusion
PCSK9 inhibitor are potent, generally well-tolerated therapeutic agents for lowering LDL-C and have been shown in cardiovascular outcomes trials to reduce risk for ASCVD in secondary prevention patients. Current guidelines recommend the use of PCSK9 inhibitors in very high-risk patients who are not at LDL-C goal while on maximally tolerated statins therapy and usually after also adding ezetimibe. Cost remains a major consideration for PCSK9 inhibitor use though a recent price reduction may see an increase in uptake. Finally, novel PCSK9 inhibitors are being studied that will further increase the arsenal for ASCVD prevention.
Disclosure
Dr. Christie Ballantyne reports grants from Akcea, Amgen, Arrowhead, Esperion, Ionis, Novartis, Regeneron, personal fees from Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Denka Seiken, Esperion, Genentech, Gilead, Illumina, Matinas BioPharma Inc, Merck, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Sanofi-Synthelabo, outside the submitted work. Dr. Salim Virani reports Grant support: Department of Veterans Affairs, World Heart Federation, NIH, Tahir and Jooma Family Honorarium: American College of Cardiology (Associate Editor for Innovations, acc.org). The authors report no conflicts of interest in this work.
References
1. Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. 2014;114(6):1022–1036. doi:10.1161/CIRCRESAHA.114.301621
2. Handelsman Y, Lepor NE. PCSK9 inhibitors in lipid management of patients with diabetes mellitus and high cardiovascular risk: a review. J Am Heart Assoc. 2018;7(13). doi:10.1161/JAHA.118.008953
3. Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37(2):161–165. doi:10.1038/ng1509
4. Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–156. doi:10.1038/ng1161
5. Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–1272. doi:10.1056/NEJMoa054013
6. Zhao Z, Tuakli-Wosornu Y, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79(3):514–523. doi:10.1086/507488
7. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi:10.1056/NEJMoa1615664
8. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi:10.1056/NEJMoa1801174
9. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi:10.1093/eurheartj/ehz455
10. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. doi:10.1161/CIR.0000000000000625
11. Jia X, Liu J, Mehta A, Ballantyne CM, Virani SS. Lipid-lowering biotechnological drugs: from monoclonal antibodies to antisense therapies-a clinical perspective. Cardiovasc Drugs Ther. 2021;35(6):1269–1279. doi:10.1007/s10557-020-07082-x
12. Rifai MA, Ballantyne CM. PCSK9-targeted therapies: present and future approaches. Nat Rev Cardiol. 2021;18(12):805–806. doi:10.1038/s41569-021-00634-0
13. Chaudhary R, Garg J, Shah N, Sumner A. PCSK9 inhibitors: a new era of lipid lowering therapy. World J Cardiol. 2017;9(2):76–91. doi:10.4330/wjc.v9.i2.76
14. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–1499. doi:10.1056/NEJMoa1501031
15. Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370(19):1809–1819. doi:10.1056/NEJMoa1316222
16. Farnier M, Jones P, Severance R, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138–146. doi:10.1016/j.atherosclerosis.2015.11.010
17. Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol. 2014;176(1):55–61. doi:10.1016/j.ijcard.2014.06.049
18. Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2531–2540. doi:10.1016/j.jacc.2014.03.018
19. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541–2548. doi:10.1016/j.jacc.2014.03.019
20. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580–1590. doi:10.1001/jama.2016.3608
21. Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9). doi:10.1161/JAHA.116.003421
22. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758–769. doi:10.1016/j.jacl.2015.08.006
23. Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation. 2013;128(9):962–969. doi:10.1161/CIRCULATIONAHA.113.001969
24. O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139(12):1483–1492. doi:10.1161/CIRCULATIONAHA.118.037184
25. Ruscica M, Sirtori CR, Corsini A, Watts GF, Sahebkar A. Lipoprotein(a): knowns, unknowns and uncertainties. Pharmacol Res. 2021;173:105812. doi:10.1016/j.phrs.2021.105812
26. Ray KK, Vallejo-Vaz AJ, Ginsberg HN, et al. Lipoprotein(a) reductions from PCSK9 inhibition and major adverse cardiovascular events: pooled analysis of alirocumab phase 3 trials. Atherosclerosis. 2019;288:194–202. doi:10.1016/j.atherosclerosis.2019.06.896
27. Saeed A, Kinoush S, Virani SS. Lipoprotein (a): recent updates on a unique lipoprotein. Curr Atheroscler Rep. 2021;23(8):41. doi:10.1007/s11883-021-00940-5
28. Bittner VA, Szarek M, Aylward PE, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75(2):133–144. doi:10.1016/j.jacc.2019.10.057
29. Schwartz GG, Steg PG, Szarek M, et al. Peripheral artery disease and venous thromboembolic events after acute coronary syndrome: role of lipoprotein(a) and modification by alirocumab: prespecified analysis of the ODYSSEY OUTCOMES randomized clinical trial. Circulation. 2020;141(20):1608–1617. doi:10.1161/CIRCULATIONAHA.120.046524
30. Marston NA, Gurmu Y, Melloni GEM, et al. The effect of PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibition on the risk of venous thromboembolism. Circulation. 2020;141(20):1600–1607. doi:10.1161/CIRCULATIONAHA.120.046397
31. Paciullo F, Petito E, Falcinelli E, Gresele P, Momi S. Pleiotropic effects of PCSK9-inhibition on hemostasis: anti-PCSK9 reduce FVIII levels by enhancing LRP1 expression. Thromb Res. 2022;213:170–172. doi:10.1016/j.thromres.2022.03.021
32. Bohula EA, Giugliano RP, Leiter LA, et al. Inflammatory and cholesterol risk in the fourier trial. Circulation. 2018;138(2):131–140. doi:10.1161/CIRCULATIONAHA.118.034032
33. Ruscica M, Corsini A, Ferri N, Banach M, Sirtori CR. Clinical approach to the inflammatory etiology of cardiovascular diseases. Pharmacol Res. 2020;159:104916. doi:10.1016/j.phrs.2020.104916
34. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373–2384. doi:10.1001/jama.2016.16951
35. Raber L, Ueki Y, Otsuka T, et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA. 2022;327(18):1771–1781. doi:10.1001/jama.2022.5218
36. Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376(16):1527–1539. doi:10.1056/NEJMoa1701488
37. Jia X, Al Rifai M, Birnbaum Y, Smith SC, Virani SS. The 2018 cholesterol management guidelines: topics in secondary ASCVD prevention clinicians need to know. Curr Atheroscler Rep. 2019;21(6):20. doi:10.1007/s11883-019-0784-8
38. Jia X, Al Rifai M, Ramsey DJ, et al. Association between lipid testing and statin adherence in the veterans affairs health system. Am J Med. 2019;132(9):e693–e700. doi:10.1016/j.amjmed.2019.04.002
39. Rana JS, Virani SS, Moffet HH, et al. Association of low-density lipoprotein testing after an atherosclerotic cardiovascular event with subsequent statin adherence and intensification. Am J Med. 2021;135:603–606.
40. Koskinas KC, Windecker S, Pedrazzini G, et al. Evolocumab for early reduction of LDL cholesterol levels in patients with acute coronary syndromes (EVOPACS). J Am Coll Cardiol. 2019;74(20):2452–2462. doi:10.1016/j.jacc.2019.08.010
41. Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. 2021;128(12):1818–1832. doi:10.1161/CIRCRESAHA.121.318535
42. Giugliano RP, Pedersen TR, Saver JL, et al. Stroke prevention with the PCSK9 (Proprotein Convertase Subtilisin-Kexin Type 9) inhibitor evolocumab added to statin in high-risk patients with stable atherosclerosis. Stroke. 2020;51(5):1546–1554. doi:10.1161/STROKEAHA.119.027759
43. Jukema JW, Zijlstra LE, Bhatt DL, et al. Effect of alirocumab on stroke in ODYSSEY OUTCOMES. Circulation. 2019;140(25):2054–2062. doi:10.1161/CIRCULATIONAHA.119.043826
44. Bajaj NS, Patel N, Kalra R, et al. Neurological effects of proprotein convertase subtilisin/kexin type 9 inhibitors: direct comparisons. Eur Heart J Qual Care Clin Outcomes. 2018;4(2):132–141. doi:10.1093/ehjqcco/qcx037
45. Bonaca MP, Nault P, Giugliano RP, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the fourier trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk). Circulation. 2018;137(4):338–350. doi:10.1161/CIRCULATIONAHA.117.032235
46. McBride CL, Akeroyd JM, Ramsey DJ, et al. Statin prescription rates and their facility-level variation in patients with peripheral artery disease and ischemic cerebrovascular disease: insights from the Department of Veterans Affairs. Vasc Med. 2018;23(3):232–240. doi:10.1177/1358863X18758914
47. Hira RS, Cowart JB, Akeroyd JM, et al. Risk factor optimization and guideline-directed medical therapy in US veterans with peripheral arterial and ischemic cerebrovascular disease compared to veterans with coronary heart disease. Am J Cardiol. 2016;118(8):1144–1149. doi:10.1016/j.amjcard.2016.07.027
48. Colantonio LD, Hubbard D, Monda KL, et al. Atherosclerotic risk and statin use among patients with peripheral artery disease. J Am Coll Cardiol. 2020;76(3):251–264. doi:10.1016/j.jacc.2020.05.048
49. Xian Y, Navar AM, Li S, et al. Intensity of lipid lowering with statin therapy in patients with cerebrovascular disease versus coronary artery disease: insights from the PALM registry. J Am Heart Assoc. 2019;8(19):e013229. doi:10.1161/JAHA.119.013229
50. Arya S, Khakharia A, Binney ZO, et al. Association of statin dose with amputation and survival in patients with peripheral artery disease. Circulation. 2018;137(14):1435–1446. doi:10.1161/CIRCULATIONAHA.117.032361
51. Marz W, Dippel FW, Theobald K, Gorcyca K, Iorga SR, Ansell D. Utilization of lipid-modifying therapy and low-density lipoprotein cholesterol goal attainment in patients at high and very-high cardiovascular risk: real-world evidence from Germany. Atherosclerosis. 2018;268:99–107. doi:10.1016/j.atherosclerosis.2017.11.020
52. Braamskamp M, Langslet G, McCrindle BW, et al. Effect of rosuvastatin on carotid intima-media thickness in children with heterozygous familial hypercholesterolemia: the CHARON study (hypercholesterolemia in children and adolescents taking rosuvastatin open label). Circulation. 2017;136(4):359–366. doi:10.1161/CIRCULATIONAHA.116.025158
53. Luirink IK, Wiegman A, Kusters DM, et al. 20-year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med. 2019;381(16):1547–1556. doi:10.1056/NEJMoa1816454
54. Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331–340. doi:10.1016/S0140-6736(14)61399-4
55. Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126(20):2408–2417. doi:10.1161/CIRCULATIONAHA.112.144055
56. Hovingh GK, Raal FJ, Dent R, et al. Long-term safety, tolerability, and efficacy of evolocumab in patients with heterozygous familial hypercholesterolemia. J Clin Lipidol. 2017;11(6):1448–1457. doi:10.1016/j.jacl.2017.09.003
57. Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36(43):2996–3003. doi:10.1093/eurheartj/ehv370
58. Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):341–350. doi:10.1016/S0140-6736(14)61374-X
59. Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128(19):2113–2120. doi:10.1161/CIRCULATIONAHA.113.004678
60. Gurgoze MT, Muller-Hansma AHG, Schreuder MM, Galema-Boers AMH, Boersma E, Roeters van Lennep JE. Adverse events associated with PCSK9 inhibitors: a real-world experience. Clin Pharmacol Ther. 2019;105(2):496–504. doi:10.1002/cpt.1193
61. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–1509. doi:10.1056/NEJMoa1500858
62. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377(7):633–643. doi:10.1056/NEJMoa1701131
63. Korthauer LE, Giugliano RP, Guo J, et al. No association between APOE genotype and lipid lowering with cognitive function in a randomized controlled trial of evolocumab. PLoS One. 2022;17(4):e0266615. doi:10.1371/journal.pone.0266615
64. Janik MJ, Urbach DV, van Nieuwenhuizen E, et al. Alirocumab treatment and neurocognitive function according to the CANTAB scale in patients at increased cardiovascular risk: a prospective, randomized, placebo-controlled study. Atherosclerosis. 2021;331:20–27. doi:10.1016/j.atherosclerosis.2021.06.913
65. Hirsh Raccah B, Yanovsky A, Treves N, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and the risk for neurocognitive adverse events: a systematic review, meta-analysis and meta-regression. Int J Cardiol. 2021;335:7–14. doi:10.1016/j.ijcard.2021.04.025
66. Gencer B, Marston NA, Im K, et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2020;396(10263):1637–1643. doi:10.1016/S0140-6736(20)32332-1
67. Sever P, Gouni-Berthold I, Keech A, et al. LDL-cholesterol lowering with evolocumab, and outcomes according to age and sex in patients in the Fourier Trial. Eur J Prev Cardiol. 2021;28(8):805–812. doi:10.1177/2047487320902750
68. Sinnaeve PR, Schwartz GG, Wojdyla DM, et al. Effect of alirocumab on cardiovascular outcomes after acute coronary syndromes according to age: an ODYSSEY OUTCOMES trial analysis. Eur Heart J. 2020;41(24):2248–2258. doi:10.1093/eurheartj/ehz809
69. Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375(22):2144–2153. doi:10.1056/NEJMoa1604304
70. Goldman A, Raschi E, Cukierman-Yaffe T, et al. Hyperglycaemic disorders associated with PCSK9 inhibitors: a real-world, pharmacovigilance study. Eur J Prev Cardiol. 2021. doi:10.1093/eurjpc/zwab209
71. Warden BA, Fazio S, Shapiro MD. The PCSK9 revolution: current status, controversies, and future directions. Trends Cardiovasc Med. 2020;30(3):179–185. doi:10.1016/j.tcm.2019.05.007
72. Bays HE, Rosenson RS, Baccara-Dinet MT, Louie MJ, Thompson D, Hovingh GK. Assessment of the 1% of patients with consistent < 15% reduction in low-density lipoprotein cholesterol: pooled analysis of 10 Phase 3 ODYSSEY alirocumab trials. Cardiovasc Drugs Ther. 2018;32(2):175–180. doi:10.1007/s10557-018-6784-z
73. Kazi DS, Penko J, Coxson PG, et al. Updated cost-effectiveness analysis of PCSK9 inhibitors based on the results of the fourier trial. JAMA. 2017;318(8):748–750. doi:10.1001/jama.2017.9924
74. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(10):1069–1078. doi:10.1001/jamacardio.2017.2762
75. Arrieta A, Hong JC, Khera R, Virani SS, Krumholz HM, Nasir K. Updated cost-effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the fourier trial. JAMA Cardiol. 2017;2(12):1369–1374. doi:10.1001/jamacardio.2017.3655
76. Virani SS, Akeroyd JM, Nambi V, et al. Estimation of eligibility for proprotein convertase subtilisin/kexin type 9 inhibitors and associated costs based on the fourier trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk): insights from the department of veterans affairs. Circulation. 2017;135(25):2572–2574. doi:10.1161/CIRCULATIONAHA.117.028503
77. Virani SS, Akeroyd JM, Nambi V, et al. Applicability and cost implications for proprotein convertase subtilisin/kexin type 9 inhibitors based on the ODYSSEY outcomes trial. Circulation. 2019;139(3):410–412. doi:10.1161/CIRCULATIONAHA.118.034993
78. Navar AM, Taylor B, Mulder H, et al. Association of prior authorization and out-of-pocket costs with patient access to PCSK9 inhibitor therapy. JAMA Cardiol. 2017;2(11):1217–1225. doi:10.1001/jamacardio.2017.3451
79. Hess GP, Natarajan P, Faridi KF, Fievitz A, Valsdottir L, Yeh RW. Proprotein convertase subtilisin/kexin type 9 inhibitor therapy: payer approvals and rejections, and patient characteristics for successful prescribing. Circulation. 2017;136(23):2210–2219. doi:10.1161/CIRCULATIONAHA.117.028430
80. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
81. Blais JE, Wei Y, Knapp M, Wong ICK, Wei L, Chan EW. Trends in PCSK9 inhibitor utilization in the United States, Europe, and other countries: an analysis of international sales data. Am Heart J. 2022;248:13–20. doi:10.1016/j.ahj.2022.02.008
82. Fonarow GC, van Hout B, Villa G, Arellano J, Lindgren P. Updated cost-effectiveness analysis of evolocumab in patients with very high-risk atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(7):691–695. doi:10.1001/jamacardio.2019.1647
83. Zafrir B, Egbaria A, Stein N, Elis A, Saliba W. PCSK9 inhibition in clinical practice: treatment patterns and attainment of lipid goals in a large health maintenance organization. J Clin Lipidol. 2021;15(1):202–211 e202.
84. Nanchen D, Carballo D, Bilz S, et al. Effectiveness, adherence, and safety of evolocumab in a swiss multicenter prospective observational study. Adv Ther. 2022;39(1):504–517. doi:10.1007/s12325-021-01962-w
85. Saborowski M, Dolle M, Manns MP, Leitolf H, Zender S. Lipid-lowering therapy with PCSK9-inhibitors in the management of cardiovascular high-risk patients: effectiveness, therapy adherence and safety in a real world cohort. Cardiol J. 2018;25(1):32–41. doi:10.5603/CJ.a2017.0137
86. Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–1519.
87. Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–1530. doi:10.1056/NEJMoa1913805
88. Tucker TJ, Embrey MW, Alleyne C, et al. A series of novel, highly potent, and orally bioavailable next-generation tricyclic peptide PCSK9 inhibitors. J Med Chem. 2021;64(22):16770–16800. doi:10.1021/acs.jmedchem.1c01599
89. Musunuru K, Chadwick AC, Mizoguchi T, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593(7859):429–434. doi:10.1038/s41586-021-03534-y
90. Musunuru K. Moving toward genome-editing therapies for cardiovascular diseases. J Clin Invest. 2022;132(1). doi:10.1172/JCI148555
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
