Back to Journals » Journal of Pain Research » Volume 15
Patterns of Viral Arthropathy and Myalgia Following COVID-19: A Cross-Sectional National Survey
Authors Herndon CM , Nguyen V
Received 11 May 2022
Accepted for publication 8 August 2022
Published 29 September 2022 Volume 2022:15 Pages 3069—3077
DOI https://doi.org/10.2147/JPR.S373295
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Christopher M Herndon,1 Van Nguyen2
1School of Pharmacy, Southern Illinois University Edwardsville, Edwardsville, IL, 62026, USA; 2Hospital Sisters Health System, O’Fallon, IL, 62269, USA
Correspondence: Christopher M Herndon, School of Pharmacy, Southern Illinois University Edwardsville, 200 University Park Drive, Box 2000, Edwardsville, IL, 62026, USA, Tel +1 618.650.5116, Email [email protected]
Introduction: Viral arthropathy is an increasingly recognized sequela of several viral pathogens including alphaviruses, hepatitis, and potentially coronaviruses. Case reports of viral arthropathy and myalgia associated with SARS-CoV-2 infection (COVID-19) both during active disease and following resolution of acute COVID-19 symptoms are becoming more prevalent. We sought to describe the prevalence of viral arthropathy and myalgia associated with COVID-19, as well as to identify factors that may predict these symptoms.
Methods: A national, cross-sectional survey was conducted using a questionnaire administered online. Subjects self-reporting previous confirmed COVID-19 were recruited using the Amazon Mechanical Turk crowdsourcing platform. Questionnaire items included demographics, frequency and severity of common COVID-19 symptoms, requirement for hospitalization or mechanical ventilation, subject recall of arthropathy or myalgia onset, duration, and severity, as well as WOMAC score. Binary logistic regression was used to identify potential predictive co-variates for the development of either arthropathy or myalgia.
Results: A total of 3222 participants completed the arthropathy/myalgia questionnaire with 1065 responses remaining for analysis following application of exclusion criteria, data integrity review, and omission of respondents with confounding conditions. Of the 1065 cases, 282 (26.5%) reported arthralgia and 566 (53.2%) reported myalgia at some point during or after COVID-19 with 9.9% and 6.0% reporting onset of arthralgia or myalgia, respectively, after resolution of acute COVID-19 symptoms. The presence of several commonly reported COVID symptoms or indicators of disease severity was predictive of arthralgia including hospitalization (OR 3.7; 95% CI 2.4 to 5.8), sore throat (OR 2.3; 95% CI 1.5 to 3.5), fatigue (OR 2.9; 95% CI 1.7 to 4.9), and ageusia/anosmia (OR 1.7; 95% CI 1.1 to 2.7).
Discussion: Based on these results, new-onset arthropathy and myalgia following COVID-19 resolution may be an increasingly encountered etiology for pain.
Keywords: COVID-19, viral arthropathy, arthritis, joint pain, long-COVID, myalgia
Introduction
Viral polyarthropathy is an uncommon but significant sequela of several types of viral infections, including parvovirus B19, hepatitis A, hepatitis B, hepatitis C, rubella, etc.1 As many as 1% of the new cases of acute arthritis are thought to be associated with viral etiologies.2 While the majority of viral arthropathies present with the onset of infection and are usually self-limiting, symptoms may persist with select patients and in cases of specific viral infections.3 Additionally, myalgia associated with viral infection has been reported and appears to be most frequently associated with influenza although epidemiologic data is lacking.4–6 Previous coronavirus outbreaks, including Middle East respiratory syndrome coronavirus (MERS) and severe acute respiratory syndrome coronavirus (SARS), were not associated with notable new-onset arthropathy; however, myalgia was a reported symptom during acute illness for both viruses.7
SARS-CoV-2 is one of seven viruses of the coronavirus family and has been previously implicated as an independent risk factor for the development of rheumatoid arthritis. However, the true incidence new-onset arthropathy or myalgia following infection with SARS-CoV-2 is poorly understood.8 In severe COVID-19, an excessive innate immune response may lead to a cytokine storm. The pattern of pro-inflammatory cytokines induced in COVID-19 has similarities to those targeted in the treatment of rheumatoid arthritis. The persistence of symptoms, such as fatigue, myalgia, dyspnea, coughing, and headache, has been reported weeks after the infection has resolved.9
Unfortunately, patients with symptomatic COVID-19 may continue to experience symptoms chronically after the resolution of their initial infection. This experience, described as “long COVID”, is a period in which certain symptoms may persist weeks or months after the infection has resolved and is still poorly understood.10 Although myalgia has been described as a predominant symptom of both acute and long-COVID, little is known about the prevalence and patient experience of arthropathies associated with both acute COVID-19 and long-COVID syndrome.11
The primary aim of this study was to provide a general descriptive analysis of the prevalence of viral polyarthropathy and myalgia in patients self-reporting a previous diagnosis with COVID-19. Secondary aims were to assess the impact of various COVID-19 symptoms and surrogate markers of disease severity on the likelihood of experiencing new-onset arthropathy or myalgia.
Methods
The Southern Illinois University Edwardsville Institutional Review Board (protocol #1165) deemed the project exempt according to the federal regulations on human subject research as allowed in 45 CFR 46.104 (d)(3). An informed consent statement outlining participants’ rights preceded the questionnaire with participants required to review and approve prior to progressing to the questions in accordance with the Declaration of Helsinki.
This cross-sectional study utilizing a web-based online survey was conducted to assess the incidence, characteristics, and associated disability of viral polyarthropathy and myalgia in people previously infected with COVID-19. Subjects from the United States were recruited to participate through the Amazon Mechanical Turk (MTurk) platform, a crowdsourcing marketplace, with a stipend offered of 0.50 USD for voluntary participation.12 The use of MTurk in survey research has been reviewed previously.13 Human Intelligence Tasks (HITS) were advertised to all users of the MTurk platform between the ages of 18 and 89 years. The invitation heading requested completion by those with either current or previously confirmed COVID-19, regardless of symptoms. The web-based online questionnaire was created by the investigators and reviewed for consistent interpretation and understanding by 10 health care professionals and 10 laypersons. The questionnaire consisted of basic demographic questions, assessment of symptoms associated with COVID-19 (eg, cough, fatigue, myalgia, dyspnea, headache, dysgeusia, and anosmia), and self-reported medical problems that are associated with myalgia, arthralgia, or chronic pain. Race and ethnicity were requested of respondents to evaluate potential differences in arthralgia and myalgia prevalence between populations.
Subjects reporting the presence of arthralgia during or after their COVID-19 disease course were additionally requested to complete the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) assessment tool.14 Medical terms were presented to lay respondents as feeling tired (fatigue), shortness of breath (dyspnea), loss of taste (dysgeusia), loss of smell (anosmia), muscle aches or pains (myalgia), and joint pain (arthralgia).
The WOMAC is validated for use in the evaluation of hip and knee osteoarthritis but has been used with rheumatoid arthritis, fibromyalgia, systemic lupus erythematosus, and low back pain.14 There are three subscales within the WOMAC: pain, joint stiffness, and physical function which subjects were requested to complete. Additionally, participants’ self-rating of joint and muscle pain severity and impact on quality of life was assessed with a 0–100 visual analog scale (VAS).
With the use of research crowdsourcing tools such as mTurk, a prior data integrity analysis is required to ensure the reliability of the results obtained. Investigators assessed the mean completion time of a cohort of 10 volunteer lay reviewers asked to complete the online questionnaire in which to compare the mTurk respondents. Here, we found a mean (standard deviation) survey completion time of 318.3 (112.4) seconds. This allowed investigators to omit responses from participants who completed the survey quicker than one standard deviation below the mean time of our reviewers.
Inclusion criteria for this study included a previous self-reported infection of COVID-19. Participants’ responses were excluded if respondent <18 years of age, the COVID-19 experience did not occur between January 1, 2020 and July 13, 2021 as reported by study subjects, survey completion did not fall within the cut-points of 427 seconds (1 standard deviation from the mean duration of the sample test run) and 1 hour, and answering “yes” to all previously related viral or comorbid conditions (indicating a non-earnest attempt to complete). Additionally, respondents self-reporting a history of co-occuring illnesses or infections which could confound the results, including hepatitis A, hepatitis B, hepatitis C, herpes simplex, parvovirus, varicella zoster, Lyme disease, Epstein-Barr, rheumatoid arthritis, systemic lupus erythematosus, Sjogren’s disease, dermatomyositis, polymyalgia rheumatica, osteoarthritis, fibromyalgia, ankylosing spondylitis, diabetic painful peripheral neuropathy, vitamin D deficiency, hypothyroidism, and anemia were all excluded (Figure 1).
 |
Figure 1 Disposition of subjects completing the questionnaire. Notes: Flow diagram adapted from: Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657-662.15 |
IBM SPSS version 28.0 was used for statistical analysis. Bivariate logistic regression analysis was used to identify predictive co-variates for the development of polyarthropathy and myalgia. Co-variates identified a priori included all general demographic variables, presence of commonly reported symptoms, and requirement for hospitalization or mechanical ventilation. Chi-Square analysis was used to compare nominal data.
Results
Demographics
A total of 3222 participants completed the survey (Figure 1).16 After excluding participants based on the exclusion criteria and confounding co-occuring conditions, there were 1065 (n = 1065) valid participants with the majority being male (56.2%) and Caucasian (60.1%). A majority of participants reported some level of college education culminating in a degree (61.4%). Most reported earning an income of less than 90,000 USD annually with 30.8% reporting income less than 30,000 USD per year (Table 1).
 |
Table 1 Demographic Data |
Arthralgia
Subjects reporting any presence of arthralgia (26.5%) were additionally asked to specify if arthralgia was experienced during COVID (69.1%), after COVID (9.9%), or both during and after COVID (20.9%). Almost one-fourth (22.7%) endorsed continued joint pain at the time of questionnaire completion (Table 2). Using an electronic 100-point visual analog scale (VAS), participants were also asked to rate the severity of their joint pain and the impact of their joint pain on quality of life. Mean (SD) scores for pain severity and quality of life impact were 61.2 (24.6) and 59.6 (24.3), respectively. Respondents indicating arthralgia onset following resolution of COVID reported this occurred following a median (IQR) of 6.5 (24.3) days, and they continued to experience arthralgia for a median (IQR) of 2 (2) months. No demographic variables were predictive for the presence of arthralgia on binary logistic regression modeling. However, the presence of several commonly reported COVID symptoms or indicators of disease severity was predictive of arthralgia including hospitalization (OR 3.7; 95% CI 2.4 to 5.8), sore throat (OR 2.3; 95% CI 1.5 to 3.5), fatigue (OR 2.9; 95% CI 1.7 to 4.9), and ageusia/anosmia (OR 1.7; 95% CI 1.1 to 2.7). Experiencing myalgia appeared to inversely predict arthralgia (OR 0.7; 95% CI 0.6 to 0.9). All corresponding results are presented in Table 3. Self-reported individual joints impacted are presented in Figure 2.
 |
Table 2 Incidence, Prevalence, and Severity of Arthralgia and Myalgia |
 |
Table 3 Predictive Factors of Developing Arthralgia and Myalgia |
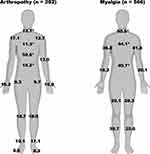 |
Figure 2 Frequency of reported sites impacted by respondents, arthralgia (%). |
Myalgia
Myalgia (described as “muscle aches or pain” within questionnaire) was more commonly reported (53.2%) than was arthralgia (Table 2). Here, myalgia was predominantly experienced during COVID (68.9%) versus after COVID (6.0%), or both during and after COVID (25.1%). Almost one-fifth (19.4%) of subjects reported continued myalgia at the time of questionnaire completion for a median (IQR) duration of 206 (233.5) days. Again, respondents were asked to rate myalgia symptom severity and impact on quality of life via VAS. The mean (SD) severity rating of pain from myalgia was 55.9 (24.2) and the mean quality of life impact from myalgia was 55.4 (24.5). Reporting being of male gender was predictive of lower risk of experiencing myalgia (OR 0.672; 95% CI 0.528–0.854). No other demographic variables were predictive of myalgia risk. Similar to arthralgia, several COVID-related symptoms were predictive of myalgia (Table 3). These included chest pain/discomfort, headaches, fever/chills, fatigue, and the presence of joint pain.
Vaccination Status
Of the 1065 participants completing the survey, 724 (68%) reported having received a full course of SARS-CoV-2 vaccination which was defined as a two-dose series for the Moderna and Pfizer/BioNtech vaccines and one dose of the Johnson & Johnson vaccine. While the temporal relationship between vaccination dose(s) and symptoms of myalgia or arthralgia was not queried, those reporting having not received vaccination were more likely to experience myalgia vs those reporting having received a full course of vaccination (57.6% versus 51.1%, respectively; p = 0.046). No difference was identified based on vaccination status and reporting of any incidence of arthralgia (p = 0.667). When comparing the onset of myalgia or arthralgia based on vaccination status, significantly more respondents without full vaccination reported arthralgia (30.1%) versus those reporting having received a full vaccination course (16.4%; p = 0.021).
Discussion
This study found that patients with a self-reported previous infection of COVID-19 experienced new onset symptoms of joint or muscle pain during after their COVID-19 experience. Arthralgia has been an increasingly cited component of long-covid syndrome, with an estimated prevalence of 19% (95% CI 7–34).17 We believe ours is the largest study to date to specifically describe the characteristics and timing of arthralgia and myalgia associated with COVID-19. A growing body of literature describing these COVID-19 sequelae exits.
In a retrospective, observational case series, Pal et al report their experiences with 23 patients presenting to a specialty rheumatology clinic in India with a history of documented confirmed COVID-19.18 Patients with positive acute phase reactants, rheumatoid factor, or anti-cyclic citrullinated peptide were excluded from the report. The mean age of patients was 42.8 (± 14.1) years and 19 (82.6%) reported symptoms consistent with COVID-19. Patients reported an average duration of 25.9 (± 12.8) days from the time of onset of fever and subsequent arthritis symptoms. Most frequently reported joint involvement was knees (16 of 23), ankles (15 of 23), and low back (7 of 23). Additionally, 9 of 23 patients experienced pain suggestive of enthesitis.
Numerous case reports of confirmed COVID-19 patients experiencing arthropathy have been reviewed in a recent case series.19 Here, Kocyigit and Akyol present an aggregated view of the joints involved, with the knee and ankle being the most frequently reported sites. Our results outlining affected joints was largely congruent with Kocyigit and Akyol, although our respondents reported a higher prevalence of axial spine involvement. Given problems with recall bias in a survey of this nature, symptom onset and proximity to active COVID-19 infection could certainly be suspect.
Timing of new onset arthropathy following COVID-19 symptom resolution has also been described. Gracia-Ramos et al report a mean (SD) onset of arthropathy following COVID-19 symptom resolution of 20.2 (11.5) days.20 This onset of arthropathy symptoms is substantially delayed in comparison to our results of 6.5 (24.3) days. However, some of the patients reported by Gracia-Ramos may have represented undiagnosed autoimmune arthritides versus a true viral arthropathy.
Several unanswered questions regarding COVID-19 arthropathy and myalgia exist. Potentially the most pertinent is the underlying etiopathogenesis and the likelihood of arthropathy following resolution of COVID-19 symptoms representing an uncovered autoimmune disease versus a true component of long-covid syndrome. The pathogenesis of the more common viral arthritides has been well described previously.1 Molecular mimicry, a phenomenon in which immune cells react with epitopes both derived from the virus and from self, is one proposed mechanism for an autoimmune etiology of new-onset arthritis. This is opposed to actual synovial intrusion by the virus. Similar pathogenesis has been described relating to the receipt of COVID-19 vaccination as well.21,22
Gracia-Ramos et al published a systematic review of 99 cases outlining new-onset autoimmune disease following resolution of COVID-19.20 These authors propose three distinct patterns of new-onset COVID-19 related arthropathy based on symptom timing (ie, prior to COVID-19 symptoms, during the active infection, or following recovery/symptom resolution). Of the 99 cases reviewed, 46 patients demonstrated some type of vasculitis. Six patients met criteria for Rheumatoid arthritis, nine met criteria for Ankylosing Spondylitis, six met criteria for systemic lupus erythematosus, and nine were considered to have an inflammatory myopathy. The remaining 17 patients were determined to have reactive arthritis, although the authors correctly comment that traditional diagnostic criteria for reactive arthritis involves bacterial versus viral etiologies and variability of onset.
Yokogawa et al additionally describe their experiences with 306 patients admitted to their institution for COVID-19. While approximately one-fourth of these patients reported joint and muscle pain, four patients developed joint aspiration confirmed crystal-associated arthritis (eg, gout) with variable timing of onset, ranging from eight to 27 days from COVID-19 symptom presentation.23
One difficulty in reviewing the literature for this particular topic is the potentially problematic use of the term, reactive arthritis (ReA), to describe COVID-19 related arthropathy. Traditionally, ReA is considered to be clinically similar to axial or peripheral spondyloarthropathies arising from bacterial gasteroentero- or genitourinary infections.8,24 More generally, this disorder is characterized by sterile synovitis in one or more large joints distant to the primary site of infection.
There were several limitations of this study. Recall bias was a major limitation considering the survey responses were based on subjective feedback from the participants and depending on the time frame from which they experienced the infection, accuracy in their responses may be diminished. Additionally, the study population was limited to the Amazon MTurk community, those with computer literacy, and those with the financial means to access the internet. The financial incentive offered to complete the questionnaire may also present a limitation. Another major drawback is the study’s lack of objective laboratory values to determine extent of inflammatory response during their COVID experience as well as self-report of COVID-19. While the invitation to participate specifically requested only those persons with documented COVID-19 positive histories complete the questionnaire (via laboratory or point of care testing), there does exist the possibility of persons completing the survey that did not have confirmed COVID-19. Lack of information regarding variability in treatment used during their COVID experience, especially with respect to glucocorticoids, also confounds the generalizability of the results. There may additionally be confusion among lay persons when asking about “joint pain” and “muscle pain.” While this was not identified as problematic during our questionnaire review, anecdotally we have noted these terms used interchangeably by patients. Also of concern is the increasing reports of post-COVID, new-onset autoimmune conditions (eg, rheumatoid arthritis). Unfortunately, the investigators did not stipulate in the questionnaire if potentially confounding conditions were diagnosed before or after the respondents’ COVID-19 experience. This could potentially have skewed results given respondents indicating the presence of these conditions were excluded from data analysis. The findings of myalgia and arthralgia prevalence and timing based on vaccination status, while interesting, cannot be interpreted as having a causal relationship, especially given the timing of vaccination in relation to the onset of COVID-19 was not captured. This may represent a significant bias in interpreting this data considering COVID-19 vaccination has been reportedly associated with both new onset and flare of several autoimmune disorders which might present with myalgia and/or arthralgia.
Future prospective studies of new-onset, post-COVID-19 arthropathy and myalgia are certainly required to more clearly elucidate this disorder. Consideration of a consensus-driven terminology to describe these two symptoms is certainly warranted in the context of viral illness (versus bacterial pathogens). Measurement of acute phase reactants, as well as specific proteins associated with autoimmune inflammatory disorders, would aid in the diagnosis, especially in those patients with no previous history of autoimmune conditions. Owing to the lack of testing capacity for the various strains of SARS-CoV-2, limitations in generalizing these results to all strains exist. Additionally, comparing incidence or prevalence in arthralgia or myalgia in those with or without vaccination, type of vaccination, and booster status should all be considered.
Conclusions
Arthropathy and myalgia are common symptoms experienced during and following COVID-19. After controlling for self-reported, pre-existing conditions, we found approximately one-fifth to one-quarter of patients reported experiencing arthralgia or myalgia, respectively. More noteworthy was new onset joint pain or myalgia following resolution of COVID-19 symptoms. Additionally, COVID-19 vaccination may also contribute to the onset of these symptoms making causal inferences difficult. Viral arthropathy due to COVID-19 infection should be considered in a differential diagnosis when encountering patients with new onset polyarthropathy or myalgia.
Data Sharing Statement
The authors will make available both the data and program codes used in analysis to any researcher for purposes of reproducing the results or replicating the analysis.
Acknowledgments
Supported/supported in part by an internal grant from Southern Illinois University Edwardsville School of Pharmacy, Department of Pharmacy Practice.
Disclosure
Dr Van Nyugen certifies that she has no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. Dr Herndon has received financial support in the form of grants and contracts from the Illinois Department of Human Services and for consultant work for the United States Department of Justice. Additionally, Dr Herndon received intramural funding to support this study from the Department of Pharmacy Practice, School of Pharmacy, Southern Illinois University Edwardsville.
References
1. Suhrbier A, Mahalingam S. The immunobiology of viral arthritides. Pharmacol Ther. 2009;124(3):301–308. doi:10.1016/j.pharmthera.2009.09.005
2. Marks M, Marks JL. Viral arthritis. Clin Med. 2016;16(2):129–134. doi:10.7861/clinmedicine.16-2-129
3. Vassilopoulos D, Calabrese LH. Virally associated arthritis 2008: clinical, epidemiologic, and pathophysiologic considerations. Arthritis Res Ther. 2008;10(5):215. doi:10.1186/ar2480
4. Kerr J, Macartney K, Britton PN. Influenza-associated myositis: a single-centre, 5-year retrospective study. Eur J Pediatr. 2021;180(2):577–584. doi:10.1007/S00431-020-03835-W
5. Saud A, Naveen R, Aggarwal R, Gupta L. COVID-19 and myositis: what we know so far. Curr Rheumatol Rep. 2021;23(8). doi:10.1007/S11926-021-01023-9
6. Nauss MD, Schmidt EL, Pancioli AM. Viral myositis leading to rhabdomyolysis: a case report and literature review. Am J Emerg Med. 2009;27(3):372.e5–372.e6. doi:10.1016/J.AJEM.2008.07.022
7. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi:10.1038/nrmicro.2016.81
8. Bekaryssova D, Yessirkepov M, Zimba O, Gasparyan AY, Ahmed S. Reactive arthritis before and after the onset of the COVID-19 pandemic. Clin Rheumatol. 2022;41(6):1641–1652. doi:10.1007/S10067-022-06120-3
9. Fragata I, Mourão AF. Coronavirus disease 19 (COVID-19) complicated with post-viral arthritis. Acta Reumatologica Portuguesa. 2020;45(4):278–280.
10. Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020;20(1). doi:10.1186/s12913-020-06001-y
11. Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199(2):113–119. doi:10.1007/s00408-021-00423-z
12. Strickland JC, Stoops WW. The use of crowdsourcing in addiction science research: amazon mechanical Turk. Exp Clin Psychopharmacol. 2019;27(1):1–18. doi:10.1037/pha0000235
13. Cunningham JA, Godinho A, Kushnir V. Using Mechanical Turk to recruit participants for internet intervention research: experience from recruitment for four trials targeting hazardous alcohol consumption. BMC Med Res Methodol. 2017;17(1):156. doi:10.1186/s12874-017-0440-3
14. McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Care Res. 2001;45(5):453–461. doi:10.1002/1529-0131(200110)45:5<453::AID-ART365>3.0.CO;2-W
15. Moher D, Schulz KF, Altman DG The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–662.. doi:10.7326/0003-4819-134-8-200104170-00011
16. Glasgow RE, Huebschmann AG, Brownson RC. Expanding the CONSORT figure: increasing transparency in reporting on external validity. Am J Prev Med. 2018;55(3):422–430. doi:10.1016/j.amepre.2018.04.044
17. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1). doi:10.1038/s41598-021-95565-8
18. Pal A, Roongta R, Mondal S, et al. Does post-COVID reactive arthritis exist? Experience of a tertiary care centre with a review of the literature. Reumatología Clínica. 2022. doi:10.1016/j.reuma.2022.03.004
19. Kocyigit BF, Akyol A. Reactive arthritis after COVID-19: a case-based review. Rheumatol Int. 2021;41(11):2031–2039. doi:10.1007/s00296-021-04998-x
20. Gracia-Ramos A, Martin-Nares E, Hernandez-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592. doi:10.3390/cells10123592
21. Vojdani A, Vojdani E, Kharrazian D, Ascherman DP, Richmond JM. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2021;11:1. doi:10.3389/fimmu.2020.617089
22. Padiyar S, Kamath N, Mathew J, et al. New-onset Adult-onset Still’s disease-like syndrome after ChAdOx1 nCoV-19 vaccination-a case series with review of literature. Clin Rheumatol. 2022;1:3. doi:10.1007/s10067-022-06065-7
23. López-González MDC, Peral-Garrido ML, Calabuig I, et al. Case series of acute arthritis during COVID-19 admission. Ann Rheum Dis. 2021;80(4):e58–e58. doi:10.1136/annrheumdis-2020-217914
24. García-Kutzbach A, Chacón-Súchite J, García-Ferrer H, Iraheta I. Reactive arthritis: update 2018. Clin Rheumatol. 2018;37(4):869–874. doi:10.1007/s10067-018-4022-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
