Back to Journals » OncoTargets and Therapy » Volume 11
Patterns of regional nodal relapse after D2 lymphadenectomy in gastric cancer: rethinking the target volume
Authors Yang W, Zhou M, Hu R, Li G, Wang Y, Shen L, Liang L, Yang J, Zhang Z
Received 15 June 2018
Accepted for publication 16 October 2018
Published 12 November 2018 Volume 2018:11 Pages 8015—8024
DOI https://doi.org/10.2147/OTT.S177315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Wang Yang,1,2,* Menglong Zhou,1,2,* Ran Hu,1,2 Guichao Li,1,2 Yan Wang,1,2 Lijun Shen,1,2 Liping Liang,1,2 Jianing Yang,1,2 Zhen Zhang1,2
1Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
*These authors contributed equally to this work
Purpose: This study mapped the localization of regional nodal recurrence to determine whether the clinical target volume (CTV) should be redefined in adjuvant radiotherapy.
Patients and methods: Between January 2004 and October 2015, a total of 129 patients with gastric cancer following D2 resection who experienced regional recurrence were retrospectively examined. The lymph nodes (LNs) were hand-drawn proportionally on template computed tomography (CT) images of a standard patient by referencing surrounding anatomic landmarks. The association between clinicopathologic factors and LNs at risk was further investigated.
Results: Based on the contour of the recurrent LNs, the authors observed high-risk regions for relapse and drew a density distribution map of 16 LN stations on the CT images. The most commonly involved recurrent LNs were stations 16b (51.2%) and 16a (39.5%). Stations 13, 12, 9, and 14 were involved in 36.4%, 33.3%, 28.7%, and 27.9% of recurrences, respectively. Other regions, including stations 1–6 (perigastric LNs) and station 10 (splenic hilar LN), were of low risk. Notably, 72% (83/116) of recurrent 16b LNs were located in the upper half of 16b1. Analysis within subgroups showed that the pathologic N stage was the only independent risk factor for LN 16 relapse.
Conclusion: This mapping suggests a new method for vessel-guided delineation of regional LNs when defining the CTV in patients after standard D2 resection. LNs around the abdominal aorta and its main branches, as well as regions around the hepatic hilar area and pancreatic head, should be the most important radiotherapeutic targets.
Keywords: gastric cancer, regional, lymph nodes, recurrence, target volume
Introduction
Gastric cancer is the fifth most common malignant cancer and the third cause of cancer-related deaths worldwide.1,2 Although surgery is a crucial treatment strategy for gastric carcinoma, 21.8%–63.4% of postsurgical patients with advanced gastric cancers experience recurrence or metastasis, even after radical resection, resulting in an unfavorable prognosis.3–5
As a component of multimodal treatments, the role of chemoradiation has been reported by several randomized trials, including intergroup trial 0116,3 particularly for locally advanced disease. Several studies demonstrated that adjuvant chemoradiation was associated with preferable local control. A phase III trial known as the ARTIST trial analyzed patients with D2 lymphadenectomy and showed that the locoregional relapse rate was 8.3% in the chemotherapy (ChT) arm and was reduced to 4.3% by adding adjuvant chemoradiotherapy (CRT).6
Therefore, as a local treatment, radiotherapy after curative D2 resection should focus on the locoregional area, especially the regional lymph nodes (LNs). Although Smalley et al recommended elementary guidance for defining the clinical target volume (CTV) for adjuvant radiotherapy in 2002,7 confusion and controversy remain regarding the target volume’s definition in gastric cancer. With the development of surgical techniques and radiotherapy, the guidelines based on recurrence patterns over the past few decades no longer apply. However, a detailed guideline for regional LN delineation is lacking, and target volume varies in most studies examining postoperative radiotherapy. Hence, delineating elective regional LNs, especially for lower CTV borders, remains a challenge. Herein, the authors documented the precise localization of regional relapse in patients after D2 lymphadenectomy, investigating patients at high risk for recurrence.
Patients and methods
Patient identification
Patients with curatively resected gastric carcinoma and D2 lymphadenectomy at Fudan University Shanghai Center between January 2004 and October 2014 were retrospectively identified. Patient eligibility criteria included the following: 1) diagnosed with regional relapse after radical surgery; 2) underwent R0 gastrectomy and D2 or D2+ lymphadenectomy; 3) pathologically confirmed as adenocarcinoma; 4) complete medical record and abdominal imaging data available; and 5) no history of other carcinomas. In total, 129 patients met these criteria and were included in this analysis. All patients in the study signed informed consent forms. The study protocol was approved by the ethics committee of Fudan University Shanghai Cancer Center.
Treatment and follow-up
All patients underwent total or subtotal gastrectomy and D2 lymphadenectomy with resections of perigastric LN stations along the lesser curvature (stations 1, 3, and 5) and greater curvature (stations 2, 4, and 6) and LNs along the left gastric artery (station 7), common hepatic artery (station 8), celiac artery (station 9), and splenic artery (stations 10 and 11). Radical resection with pathologically confirmed negative margins was also required (R0 resection). Five surgeons specializing in gastric surgery with more than 10 years of experience each performed the surgery to ensure quality control of the primary operation. The annual gastric cancer surgeon volume for all surgeons was more than 100 cases.
Enrolled patients received various therapies, including surgery alone (n=10), (neo)adjuvant ChT (n=86), and (neo)adjuvant radiotherapy (n=33). Adjuvant ChT alone, including fluoropyrimidine and platinum-based drug regimens, was administered to 80 individuals, and neoadjuvant ChT was administered to the other 6. Adjuvant CRT was delivered to 27 individuals, and neoadjuvant CRT was performed in the other 6, using either three-dimensional conformal radiotherapy or intensity-modulated radiation therapy (IMRT) with a median dose of 45 Gy (range, 45.0–50.4 Gy) in 1.8 Gy daily fractions. The postoperative radiation CTV encompassed the tumor bed, anastomosis site, duodenal stump, and selective regional LNs. Preoperative radiation was delivered to the primary tumor, any perigastric tumor extension, subclinical involvement, and regional LNs. Concurrent ChT regimens included 5-fluorouracil, capecitabine, or S1.
After radical dissection, patients were followed every 3 months for the first 2 years, every 6 months for the next 5 years, and annually thereafter. The follow-up program consisted of a physical examination, laboratory tests, computed tomography (CT), or magnetic resonance (MR) scans of the chest, abdomen, and pelvis and endoscopy at each visit.
Definition of regional LN recurrence
Regional recurrence was defined as recurrence at the celiac regional LNs within stations 1–16, such as in the perigastric, porta hepatis, peripancreatic, and para-aortic LNs. Anatomical definitions of LN stations were based on the third classification by the Japanese Gastric Cancer Association (JGCA).8 Distant LNs, such as the supraclavicular LNs, were not considered regional recurrences. All images were reevaluated by two experienced radiation oncologists specializing in the gastrointestinal tract. Regional LNs were considered to represent recurrence if they were larger than 8 mm in short-axis diameter. In addition to size criteria, features supporting a consideration for malignancy included a rounded shape, central necrosis, marked or heterogeneous enhancement, clustered LNs, FDG avidity (if available), and responsiveness to anticancer treatment regardless of size.9–13
Description of LN mapping
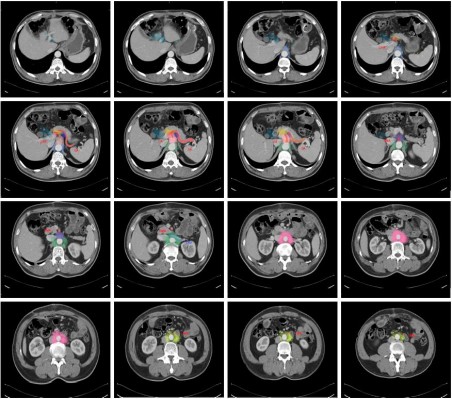
A 59-year-old man (165 cm, 65 kg, BMI 23.9) with locally advanced gastric cancer (pT3N1M0) who underwent subtotal gastrectomy was selected as the standard patient. All recurrent LNs were hand-drawn on this patient’s abdominally enhanced CT images. To describe mapping patterns of regional recurrence, the authors delineated the atlas of image-positive nodes for all patients at equivalent locations from the diagnostic CT onto the template CT by referencing the surrounding main vessels. In addition to location, proportional scales of the LNs relative to their original reference were precisely registered by measuring the distance between the nodes and landmarks to overcome anatomical variations. To show the geographic localization of recurrent station 16b nodes visually, we used a circle with a 5 mm diameter to replace the central position of every node to avoid the mass effect of enlarged nodes and created a three-dimensional model using MIM software™ (MIM Software Inc., Cleveland, OH, USA) with CT and digitally reconstructed radiograph images.
Statistical analysis
Data were recorded as categorical or continuous variables. The chi-squared test and Fisher’s exact test were used to detect differences in clinical factors and recurrence of regional LNs. Independent risk factors that influenced recurrence were analyzed by logistic analysis. All P-values were two-sided, and P-values <0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics, version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Patient characteristics are listed in Table 1. Of the 129 patients enrolled in this study, over three-quarters (75.2%) were male, with a median age of 57 years. Primary tumors were located in the upper 1/3 of the stomach in 23 patients, the middle 1/3 in 38 patients, the lower 1/3 in 59 patients, and the whole stomach in four patients. Most matched patients were stage III (67.4%), and over half the enrolled patients had N3 disease (50.4%) per the eighth edition of the American Joint Committee on Cancer staging criteria.14
  | Table 1 Characteristics of 129 gastric cancer patients with regional LN recurrence |
Treatment and outcomes after regional relapse
Enrolled patients received various therapies after regional relapse was diagnosed; among them, 28 patients (21.7%) received involved-field radiotherapy alone, 49 patients (38.0%) received CRT, 34 (26.4%) received ChT or targeted therapy alone, and the remaining 18 (14.0%) received no treatment. Gross target volume (GTV) encompassed recurrence lesions and enlarged LNs for patients receiving radiotherapy with or without ChT. The ChT regimens mostly consisted of combination therapy involving 5-FU or oral fluorouracil derivatives as the backbone with added oxaliplatin, epirubicin, irinotecan, or docetaxel.
At the time of analysis, the median follow-up was 28 months. Forty-seven patients died, and 91 developed local recurrence (n=23), peritoneal metastasis (n=26), or distant metastasis (n=65) apart from regional relapse. The estimated 3-year overall survival (OS) and disease-free survival (DFS) were 65.1% and 53.1%, respectively. A significant difference was observed among various types of treatment after regional recurrence, and the estimated 3-year OS in patients receiving (chemo)radiotherapy, ChT alone, or no treatment was 76.3%, 57.7%, and 41.2%, respectively (P=0.038, Figure 1).
  | Figure 1 Kaplan–Meier estimate of OS of patients treated with different treatments after regional recurrence. |
Prevalence and distribution of recurrent regional LNs
One hundred five of 129 recurrent patients (81.4%) were diagnosed by CT, 7 (5.4%) by MRI, and 17 (13.2%) by positron-emission tomography-CT. The median time to regional progression was 12 months (range, 1–93 months) and 91 of 129 patients (70.5%) experienced relapse within 24 months. Figure 2 illustrates the frequency of recurrent regional radiological-positive LNs after D2 lymphadenectomy. Per the JGCA criteria, the most common relapse site was 16b (para-aortic LNs between the lower border of the left renal vein [LRV] and the aortic bifurcation, 51.2%) and 16a (para-aortic LNs between the diaphragmatic aortic hiatus and the lower border of the LRV, 39.5%), followed by station 13 (LNs on the posterior surface of the pancreatic head cranial to the duodenal papilla, 36.4%), station 12 (hepatoduodenal ligament LNs, 33.3%), station 9 (celiac artery LNs, 28.7%), and station 14 (LNs along the superior mesenteric artery or vein, 27.9%). Among patients with para-aortic LN recurrence, the recurrence area was mainly concentrated in station 16a2 (para-aortic LNs between the upper margin of the origin of the celiac artery and the lower border of the LRV, 37.2%) and station 16b1 (para-aortic LNs between the lower border of the LRV and the upper border of the inferior mesenteric artery [IMA], 51.2%). In contrast, only five of 129 patients (3.9%) experienced regional relapse limited to the perigastric LNs (Nos 1–6). Recurrence of LNs located in stations 8 (LNs along the common hepatic artery, 5.4%), 11 (splenic artery LNs, 5%), 10 (splenic hilar LNs, 0.8%), 7 (LNs along the trunk of left gastric artery, 0%), and 15 (LNs along the middle colic vessels, 0%) was also rare. At surgery, most metastatic LNs were located around the stomach, whereas most recurrent nodes were outside the D2 dissection field (Figure S1).
  | Figure 2 Distribution of recurrent lymph nodes in 129 patients. |
GTV delineation of recurrent LNs
The authors contoured vessel-based GTV on axial images to represent the recurrent LN regions and depicted the radiographic delineation of the recurrent 16 LN stations on a standard patient. As shown in Figure 3, LNs around the abdominal aorta and its main branches, such as the celiac trunk and superior mesenteric artery (stations 16, 9, and 14), are considered high-risk regions. Furthermore, regions around the hepatic hilar area, duodenal papilla, and pancreatic head (stations 12 and 13) are also commonly involved. Conversely, areas farther from main vessels, such as perigastric LNs or splenic hilar LNs, are seldom involved in relapse. Figure 4 depicts three-dimensional mapping of recurrent station 16b and divides it into three zones, including the upper half of 16b1, the lower half of 16b1 and 16b2. In total, 116 relapsed nodes were in station 16b in 66 patients. Notably, 83 of the 116 nodes (71.6%) were concentrated in the upper half of 16b1 in these patients, accounting for 84.7% of the total 16b1 recurrences. The most commonly involved site was the left side of the abdominal aorta, followed by the region between the inferior vena cava and abdominal aorta.
Patterns of relapse for different clinical factors
A subgroup analysis was conducted stratified by different treatment strategies, primary tumor localization, and pathologic N stage. No significant differences were observed within the various treatment arms (Figure S2, Table S1). Interestingly, adjuvant CRT was administered in two patients, while the other three patients experiencing perigastric LN relapse were treated with adjuvant ChT. No significant difference was observed in the proportion of perigastric LN relapses between irradiated and nonirradiated patients (6.1% vs 3.1%, P=0.602). Similar to the distribution of recurrent nodes in all patients, the regions at highest risk for different tumor localizations were located in stations 16b, 16a, 13, 12, 9, and 14. However, a relatively higher relapse rate was seen in stations 12 and 13 if the tumor was located in the lower 1/3 of the stomach (Table S2). Among these patients, commonly involved LNs were stations 13 (51.7%), 16b (48.3%), 12 (37.9%), 16a (34.4%), 9 (31.0%), and 14 (29.3%), which differed slightly from the recurrence patterns observed in other primary tumor localizations. Regarding the association between pathologic N stage and patterns of regional recurrence, we found the following distribution of most commonly involved LNs for patients with N2–3 disease: stations 16b (56.0%) and 16a (45.1%), followed by stations 12 (35.2%), 13 (34.1%), 14 (29.7%), and 9 (28.6%). However, stations 16a and 16b LNs (para-aortic LNs) were not at the highest risk of recurrence for patients with N0–1 disease. The risk for station 16 recurrence was significantly higher in N2–3 than in N0–1 patients, regardless of station 16a or 16b involvement (P=0.025 and 0.019, respectively) (Table S3). Pathologic N stage was the only independent risk factor for station 16b1 failure based on univariate and multivariate analyses (Table 2).
  | Table 2 Univariate and multivariate analyses of No 16b relapse |
Discussion
Although the ARTIST trial performed in Korea enrolled all patients who underwent D2/R0 dissection and demonstrated no marked improvement in either OS or DFS in the CRT group, an exploratory subgroup analysis revealed an improved DFS after adjuvant CRT in patients with LN metastasis (pN+).6 Moreover, a study designed to evaluate the influence of adjuvant RT on recurrence by analyzing the results of the ARTIST trial also illustrated that adjuvant radiation after D2 resection reduced locoregional recurrence and prolonged locoregional recurrence-free survival, especially in patients with LN metastasis.20
Delineation of the target volume in gastric cancer after radical dissection has undergone many changes and remains controversial. Based on studies of failure patterns after radical surgery,15,16 Smalley and Tepper recommended guidance for adjuvant radiotherapy in 2002.7,17 Afterward, tumor bed, remnant stomach, resection margins, anastomosis site, duodenal stump, and regional LNs were included in the radiation target volume in clinical practice and most clinical studies, including the intergroup trial INT-0116.3 However, grade 3 toxic effects occurred in 41% of patients due to a two-dimensional radiotherapy technique and target volume with a wider coverage in INT-0116. Hence, optimizing the design of CTV to reduce radiation-related toxicity became an essential research avenue. Considering the mobility and distensible nature, an additional margin resulting in a larger radiation field and higher incidence of toxicity is unavoidable for remnant stomach irradiation. In 2008, a retrospective analysis in Korea suggested that the remnant stomach should be excluded from the radiation target volume.18 However, no clear standard was suggested regarding elective LNs and their margins.
Previous studies have indicated that most metastatic LNs involved at initial surgery embraced the stomach (stations 1–6), followed by stations 7–11.5,19 However, the distribution of metastatic LNs at recurrence was completely divergent from the pattern involved at the time of surgery, falling mostly outside the D2 dissection field.5 Similarly, radiation oncologists retrospectively analyzed results from the ARTIST trial and illustrated that LNs in groups 2 and 3 might be the high-risk areas for recurrence.20 Notably, recurrence was less common in the remnant stomach and perigastric and splenic hilar LNs, although these areas were not encompassed within the target volume in the ARTIST trial.
As our work showed, the atlas confirmed that LN metastasis frequently occurs in LNs around the abdominal aorta and its main branches, such as the celiac trunk and superior mesenteric artery (stations 16a2, 16b1, 9, and 14) and regions around the hepatic hilar area, duodenal papilla, and pancreatic head (stations 12 and 13), which is consistent with a previous study in Korea. Therefore, these areas should be the most important radiotherapeutic targets, whereas stations 10 and 16b2 can be excluded from the radiation field.
Although some patients received radiotherapy in this study, the proportion of perigastric LN relapse did not significantly differ between irradiated and nonirradiated patients. Therefore, the low incidence of perigastric progression may not have been led by radiotherapy. For patients after standard D2 lymphadenectomy performed by well-trained surgeons, or in poor physical condition, skipping the inclusion of perigastric LNs in the CTV to reduce gastrointestinal toxicity may be considered.
Defining the lower border of the CTV for patients after D2 lymphadenectomy has been a major issue during the past few decades. Although para-aortic LNs below the lower border of the LRV (No 16b1) were a high-risk area, many researchers exclude this region in consideration of radiation-induced toxicity. However, modern techniques, such as IMRT, have been shown to achieve better conformity, dose homogeneity, and organ preservation.21–23 Our work showed that N stage was the only independent risk factor for station 16b1 recurrence, and the presence of ≥3 positive LNs conveyed an increased risk for recurrence in station 16b1. Consequently, we believe that the lower CTV border may be reasonably modified to the middle of the LRV and IMA for patients with N2 or N3 disease, while station 16b1 should be excluded from CTV for stage N0–1.
Many studies have stated that recurrent areas depend on the original tumor site.5,7,17 However, the data in our study showed that stations 16, 13, 12, 9, and 14 were the most commonly involved regions for all patients regardless of primary localization, although a higher trend of relapse in stations 12 and 13 was seen in patients with distal-third cancer. Thus, we believe that recommending a guide based on primary localization is unnecessary, and our suggestions may minimize inconsistencies among delineators with high feasibility.
Several reports advocate more generous CTV for (neo)adjuvant radiotherapy.7,17,24 However, the guidance for adjuvant radiotherapy in 2002 was based on a series of studies reported in the last century. With the development of surgical techniques over the past few decades, regional node resection has improved, particularly for perigastric and splenic hilar LNs. Moreover, the previous consensus was reached based on a Western population, whereas the current study was performed in patients after D2 dissection in a Chinese cohort.
This study has several limitations, one of which is the nature of the retrospective analysis and that patients were not enrolled beforehand. Complete medical data and follow-up for all patients who underwent radial dissection during the study period were not available. Thus, the authors discussed the distribution and proportion of recurrent LNs as a surrogate. Information was lacking on the pathologic assessments for diagnoses; therefore, we could not show that all identified LNs contained metastatic disease. Additionally, relatively small subgroups and small event numbers on perigastric nodes influenced the statistical power of the analysis. Although the current study illustrated the failure pattern of regional LNs, it was not strong enough to determine whether a limited-field CTV in adjuvant radiotherapy is effective by analyzing the nodal recurrence sites alone. Finally, all findings in the current study were based on data from a D2 dissection cohort, and the CTV suggested may not apply for Western patients who have undergone D1 dissection.
To our knowledge, studies investigating the high risk of regional nodal recurrence in detail after D2 dissection are lacking. We believe that the mapping in this study provides convincing evidence for redefining the CTV in adjuvant radiotherapy for gastric cancer. Moreover, interobserver variation in LN delineation may be mitigated by normalizing and simplifying the contour.
Acknowledgments
The authors appreciate Dr Wang YN, Dr Huang H, Dr Liu XW, Dr Zhou Y, and Dr Zhao GF, Department of Gastric Surgery, Fudan University Shanghai Cancer Center, for their performance and quality control of the primary operations. This project was supported by the National Natural Science Foundation of China (Grant No 81773357).
The abstract of this paper was presented at the ESTRO37 Conference as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts”: https://www.postersessiononline.eu/173580348_eu/congresos/ESTRO37/aula/-PO_768_ESTRO37.pdf.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. | ||
Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. | ||
Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87(2):236–242. | ||
Chang JS, Lim JS, Noh SH, et al. Patterns of regional recurrence after curative D2 resection for stage III (N3) gastric cancer: implications for postoperative radiotherapy. Radiother Oncol. 2012;104(3):367–373. | ||
Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30(3):268–273. | ||
Smalley SR, Gunderson L, Tepper J, et al. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52(2):283–293. | ||
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14(2):113–123. | ||
Fukuya T, Honda H, Hayashi T, et al. Lymph-node metastases: efficacy for detection with helical CT in patients with gastric cancer. Radiology. 1995;197(3):705–711. | ||
Chen CY, Hsu JS, Wu DC, et al. Gastric cancer: preoperative local staging with 3D multi-detector row CT – correlation with surgical and histopathologic results. Radiology. 2007;242(2):472–482. | ||
Feng XY, Wang W, Luo GY, et al. Comparison of endoscopic ultrasonography and multislice spiral computed tomography for the preoperative staging of gastric cancer – results of a single institution study of 610 Chinese patients. PLoS One. 2013;8(11):e78846. | ||
Kim YN, Choi D, Kim SH, et al. Gastric cancer staging at isotropic MDCT including coronal and sagittal MPR images: endoscopically diagnosed early vs. advanced gastric cancer. Abdom Imaging. 2009;34(1):26–34. | ||
Habermann CR, Weiss F, Riecken R, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004;230(2):465–471. | ||
Amin MB, Edge S, Greene F. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017. | ||
Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8(1):1–11. | ||
Landry J, Tepper JE, Wood WC, Moulton EO, Koerner F, Sullinger J. Patterns of failure following curative resection of gastric carcinoma. Int J Radiat Oncol Biol Phys. 1990;19(6):1357–1362. | ||
Tepper JE, Gunderson LL. Radiation treatment parameters in the adjuvant postoperative therapy of gastric cancer. Semin Radiat Oncol. 2002;12(2):187–195. | ||
Nam H, Lim DH, Kim S, et al. A new suggestion for the radiation target volume after a subtotal gastrectomy in patients with stomach cancer. Int J Radiat Oncol Biol Phys. 2008;71(2):448–455. | ||
Saito H, Fukumoto Y, Osaki T, et al. Distinct recurrence pattern and outcome of adenocarcinoma of the gastric cardia in comparison with carcinoma of other regions of the stomach. World J Surg. 2006;30(10):1864–1869. | ||
Yu JI, Lim DH, Ahn YC, et al. Effects of adjuvant radiotherapy on completely resected gastric cancer: a radiation oncologist’s view of the ARTIST randomized phase III trial. Radiother Oncol. 2015;117(1):171–177. | ||
Minn AY, Hsu A, La T, et al. Comparison of intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy as adjuvant therapy for gastric cancer. Cancer. 2010;116(16):3943–3952. | ||
Trip AK, Nijkamp J, van Tinteren H, et al. IMRT limits nephrotoxicity after chemoradiotherapy for gastric cancer. Radiother Oncol. 2014;112(2):289–294. | ||
Wang X, Li G, Zhang Y, et al. Single-arc volumetric-modulated arc therapy (sVMAT) as adjuvant treatment for gastric cancer: dosimetric comparisons with three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT). Med Dosim. 2013;38(4):395–400. | ||
Matzinger O, Gerber E, Bernstein Z, et al. EORTC-ROG expert opinion: radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother Oncol. 2009;92(2):164–175. |
Supplementary materials
  | Figure S1 Distribution of lymph nodes at the surgery and recurrence. |
  | Figure S2 Patterns of regional nodal relapse for irradiated patients and nonirradiated patients. |
  | Table S1 Relationship between patterns of relapse and various treatments |
  | Table S2 Relationship between patterns of relapse and primary tumor location |
  | Table S3 Relationship between patterns of relapse and pathologic N stage |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.