Back to Journals » International Journal of General Medicine » Volume 15
Patterns of Dyslipidemia in the Anemic and Nonanemic Hypertensive Saudi Population: A Cross-Sectional Study
Authors Alfhili MA , Alsughayyir J, Basudan AM , Ghneim HK, Alfaifi M, Alamri HS, Awan ZA , Algethami MR
Received 1 July 2022
Accepted for publication 21 September 2022
Published 21 October 2022 Volume 2022:15 Pages 7895—7906
DOI https://doi.org/10.2147/IJGM.S379597
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mohammad A Alfhili,1 Jawaher Alsughayyir,2 Ahmed M Basudan,1 Hazem K Ghneim,1 Mohammed Alfaifi,3 Hassan S Alamri,4 Zuhier A Awan,5,6 Mohammed R Algethami7
1Chair of Medical and Molecular Genetics Research, Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia; 2Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia; 3Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia; 4Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud Bin Abdulaziz University for Health Sciences (KSAU-HS), King Abdullah International Medical Research Center (KAIMRC), Riyadh, Saudi Arabia; 5Department of Clinical Biochemistry, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 6Department of Clinical Pathology, Al-Borg Medical Laboratories, Jeddah, Saudi Arabia; 7Ministry of Health, Jeddah, Saudi Arabia
Correspondence: Mohammad A Alfhili, Chair of Medical and Molecular Genetics Research, Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia, Tel +966-504-262-597, Email [email protected]
Background: Risk factors of cardiovascular disease include dyslipidemia, hypertension (HTN), and anemia. Our objective is to assess the patterns of dyslipidemia in the anemic and non-anemic hypertensive Saudi population.
Methods: A retrospective, cross-sectional study of the gender, blood pressure, lipid markers, and CBC parameters of 3111 subjects, which were retrieved from the database of Al-Borg Medical Laboratories over a six-year period (2014– 2019), was carried out. Means were compared among study groups and the prevalence, association, and diagnostic accuracy of lipid markers for HTN were evaluated.
Results: TG, LDL/HDL, and TG/HDL were significantly higher (P < 0.0001) in hypertensives. Anemia reduces TC and LDL (P < 0.0001) in both genders, and reduces all markers and increases HDL (P < 0.01) in male hypertensives. HTN was more prevalent in anemics with high TC than normal TC (38.23% vs 11.17%, P < 0.001) and in non-anemics with high TG than normal TG (56.31% vs 21.22%, P < 0.001). Furthermore, non-anemics with high TG/HDL had the highest risk for HTN (RR = 1.20, 95% CI = 1.1551– 1.2473, P < 0.0001). Elevated TC (P = 0.0142), TG (P < 0.0001), TC/HDL (P < 0.0001), LDL/HDL (P < 0.0001), and TG/HDL (P < 0.0001), and low HDL (P < 0.0001) were risk factors for HTN as shown by ORs. In anemics, high TC/HDL, LDL/HDL, and TG/HDL were not. Importantly, only TG and TG/HDL had a discriminating capacity for HTN.
Conclusion: The anemic state of hypertensive Saudi patients influences dyslipidemia which warrants further investigation.
Keywords: hypertension, dyslipidemia, anemia, prevalence, Saudi Arabia
Plain Language Summary
The risk for cardiovascular disease significantly increases with increased blood lipids, blood pressure (hypertension), and anemia. This study investigates changes in blood levels of different lipids in relation to hypertension in the Saudi population. The isolated and combined effect of anemia and hypertension on blood lipids was also examined. Gender, age, and laboratory results of 3111 subjects were retrieved from the database of Al-Borg Medical Laboratories and analyzed. We found that distinct lipid markers are differentially altered based on gender, anemic state, and/or hypertension. In particular, HDL, TG, LDL/HDL, and TG/HDL were significantly higher in hypertensive subjects. In males with hypertension, the presence of anemia reduces TG, TC/HDL, LDL/HDL, and TG/HDL and increases HDL. Hypertension was more common in subjects with high LDL (60.95% vs 15.22%), LDL/HDL (62.82% vs 25.43%), and TG/HDL (43.52% vs 25.26%) compared to those with normal results. Hypertension was also more common in subjects with anemia who have high TC (38.23%) compared to those with normal TC (11.17%). In non-anemic subjects, hypertension was more common with high TG than normal TG (56.31% vs 21.22%), and subjects who had high TG/HDL had the highest risk for hypertension. We identified increased TC, TG, TC/HDL, LDL/HDL, and TG/HDL, and low HDL as risk factors for hypertension. Among all lipid markers, only TG and TG/HDL may be useful to diagnose hypertension. Altogether, our findings highlight the importance of assessing the anemic state of hypertension patients along with measuring blood lipids in order to reduce the burden of cardiovascular disease.
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide with an estimated total of 18 million deaths in 2019 making up 32% of the global death toll.1 CVD accounts for 50% of deaths in the United States and causes around 4 million annual deaths in Europe representing 45% of all mortalities.2 In Saudi Arabia, 42% of deaths were due to CVD whose prevalence is projected to increase by 2035 with concomitant rise in costs to approximately $10 billion.3 Established risk factors for CVD include poor dietary habits, sedentary lifestyle, obesity, dyslipidemia, hypertension (HTN), tobacco smoking, alcohol consumption, and anemia.4,5 In fact, anemia is detected in one in three individuals with acute coronary syndrome,6 and one in two cases of chronic heart failure.7 More alarmingly, the presence of anemia increases the risk of death from chronic heart failure from 16% to 28%.8
Dyslipidemia is defined as an abnormal blood level of any lipid species brought about by either genetic or lifestyle factors. These include obesity, tobacco use, physical inactivity, and bad dietary habits. Obesity, as a predisposing factor for dyslipidemia, is projected to exert an enormous national and personal economic burden given the projected increase in prevalence in Western and Eastern countries.9 In a systematic review, it was revealed that the prevalence of dyslipidemia had a median of 43.5% and 63% in middle- and low-income countries, respectively. Conceivably, childhood obesity-related HTN was more prevalent in middle- and low-income countries in comparison to high-income countries (35.6% vs 12.7%). Moreover, Asia had the highest prevalence of childhood obesity-related HTN at 38.6% followed by South America (25.3%) and Europe (20.1%).10 For instance, in Jordan, Vietnam, and China, the odds of having dyslipidemia are higher in overweight individuals than in those with normal weight.9 In a case-control study of Vietnamese children, Hanh et al found that obesity significantly increased the risk of hypertriglyceridemia with prenatal nutrition, parental BMI, and high birth weight acting as precipitating factors.11 The differential diagnosis of dyslipidemia must include common comorbidities such as nephrotic syndrome, biliary obstruction, and hypothyroidism.12 Dyslipidemia is complicated by CVD whose risk could be significantly attenuated by lipid-lowering medications and lifestyle improvement.13
HTN is a global pandemic affecting 1.28 billion individuals aged 30–79 years, 46% of whom are unaware of their condition, and 79% has it uncontrolled. A principal cause of mortality, HTN is complicated by brain, heart, kidney, and multiple organ dysfunction.14 In addition to CVD risk factors, genetic predisposition, aging, diabetes, and kidney disease also contribute to HTN development and prognosis. Since only 42% of HTN cases are diagnosed and treated, measures must be taken to improve screening and detection efforts. Thus, understanding the biochemical determinants of HTN and the shared and distinct patterns of comorbidities is of utmost importance. In this study, we aim to examine the prevalence of HTN in the Saudi population in association with dyslipidemia and anemia.
Materials and Methods
Study Population
This study was conducted according to the Declaration of Helsinki and approved by the Biomedical Ethics Unit of Al-Borg Medical Laboratories. Consent for participation was waived by the Biomedical Ethics Unit due to the retrospective nature of the study and lack of access to participant identifiers. Laboratory results (2014–2019) were extracted from the database for 3111 subjects. Males and females were separated and age groups were formed as shown in Table 1. Young (<17 years), young adults (18–39 years), adults (40–64 years), and elderlies (≥65 years) were included. Dyslipidemia was defined by a TC of ≥200 mg/dl, LDL of ≥100 mg/dl, HDL of <40 mg/dl, or TG of ≥150 mg/dl.15 Consequently, a TC/HDL ratio of ≥6, a LDL/HDL ratio of >2.5, and a TG/HDL ratio of >2 were considered high. HTN was defined, according to the most recent guidelines set forth by the American College of Cardiology, by systolic or diastolic blood pressure (BP) of ≥120 and ≥80 mmHg, respectively.16 If systolic BP is ≥130 mmHg, then diastolic BP of >80 mmHg is diagnostic.17,18 Anemia was defined by a hemoglobin (Hgb) level of <12 g/dl.19 Subjects with missing data necessary for a particular analysis were excluded.
 |
Table 1 Distribution of Study Subjects |
Statistical Analysis
Data are shown as means ± confidence interval (95% CI). Two-tailed, unpaired Student’s t-test and one-way ANOVA were used to statistically compare two and three or more groups, respectively. Association among variables was assessed by the relative risk (RR) and odds ratio (OR). GraphPad Prism v9.2.0 (GraphPad Software, Inc., San Diego, CA, USA) was used for analysis, and statistical significance was set at a P value of <0.05.
Results
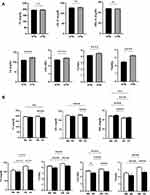
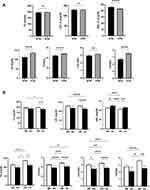
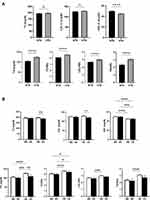
Our analysis revealed differential influence of isolated and combined presence of HTN and anemia on lipid markers. When both genders were considered together, hypertensives were found to have significantly elevated TG, TC/HDL, LDL/HDL, and TG/HDL (Figure 1A). In the normotension group (NTN), LDL/HDL was significantly lower in anemics, whereas in HTN, all markers were significantly reduced in anemics except HDL (Figure 1B). In male hypertensive patients, HDL was significantly lower but all other markers were significantly elevated (Figure 2A). The presence of anemia in male normotensives caused HDL to be significantly higher and LDL/HDL to be significantly lower compared to non-anemics (Figure 2B). Additionally, all markers were significantly reduced except HDL which was significantly higher in the HTN anemic group (Figure 2B). In hypertensive females, only HDL was significantly reduced whereas TG, TC/HDL, LDL/HDL, and TG/HDL were significantly increased (Figure 3A). The combined presence of anemia and HTN resulted in significantly lower TC, LDL, and TG values compared to HTN alone (Figure 3B).
Moreover, although TC (Figure 4A), LDL (Figure 4B), and HDL (Figure 4C) displayed modest diagnostic ability for HTN, TG showed the best performance (AUC = 0.612, P < 0.0001) in discriminating individuals with NTN and those with HTN (Figure 4D). Similar to TG, TG/HDL had the highest discriminating capacity among lipid ratios for NTN and HTN (AUC = 0.621, P < 0.0001) compared to TC/HDL (Figure 4E) and LDL/HDL (Figure 4F).
Notably, HTN was more prevalent in anemics with elevated TC (Table 2) although hypertensive anemics had lower mean TC compared to non-anemics (Figures 1B, 2B, and 3B). Surprisingly, all abnormal markers except high LDL were found to be risk factors for HTN (Table 3) despite it being more prevalent in those with elevated LDL (Table 2).
 |
Table 2 Prevalence (%) of HTN in Studied Population |
 |
Table 3 Risk Assessment of Dyslipidemia for HTN |
Among all lipid markers, TG exhibited the most consistent alterations as it was higher in hypertensive males (Figure 2B) and females (Figure 3B) which was significantly lowered in presence of anemia. In congruence, HTN was more prevalent in non-anemics with hypertriglyceridemia (Table 2). Importantly, elevated TG/HDL, along with low HDL, carried the greatest risk for HTN in non-anemics as revealed by calculated ORs (Table 3).
Discussion
The prevalence of HTN has alarmingly increased by 81% from 594 million to 1.13 billion over the period 1975–2015, constituting a major threat to public health particularly in low- and middle-income countries. This geographical disparity may be attributed to varying levels of both physician20 and patient14,21 awareness and instituted preventive measures of modifiable risk factors in developed countries.22 In this report, we highlight the differential dysregulation of lipid metabolism in light of HTN and anemic status in the Saudi population and notably underscore the association of hypertriglyceridemia and elevated TG/HDL, but not TC, with HTN.
A recent study reported a prevalence of 61.6% for HTN and 51.4% for abnormal BMI in Saudi young adults, and, compared to lean children, the obese were almost six times more likely to develop HTN.18 Dyslipidemia and tobacco smoking were more common in men than women,23,24 but anemia was consistently more prevalent in women especially those with low income.19 In contrast, unhealthy dietary habits and physical inactivity show no significant gender disparity.25 It was, however, evident in the awareness to and prevalence of HTN in favor of females.26,27 Response to stress and anxiety differ between genders with females being more susceptible,28 necessitating a multidisciplinary approach to study HTN. Furthermore, whereas males in an Indian urban setting had higher odds of HTN,29 being a female increased the risk of HTN in Bangladesh.30 The impact of HTN and diabetes mellitus on the risk of myocardial infarction was more pronounced in females.31 Because BP rises after menopause, premenopausal females had a lower prevalence of HTN than age-matched males.32 Beyond 65 years of age, HTN is more prevalent in females.33 These differences strongly point to the involvement of distinct BP regulatory mechanisms in men and women. Sex steroids are particularly involved in the differential susceptibility of males and females to chronic conditions,34 along with the renin-angiotensin system.27 Differences in the efficacy of preventive measures and in the response to treatment remain, nonetheless, to be investigated. Intriguingly, tobacco smoking was significantly lower in Saudis with CVD,22 further highlighting the ethnic-specific patterns of CVD risk factors.
In one study, it was found that over half of the Saudi population had hypercholesterolemia35 at least in part attributed to unhealthy dietary habits.4,36 In adult Saudi diabetics, 66.5% had dyslipidemia15 and correcting dyslipidemia and HTN has been demonstrated to improve bone markers by promoting osteoblast growth and function as well as preventing excess urinary loss of calcium.37
Age-standardized disability-adjusted life years caused in particular by high LDL were highest in Europe and are rising in Saudi Arabia38 in parallel to the annual increase in CVD risk factors in that country.39 For instance, hypertriglyceridemia was common in 40.3% of Saudis.35 Furthermore, the PURE-Saudi study revealed that 32.1% of 2047 participants had dyslipidemia, and 30.3% had HTN, which was more common in elderlies and in rural as opposed to urban areas.4 In contrast, hypertriglyceridemia and smoking were more prevalent in urban Saudis,35,40 as was HTN in a larger study.41 Altogether, these observations corroborate our findings that TG and TG/HDL may serve as the best predictors of HTN in our population.
It was recently reported that impaired renal function and anemia cooperatively increase CVD risk.5 Anemia in CVD is caused by malnutrition, bone marrow suppression, impaired renal function leading to low erythropoietin, medications (eg, acetylcholinesterase inhibitors), and aging.5,42 It remains elusive, however, whether anemia reflects disease severity or precipitates outcomes. In any case, inflammation and related oxidative stress observed in anemic states5,43 may account for the vascular injury typical of CVD. Indeed, the hematocrit was significantly higher in hypertensive than in normotensive subjects (40.79%, 95% CI 40.47–41.12 vs 42.46%, 95% CI 42.25–42.68, P < 0.0001), but after adjustment for Hgb, anemic hypertensives had significantly lower hematocrit than non-anemics (43.68% 95% CI 43.54–43.84 vs 32.91%, 95% CI 32.45–33.38, P < 0.0001). Therefore, reduced red cell mass may explain at least in part the apparent protective role of anemia against HTN observed in the current study (Figures 1–3, Table 3). In fact, nitric oxide-induced peripheral vasodilation seen in anemia contributes to reduced BP,42 but this, however, does not explain why anemics also had significantly lower lipid markers irrespective of their BP status (Figures 1–3) nor does it demonstrate how anemia seemed to displace the association of abnormal lipid markers with HTN (Table 3). Since an equilibrium between serum and red cell TC does exist,43 diminished serum lipids are more likely a result rather than an impetus of anemia.
Along those lines, it is worth noting that diminished HDL was the only marker resistant to the protective effect of anemia as it was consistently positively associated with HTN regardless of the anemic status (Table 3). Also unique to this marker was that it was significantly improved by anemia only in hypertensive males (Figure 2), whereas TC, LDL, and TG were improved in hypertensive females (Figure 3). In fact, HTN was more prevalent in anemics with hypercholesterolemia and in non-anemics with hypertriglyceridemia (Table 2). Differential regulation of specific lipid species has been observed in previous studies. For example, TC, LDL, and HDL were significantly lowered in sickle cell disease patients compared to healthy controls, whereas TG was significantly increased and even positively correlated with the degree of hemolysis and pulmonary HTN. Similarly, elevated TG/HDL positively correlated with endothelial dysfunction.43 In light of current evidence, alterations in lipoprotein metabolism and dynamics and the potential modulatory effect of sex steroid hormones, nitric oxide, and fatty acid mobilization, in relation to BP and anemic status deserve further investigation.
Prevention of HTN relies on several factors. These include physician and patient awareness, provision of medications, enforcing dietary restrictions, and promoting physical activity, alongside surveillance programs to facilitate early intervention and management. The need for physician education regarding appropriate clinical management of HTN is highlighted in several studies. Concerns were specifically raised regarding lack of adherence to published guidelines, use of incorrect reference values, and following inappropriate therapeutic approaches.44–46 Inordinate sodium intake and insufficient potassium in the diet are also known to contribute to the development of HTN. The Dietary Approaches to Stop Hypertension (DASH); a dietary regimen rich in whole grains, protein, and low-fat dairy foods, combined with lowered sodium, is effective for reducing BP.47,48 Another important lifestyle modification is increased physical activity as it is consistently associated with reduced BP. In particular, improved renal and endothelial function, angiogenesis, and insulin sensitivity, along with reduced inflammation, oxidative stress, and psychosocial stress are some of the proposed mechanisms through which energy expenditure through physical activity lowers BP.49
Strengths of this study include the natural sampling design, large sample size, limited analytical variability due to automated data acquisition, suitability for chronic conditions such as HTN, and identification of associated risk of multiple forms of dyslipidemia in relation to HTN and anemia. Limitations include missing data regarding anthropometric variables, lifestyle habits, personal and family history of disease, and supplement and medication intake. Also, given the cross-sectional design of the study, it was unable to derive the incidence of or the cause and effect relationship among dyslipidemia, HTN, and anemia.
Conclusion
In summary, this report shows that TG and TG/HDL perform better than other lipid markers in discriminating hypertensive from normotensive subjects. Both parameters also resist the masking effect of anemia more strongly than other markers, further arguing for their clinical value in management of HTN and related conditions. CVD is the leading cause of death in Saudi Arabia, precipitated by high BMI, hyperglycemia, and HTN.29 The high prevalence of risk factors has led to an earlier onset of CVD in the Saudi population4 which necessitates stringent adherence to preventive measures.
Abbreviations
CVD, cardiovascular disease; HTN, hypertension; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; BP, blood pressure; Hgb, hemoglobin; CI, confidence intervals; RR, relative risk; OR, odds ratio; NTN, normotension.
Data Sharing Statement
Data is available from the corresponding author upon reasonable request, and with permission of Al-Borg Medical Laboratories.
Consent to Participate
Consent for participation was waived by the Biomedical Ethics Unit due to the retrospective nature of the study and lack of access to participant identifiers.
Consent to Publish
Consent to publish was not required by the Biomedical Ethics Unit as no participant identifiers were used in the study.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University for funding this research project through the Vice Deanship of Scientific Research Chairs; Research Chair of Medical and Molecular Genetics.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was financially supported by the Deanship of Scientific Research, King Saud University through the Vice Deanship of Scientific Research Chairs; Research Chair of Medical and Molecular Genetics.
Disclosure
Mohammed R. Algethami serves as Vice Chairman of the Biomedical Ethics Unit at Al-Borg Medical Laboratories. The authors report no other conflicts of interest in this work.
References
1. World Health Organization. Cardiovascular diseases (CVDs). Available from: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
2. European Heart Network. European cardiovascular disease statistics 2017. Available from: https://ehnheart.org/cvd-statistics.html. Accessed
3. Isabelle Gagnon-Arpin MH, AlAyoubi F, Sutherland G, Dobrescu A, Villa G, Habib KA. Modelling the burden of cardiovascular disease in Saudi Arabia and the impact of reducing modifiable risk factors. J Saudi Heart Assoc. 2018;30(4):365. doi:10.1016/j.jsha.2018.05.025
4. Alhabib KF, Batais MA, Almigbal TH, et al. Demographic, behavioral, and cardiovascular disease risk factors in the Saudi population: results from the Prospective Urban Rural Epidemiology study (PURE-Saudi). BMC Public Health. 2020;20(1):1213. doi:10.1186/s12889-020-09298-w
5. Ishigami J, Grams ME, Naik RP, et al. Hemoglobin, albuminuria, and kidney function in cardiovascular risk: the ARIC (Atherosclerosis Risk in Communities) Study. J Am Heart Assoc. 2018;7(2). doi:10.1161/JAHA.117.007209
6. Meneveau N, Schiele F, Seronde MF, et al. Anemia for risk assessment of patients with acute coronary syndromes. Am J Cardiol. 2009;103(4):442–447. doi:10.1016/j.amjcard.2008.10.023
7. Maggioni AP, Opasich C, Anand I, et al. Anemia in patients with heart failure: prevalence and prognostic role in a controlled trial and in clinical practice. J Card Fail. 2005;11(2):91–98. doi:10.1016/j.cardfail.2004.05.004
8. Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE). J Am Coll Cardiol. 2003;41(11):1933–1939. doi:10.1016/s0735-1097(03)00425-x
9. Chu DT, Minh Nguyet NT, Dinh TC, et al. An update on physical health and economic consequences of overweight and obesity. Diabetes Metab Syndr. 2018;12(6):1095–1100. doi:10.1016/j.dsx.2018.05.004
10. Obita G, Alkhatib A. Disparities in the prevalence of childhood obesity-related comorbidities: a systematic review. Front Public Health. 2022;10:923744. doi:10.3389/fpubh.2022.923744
11. Hanh NTH, Tuyet LT, Dao DTA, Tao Y, Chu DT. Childhood obesity is a high-risk factor for hypertriglyceridemia: a case-control study in Vietnam. Osong Public Health Res Perspect. 2017;8(2):138–146. doi:10.24171/j.phrp.2017.8.2.06
12. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25Pt B):2889–2934. doi:10.1016/j.jacc.2013.11.002
13. Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US preventive services task force. JAMA. 2016;316(19):2008–2024. doi:10.1001/jama.2015.15629
14. World Health Organization. Hypertension. https://www.who.int/news-room/fact-sheets/detail/hypertension. Available from:
15. Alzaheb RA, Altemani AH. Prevalence and associated factors of dyslipidemia among adults with type 2 diabetes mellitus in Saudi Arabia. Diabetes Metab Syndr Obes. 2020;13:4033–4040. doi:10.2147/DMSO.S246068
16. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. doi:10.1161/HYP.0000000000000066
17. Haddadin F, Sud K, Munoz Estrella A, et al. The prevalence and predictors of resistant hypertension in high-risk overweight and obese patients: a cross-sectional study based on the 2017 ACC/AHA guidelines. J Clin Hypertens. 2019;21(10):1507–1515. doi:10.1111/jch.13666
18. El-Ashker S, Pednekar MS, Narake SS, Albaker W, Al-Hariri M. Blood pressure and cardio-metabolic risk profile in young Saudi males in a university setting. Medicina. 2021;57(8):755. doi:10.3390/medicina57080755
19. Alquaiz AJ, Khoja TA, Alsharif A, et al. Prevalence and correlates of anaemia in adolescents in Riyadh city, Kingdom of Saudi Arabia. Public Health Nutr. 2015;18(17):3192–3200. doi:10.1017/S1368980015001214
20. Al-Gelban KS, Khan MY, Al-Khaldi YM, et al. Adherence of primary health care physicians to hypertension management guidelines in the Aseer region of Saudi Arabia. Saudi J Kidney Dis Transpl. 2011;22(5):941–948.
21. Al-Saleem SA, Al-Shahrani A, Al-Khaldi YM. Hypertension care in Aseer region, Saudi Arabia: barriers and solutions. Saudi J Kidney Dis Transpl. 2014;25(6):1328–1333. doi:10.4103/1319-2442.144313
22. Al-Baghli NA, Al-Ghamdi AJ, Al-Turki KA, et al. Awareness of cardiovascular disease in eastern Saudi Arabia. J Family Community Med. 2010;17(1):15–21. doi:10.4103/1319-1683.68784
23. Ibrahim NK, Mahnashi M, Al-Dhaheri A, et al. Risk factors of coronary heart disease among medical students in King Abdulaziz University, Jeddah, Saudi Arabia. BMC Public Health. 2014;14(1):411. doi:10.1186/1471-2458-14-411
24. Mohieldein AH, Hasan M, Al-Harbi KK, Alodailah SS, Azahrani RM, Al-Mushawwah SA. Dyslipidemia and reduced total antioxidant status in young adult Saudis with prediabetes. Diabetes Metab Syndr. 2015;9(4):287–291. doi:10.1016/j.dsx.2014.04.017
25. Kalaf H, AlMesned A, Soomro T, Lasheen W, Ewid M, Al-Mohaimeed AA. Cardiovascular disease risk profile among young Saudi women of Al-Qassim, Saudi Arabia: a cross-sectional study. Int J Health Sci. 2016;10(1):29–37. doi:10.12816/0031214
26. Doumas M, Papademetriou V, Faselis C, Kokkinos P. Gender differences in hypertension: myths and reality. Curr Hypertens Rep. 2013;15(4):321–330. doi:10.1007/s11906-013-0359-y
27. Reckelhoff JF. Gender differences in hypertension. Curr Opin Nephrol Hypertens. 2018;27(3):176–181. doi:10.1097/MNH.0000000000000404
28. Di Pilla M, Bruno RM, Taddei S, Virdis A. Gender differences in the relationships between psychosocial factors and hypertension. Maturitas. 2016;93:58–64. doi:10.1016/j.maturitas.2016.06.003
29. Rani R, Kannaujiya AK, Talukdar P, Sikarwar A. Gender and rural-urban differences in hypertension among youth in India: insights from a large scale survey, 2015–16. J Biosoc Sci. 2022;1–15. doi:10.1017/S0021932022000141
30. Kibria GMA, Swasey K, Das Gupta R, et al. Differences in prevalence and determinants of hypertension according to rural-urban place of residence among adults in Bangladesh. J Biosoc Sci. 2019;51(4):578–590. doi:10.1017/S0021932018000366
31. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi:10.1016/S0140-6736(04)17018-9
32. Neuhauser H, Thamm M, Ellert U. [Blood pressure in Germany 2008–2011: results of the German Health Interview and Examination Survey for Adults (DEGS1)]. Blutdruck in Deutschland 2008–2011: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56(5–6):795–801. doi:10.1007/s00103-013-1669-6
33. Gu Q, Burt VL, Paulose-Ram R, Dillon CF. Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among US adults with hypertension: data from the National Health and Nutrition Examination Survey 1999–2004. Am J Hypertens. 2008;21(7):789–798. doi:10.1038/ajh.2008.185
34. Alfhili MA, Alsughayyir J, Basudan A, et al. Isolated and combined effect of age and gender on neutrophil-lymphocyte ratio in the hyperglycemic Saudi population. Medicina. 2022;58(8):1040. doi:10.3390/medicina58081040
35. Al-Nozha MM, Arafah MR, Al-Maatouq MA, et al. Hyperlipidemia in Saudi Arabia. Saudi Med J. 2008;29(2):282–287.
36. Abdel-Megeid FY, Abdelkarem HM, El-Fetouh AM. Unhealthy nutritional habits in university students are a risk factor for cardiovascular diseases. Saudi Med J. 2011;32(6):621–627.
37. Al-Hariri M, Aldhafery B. Association of hypertension and lipid profile with osteoporosis. Scientifica. 2020;2020:7075815. doi:10.1155/2020/7075815
38. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
39. El Bcheraoui C, Memish ZA, Tuffaha M, et al. Hypertension and its associated risk factors in the kingdom of Saudi Arabia, 2013: a national survey. Int J Hypertens. 2014;2014:564679. doi:10.1155/2014/564679
40. Aljefree N, Ahmed F. Prevalence of cardiovascular disease and associated risk factors among adult population in the gulf region: a systematic review. Adv Public Health. 2015;2015:235101. doi:10.1155/2015/235101
41. Al-Nozha MM, Abdullah M, Arafah MR, et al. Hypertension in Saudi Arabia. Saudi Med J. 2007;28(1):77–84.
42. Kaiafa G, Kanellos I, Savopoulos C, Kakaletsis N, Giannakoulas G, Hatzitolios AI. Is anemia a new cardiovascular risk factor? Int J Cardiol. 2015;186:117–124. doi:10.1016/j.ijcard.2015.03.159
43. Zorca S, Freeman L, Hildesheim M, et al. Lipid levels in sickle-cell disease associated with haemolytic severity, vascular dysfunction and pulmonary hypertension. Br J Haematol. 2010;149(3):436–445. doi:10.1111/j.1365-2141.2010.08109.x
44. Rawat R, Ram VS, Kumar G, et al. Awareness of general practitioners toward hypertension management. J Pharm Bioallied Sci. 2021;13(Suppl 2):S1513–S1516. doi:10.4103/jpbs.jpbs_268_21
45. Ale O, Braimoh RW. Awareness of hypertension guidelines and the diagnosis and evaluation of hypertension by primary care physicians in Nigeria. Cardiovasc J Afr. 2017;28(2):72–76. doi:10.5830/CVJA-2016-048
46. Feldman RD, Liu L, Wu Z, Zhang Y, Yu X, Zhang XH. Hypertension attitude perspectives and Needs (HAPPEN): a real-world survey of physicians and patients with hypertension in China. J Clin Hypertens. 2017;19(3):256–264. doi:10.1111/jch.12912
47. Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and control of hypertension: JACC health promotion series. J Am Coll Cardiol. 2018;72(11):1278–1293. doi:10.1016/j.jacc.2018.07.008
48. Juraschek SP, Miller ER
49. Diaz KM, Shimbo D. Physical activity and the prevention of hypertension. Curr Hypertens Rep. 2013;15(6):659–668. doi:10.1007/s11906-013-0386-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.