Back to Journals » Cancer Management and Research » Volume 13
Patterns of Care and Outcomes for Non-Metastatic Prostate Cancer in the United States: Results of the CancerMPact® Survey 2018
Authors de Sá Moreira E, Robinson D , Hawthorne S , Zhao L, Hanson M, Kanas G, Turnure M , Davis C, Clark O
Received 8 October 2021
Accepted for publication 27 November 2021
Published 11 December 2021 Volume 2021:13 Pages 9127—9137
DOI https://doi.org/10.2147/CMAR.S343321
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Eloisa de Sá Moreira,1 David Robinson,2 Stephanie Hawthorne,2 Linda Zhao,2 Madelyn Hanson,2 Gena Kanas,2 Matthew Turnure,2 Christine Davis,2 Otavio Clark2
1Health Division, Kantar, São Paulo, Brazil; 2Health Division, Kantar, New York, NY, USA
Correspondence: Matthew Turnure
Kantar 175 Greenwich St, WTC 3, 35th Floor, New York, NY, 10007, USA
Tel +1 314-221-4323
Email [email protected]
Purpose: We describe patterns of care and treatment outcomes for non-metastatic PCa (nmPCA), either hormone-sensitive or castration-resistant, in the United States of America (USA) in 2018.
Methods: A survey (CancerMPact®) recruited physicians nationwide to answer an online questionnaire about how they treated patients with nmPCA. Questions covered aspects of treatment at all disease stages. Board-certified urologists and oncologists with at least five years of clinical practice and who treated at least 30 PCa patients monthly were included.
Results: The survey included responses from ninety-four physicians with an average of 17.5 years of clinical practice, who had treated a combined average of 4415 patients with nmPCA per month in 2018. Approximately 40% of patients in stage I were managed with either active surveillance or observation/no therapy, decreasing to 20%, 8% and 6% in stages II, III and IV(M0), respectively. Intensity-modulated radiotherapy was favored over other radiotherapy modalities, with rates of use ranging between 60% and 69% depending on disease stage. Leuprolide as monotherapy or in combination with enzalutamide, abiraterone or bicalutamide were the most common systemic treatment options for non-metastatic hormone-sensitive PCa (nmHSPC) patients with the first or second recurrence. Only 16.5% of non-metastatic castration-resistant PCa (nmCRPC) patients did not relapse within five years of initial therapy for nmCRPC.
Conclusion: While PCa treatment recommendations are rapidly changing due to advances in treatment, we observed great concordance between their most current versions and real-world data treatment patterns reported by US physicians.
Keywords: real-world evidence, treatment, physician reported, questionnaire
Introduction
Prostate cancer (PCa) is the most common malignancy among men in the United States (USA)1 and the second-most common worldwide.2 Among men living in the USA in 2019, PCa accounted for 20% of the estimated 870,970 new diagnoses of cancer and 10% of the deaths from cancer.1 More than 90% of PCa cases are detected in the local or locally advanced stages (stages I–IV M0).3,4 Advanced age is one of the strongest risk factors, with males having a 0.8% and 8.4% probability of developing PCa by age 55 and 75, respectively.5 African American ancestry and family history are also strong risk factors for PCa.4
Selection of the best treatment or care option for non-metastatic PCa is not trivial and must be based on risk stratification according to prostate specific antigen (PSA) level, clinical stage, digital rectal exam, Gleason score and extent of prostatic and extraprostatic involvement.6–10 The importance of shared decision-making, by informing patients about short- and long-term morbidity and side effects for each approach and considering their values and preferences, has been incorporated into most treatment guidelines.8 Radical prostatectomy, external beam radiotherapy (EBRT)/brachytherapy with or without androgen deprivation therapy (ADT) or active surveillance for selected patients are all appropriate choices for low-risk cancer. Radiotherapy with or without ADT is the preferred option for patients at intermediate risk (T2b-2c, PSA level between 10 and 20 ng/mL, and Gleason score 7).6,8 Radiotherapy with adjuvant ADT is also recommended for unfavorable intermediate-risk or high-risk disease; while the first group usually receives adjuvant ADT for only 6 months, it should last about 24 months in the high-risk group.6,8 Finally, observation and watchful waiting have been recommended for men with a life expectancy of five years or less with low or intermediate risk.8,10 While luteinizing hormone-releasing hormone (LHRH) agonists represent the standard systemic ADT, there have been a number of approvals in the last few years for both second-generation hormones and drug-based therapy.
Although most men with localized PCa respond to initial treatment, 20% to 50% show a rise in PSA within 10 years.11 Patients are commonly asymptomatic in this stage and do not present clinical evidence of metastases.6 At this phase of the disease, systemic ADT is the treatment of choice for patients who are not candidates for salvage prostatectomy or salvage radiotherapy, or who have already been submitted to one of these strategies.12 After an average time of 19 months, the disease progresses again from a hormone-sensitive to a castration-resistant state.13 Non-metastatic castration-resistant PCa (nmCRPC) is generally defined as PSA recurrence while on ADT in the absence of obvious metastasis obtained through conventional imaging.14
Treatment guidelines for PCa have undergone important changes in recent years, almost keeping pace with the development of new treatment strategies and molecules.15,16 Next-generation hormone therapies, such as enzalutamide and abiraterone, first received regulatory approvals for the treatments of relapsed /refractory metastatic CRPC patients, and later expanded their indications to non-metastatic or hormone-sensitive PCa patients. However, real-world studies investigating non-metastatic PCa treatment management are limited. The aim of this study is to describe patterns of care for non-metastatic PCa, either hormone-sensitive or resistant to castration, in the United States.
Materials and Methods
An internal team of oncology experts at the Health Division of Kantar developed the CancerMPact® - Treatment Architecture – United States PCa survey. The survey is conducted annually and assesses the current clinical management of PCa patients by stage for all treatment modalities. To develop the questionnaire, the Kantar team reviewed USA PCa treatment guidelines and approvals of new drugs by the US Food and Drug Association (FDA). The Kantar team also performed a literature review of pivotal trial data from peer-reviewed medical journals and major oncology conferences to determine current practices and potential changes in PCa treatment up to 2018. We acknowledge recent changes post-2018 have occurred in treatment due to trial data and approvals in the nmCRPC setting.
Ninety-four physicians nationwide answered the online PCa survey in September 2018. Only board-certified urologists, medical oncologists, hematology oncologists and medical oncologists/ hematology oncologists with at least 5 years of clinical practice after residency, and who treated at least 30 PCa patients monthly were included in the study.
The survey included questions about the physicians’ experience (such as years in practice, practice type, number of patients seen on a monthly basis) and about treatment of their PCa patients within the last six months. Physicians were explicitly asked to disregard their personal opinions and to objectively report how they treated their patients based upon their own clinical practice and experience; definitions such as for stage or castration resistance were not provided. They answered questions about treatment modalities (radiation, surgery, systemic therapy, etc.), systemic therapy regimens used for each clinical situation, and their duration. The survey covered aspects of treatment across all stages of the disease as well as response outcomes.
Another company (Lightspeed) programmed, fielded, and hosted the online questionnaire. The anonymized raw data was securely transferred to the Health Division at Kantar, which performed data analysis and tabulation. No formal statistical treatment was given to the results and data were reported as unweighted average of all responses. This work used secondary data and therefore is exempted from ethic committee approval. In this paper, we report the survey results for non-metastatic PCa only.
Results
Physicians Profile
The detailed profile of the 94 physicians who answered is shown in Table 1. On average, respondents treated 68 PCa patients per month, with the total number of monthly cases reported by physicians as 6389 patients across all stages. Urology was the predominant specialty (43.6%) and private practice was the most common setting (36.7%). According to the physicians, non-metastatic PCa accounted for 69.1% of their cases [21.3%, 16.7%, 13.9%, and 17.2% of patients in stages I, II, III, and IV(M0)], while metastatic PCa [stage IV(M1)] corresponded to 30.9% of their cases. Based on these percentages and on the estimated total of 6389 PCa patients treated by the physicians each month, responding physicians saw approximately 4415 non-metastatic PCa cases per month.
 |
Table 1 Characteristics of Physicians Surveyed for Prostate Cancer Treatment Patterns and Response Outcomes |
Treatment of Non-Metastatic Hormone-Sensitive Prostate Cancer
Initial Therapy
Table 2 presents the utilization of the most common initial therapy modalities for nmHSPC in the USA, as estimated by the responding physicians. Around 40% of the patients in stage I were managed with either active surveillance or observation/ no therapy, with that rate decreasing to 20%, 8% and 6% in stages II, III and IV(M0), respectively. Radiotherapy-only (25.2%) and surgery-only (24.7%) were the most common modalities for stage II patients, while radiotherapy with (28.5%) or without (16.9%) systemic therapy was the main modality for stage III patients. In stage IV(M0), systemic therapy-only (56.7%), which includes hormone therapy, chemotherapy, immunotherapy, radiopharmaceuticals and other targeted therapies, was largely preferred over other treatment modalities.
 |
Table 2 Rate of Utilization of the Most Common Initial Therapy Modalities for nmHSPC, by Stage |
Utilization of EBRT by the surveyed physicians is presented in Table 3. Over two-thirds of stage I–III and over half of stage IV(M0) nmHSPC patients receiving radiotherapy were treated with Intensity Modulated Radiotherapy (IMRT).
 |
Table 3 Type of External Beam Radiotherapy Used in Treatment of Local and Locally Advanced Prostate Cancer, by Stage |
The systemic regimens utilized as initial therapy for nmHSPC, with their respective utilization rate and average duration, are presented in Table 4. LHRH analogs were the primary ADT agents used in the USA, with leuprolide as a monotherapy and combination regimens containing leuprolide and/or bicalutamide being the preferred regimens. Regimens containing the second-generation hormone therapies enzalutamide and abiraterone were more commonly used in stage IV(M0) than in earlier stages. This was also true for docetaxel.
 |
Table 4 Rate of Utilization and Duration of Systemic Therapies for Newly Diagnosed nmHSPC, by Stage |
Table 5 displays response and recurrence rates by time period after first-line therapy for nmHSPC stratified by stage. Approximately 50% of patients with stage I disease, 40% with stage II disease, and approximately one-quarter of patients in stage III did not relapse within ten years of therapy. That figure was less than 10% among stage IV(M0) patients.
 |
Table 5 Results of Initial Therapy for nmHSPC, by Stage |
Regarding type of first recurrence, stage I and II patients were most likely to experience a biochemical (PSA) progression only (64.0% and 56.1% of patients, respectively) (data not shown). Metastatic recurrence was considerably more frequent among stage IV(M0) patients (41.2%) as compared to patients in stage I, II or III (13.2%, 16.2% and 24.3%, respectively). Nearly 25% of stage IV(M0) patients developed metastatic CRPC upon recurrence after receiving first-line hormone therapy. Physicians also reported the stage of first recurrence for stage I–III patients with a diagnosed local recurrence. Approximately 65% of stage I, 55% of stage II, and 43% of stage III patients recurred with localized disease within stage I–III and the remainder recurred with stage IV(M0) disease.
Treatment After First Recurrence
Table 6 presents the overall utilization of the most common treatment modalities for nmHSPC patients who experienced a recurrence after initial treatment, either with a rising level of PSA or a diagnosed local recurrence. At least half of patients with a recurrence received systemic therapy with or without radiotherapy regardless of the initial treatment. Active surveillance accounted for roughly 10% of patients with a recurrence who underwent radiotherapy or surgery as initial treatment.
 |
Table 6 Treatment Utilization by Modality Regimen and Stage Among Patients with nmHSPC Prostate Cancer Who Experienced a First Recurrence |
After observation or active surveillance as the initial approach after a first recurrence, 51.1% or 65.9% of patients proceeded to receive treatment, within a mean time of 14.4 or 15.1 months under observation or active surveillance, respectively.
Table 7 displays the most common systemic regimens utilized, along with their average duration, for recurrent nmHSPC. Over 50% of patients received leuprolide with or without enzalutamide or bicalutamide at first recurrence, regardless of disease stage. More stage IV(M0) than stage I–III patients received abiraterone or enzalutamide regimens at first recurrence.
 |
Table 7 Rate of Utilization and Duration of Systemic Regimens for Recurrent nmHSPC, Stages I–III (A) and Stage IV(M0) (B) |
Table 8 presents the type of second recurrence by stage of disease after the first recurrence. Recurrent Stage IV(M0) patients were more likely to develop a metastatic recurrence as a second recurrence than stage I–III patients. Approximately 40% of stage I–III patients experienced a second local recurrence as stage I–III disease. The remainder of patients who had a second local recurrence experienced locally advanced, stage IV(M0) disease.
 |
Table 8 Types of Second Recurrence for nmHSPC and Their Respective Rates, by Stage After the First Recurrence |
Table 9 presents the utilization of the most common treatment modalities for nmHSPC patients who had a second recurrence. Approximately 60% of stage I–III and stage IV(M0) patients received systemic therapy for the second recurrence.
 |
Table 9 Treatment Utilization by Modality Regimen and Stage Among Patients with nmHSPC Prostate Cancer Who Experienced a Second Recurrence |
The most common systemic regimens and the average duration of treatment for nmHPSC at second local recurrence, by stage, are described in Table 10. While many patients received traditional hormone therapies such as bicalutamide plus leuprolide or leuprolide alone, almost 35% of stage IV patients received abiraterone or enzalutamide in combination with leuprolide.
 |
Table 10 Rate of Utilization and Duration of Systemic Regimens at Second Local Recurrence for nmHSPC Stages I–III (A) and Stage IV(M0) (B) |
Treatment of Non-Metastatic Castration-Resistant Prostate Cancer
Utilization of the most common therapy modalities for nmCRPC in the USA is described in Table 11. According to the respondent physicians, approximately 60% of nmCRPC patients received systemic therapy alone, while approximately 12% of patients remained under observation or active surveillance.
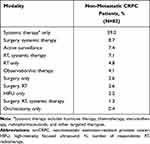 |
Table 11 Treatment Utilization by Modality Regimen Among Patients with nmCRPC |
Among the patients whose disease recurred as nmCRPC and underwent observation or active surveillance, 41.1% and 56.5% eventually received treatment, respectively; mean duration of observation and active surveillance was 11.4 and 13.4 months, respectively. Physicians also estimated that 35% of their nmCRPC patients remained untreated until they became metastatic (data not shown).
Systemic Therapy
Table 12 displays the most common regimens utilized as initial systemic therapy for nmCRPC patients in the USA, as well as their respective duration. Over 50% of patients received a second-generation hormone (enzalutamide, apalutamide) as initial therapy for nmCRPC disease.
 |
Table 12 Rate of Utilization and Duration of Systemic Regimens Among Patients with nmCRPC |
Treatment Results
Responding physicians report that 15.5% of their patients did not respond to the initial therapy for nmCRPC (Table 13). In addition, 26.6% of patients responded but relapsed within one year of therapy and 41.3% between one and five years of therapy. Only 16.5% of nmCRPC patients did not relapse within five years of initial therapy for nmCRPC. Among patients who recurred, 30.1% developed a local recurrence and 69.9% developed a metastatic recurrence; they remained non-metastatic for a mean of 17.2 months before developing metastatic CRPC.
 |
Table 13 Results of Initial Therapy for Patients with nmCRPC |
Discussion
We report the results of a nationwide survey fielded in 2018 with USA physicians who treated non-metastatic PCa in different practice settings. The survey comprised 94 physicians who treated an estimated average of 4415 non-metastatic patients per month. Through their responses to the survey, these physicians described their treatment patterns and response outcomes among their non-metastatic PCa patients. These data provide an updated overview of the current non-metastatic PCa treatment landscape and outcomes for both hormone-sensitive and castration-resistant patients in the USA.
Non-metastatic patients comprise about 90% of newly diagnosed prostate cancer cases, but only 70% of patients treated by physician respondents in our survey. This is likely due to early-stage patients being less likely to receive treatment upon initial diagnosis. Observation and active surveillance were the main approaches adopted by physicians in the initial management of stage I PCa (40% of patients) and the third-most common choice for the management of stage II patients. These findings are in agreement with a retrospective analysis of the National Cancer Data Base17 and a recent nationwide study in the Netherlands.18 The former study showed that the use of observation among PCa patients with low-risk disease has consistently increased since 2004, corresponding to roughly 16% of the cases in 2004–2005 and 32% of the cases in 2012–2013, although radical prostatectomy was still the most common approach in this last period. In the Netherlands, 45% to 65% of low-risk PCa patients, depending on the age group, received no active treatment between 2005 and 2015.18 These results show a reduction in overtreatment and reflect current PCa treatment guidelines.8,16,19–21
IMRT was largely favored by physicians over other EBRT modalities. Although IMRT is expensive, it reduces the risk of gastrointestinal toxicities, as well as the rates of salvage therapy.22 Intensity Modulated Radiotherapy is able to deliver high doses of radiation that result in durable responses while minimizing the radiation exposure to adjacent rectal and bladder tissues. Prostate cancer has been the most widely used application of IMRT, particularly because of the damage that other forms of EBRT can inflict on the pelvic region.23
Recurrence rates and time to recurrence agreed with data obtained in retrospective and prospective studies.24–26 We also found that a large proportion of nmHSPC patients with a recurrence (both first and second recurrence) received a second-generation hormone therapy (abiraterone or enzalutamide) combined with leuprolide (data not shown). These second-generation hormone therapies represented the most common systemic treatment options for patients with a second recurrence and were rated among the top four regimens among patients with a first recurrence for all stage subgroups [I, II, III and IV(M0)]. More stage IV(M0) patients received abiraterone or enzalutamide regimens at first local recurrence than stage I–III. Compared to the CancerMPact survey fielded in 2017 (data not shown), there was an increase in the use of enzalutamide over bicalutamide as the combination partner with leuprolide in 2018. Current guidelines do not recommend treatment with ADT and abiraterone or enzalutamide for treatment of nmHSPC.8,15,19 Nevertheless, in a randomized study with 1917 men with relapsed PCa not previously treated with hormone therapy, ADT plus abiraterone and prednisone resulted in significantly higher rates of overall and failure-free survival than ADT alone.27
Regarding nmCRPC, the two most common treatments in 2018 reported by the responding physicians were enzalutamide and apalutamide, in agreement with the most recent guidelines.15,19 Interestingly, a study using the 2015–2017 Ipsos Global Oncology Monitor Database found that leuprolide (16%) and triptorelin (10%) were the most commonly used first nmCRPC regimens in the US.28 Together these observations suggest that the real-world treatment landscape for nmCRPC is rapidly changing.
This study was subject to at least three limitations. First, because the survey respondents included only physicians with at least 5 years of clinical practice after residency and who treated at least 30 PCa patients monthly, the results may not reflect treatment practices of physicians with fewer years of practice experience or lower volume of patients. Second, these data could be subject to recall bias; however, we attempted to limit this bias by instructing survey respondents to limit their responses to patients seen in the last six months. Lastly, we did not ask physicians why they made their treatment decisions, which may limit the ability to interpret the results.
Conclusion
Results from this nationwide survey of physicians reporting on their non-metastatic PCa patients provide insights about the evolving treatment landscape for PCa and how new guidelines are incorporated into clinical practice. While PCa treatment recommendations are rapidly changing due to advances in treatment, we observed great concordance between the most recent versions and real-world data treatment patterns reported by USA physicians.
Acknowledgments
The authors would like to very much thank the entire CancerMPact team that made this work possible: Anna Boudoures, Kelly Clapp, Megan Epperson, Christina Fish, Qi Fu, Michael Gaschler, Medina Hasanagic, Sarthak Jain, Leonard Kusdra, Melissa Machado, Bhavna Murali, Knar Nersesyan, Sara Omlid, Michael Ramirez, Fadi Saadeh, Katherine Stockstill, and Cathy Su.
Disclosure
The authors declared following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors are employed by Kantar, a global consultancy company that acts in the healthcare market with clients from pharmaceutical and biotechnology companies, health insurance companies and hospitals. Otavio Clark is a partner of Kantar Health Brazil, a consultancy company that produces Health Technology Assessment work for pharmaceutical companies. He has also acted as an advisory board member of different pharmaceutical companies. The authors report no other conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi:10.3322/caac.21551
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
3. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004–2014. Ann Epidemiol. 2018;28:328–330. doi:10.1016/j.annepidem.2018.03.001
4. Sathianathen NJ, Konety BR, Crook J, et al. Landmarks in prostate cancer. Nat Rev Urol. 2018;15:627–642. doi:10.1038/s41585-018-0060-7
5. National Cancer Institute. SEER*Explorer; 2020. Available from: https://seer.cancer.gov/explorer/.
6. Facchini G, Perri F, Misso G, et al. Optimal management of prostate cancer based on its natural clinical history. Curr Cancer Drug Targets. 2018;18:457–467. doi:10.2174/1568009617666170209093101
7. Imber BS, Varghese M, Ehdaie B, et al. Financial toxicity associated with treatment of localized prostate cancer. Nat Rev Urol. 2020;17:28–40. doi:10.1038/s41585-019-0258-3
8. Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. part I: risk stratification, shared decision making, and care options. J Urol. 2018;199:683–690. doi:10.1016/j.juro.2017.11.095
9. Wilcox SW, Aherne NJ, Benjamin LC, et al. Long-term outcomes from dose-escalated image-guided intensity-modulated radiotherapy with androgen deprivation: encouraging results for intermediate- and high-risk prostate cancer. Onco Targets Ther. 2014;7:1519–1523. doi:10.2147/OTT.S65238
10. Bekelman JE, Rumble RB, Chen RC, et al. Clinically localized prostate cancer: ASCO clinical practice guideline endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J Clin Oncol. 2018;36:3251–3258. doi:10.1200/JCO.18.00606
11. Artibani W, Porcaro AB, De Marco V, et al. Management of biochemical recurrence after primary curative treatment for prostate cancer: a review. Urol Int. 2018;100:251–262. doi:10.1159/000481438
12. Gul A, Garcia JA, Barata PC. Treatment of non-metastatic castration-resistant prostate cancer: focus on apalutamide. Cancer Manag Res. 2019;11:7253–7262. doi:10.2147/CMAR.S165706
13. El-Amm J, Aragon-Ching JB. The current landscape of treatment in non-metastatic castration-resistant prostate cancer. Clin Med Insights Oncol. 2019;13:1179554919833927. doi:10.1177/1179554919833927
14. Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–1418. doi:10.1200/JCO.2015.64.2702
15. Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479–505. doi:10.6004/jnccn.2019.0023
16. Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi:10.6004/jnccn.2016.0004
17. Loppenberg B, Sood A, Dalela D, et al. Variation in locoregional prostate cancer care and treatment trends at commission on cancer designated facilities: a national cancer data base analysis 2004 to 2013. Clin Genitourin Cancer. 2017;15:e955–e968. doi:10.1016/j.clgc.2017.04.014
18. Vernooij RWM, van Oort I, de Reijke TM, et al. Nationwide treatment patterns and survival of older patients with prostate cancer. J Geriatr Oncol. 2019;10:252–258. doi:10.1016/j.jgo.2018.06.010
19. EAU Guidelines Office, Arnhem, The Netherlands. Available from: http://uroweb.org/guidelines/compilations-of-all-guidelines/.
20. Morash C, Tey R, Agbassi C, et al. Active surveillance for the management of localized prostate cancer: guideline recommendations. Can Urol Assoc J. 2015;9:171–178. doi:10.5489/cuaj.2806
21. Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. doi:10.1016/j.eururo.2016.08.003
22. Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309:2587–2595. doi:10.1001/jama.2013.6882
23. Kwak YK, Lee SW, Kay CS, et al. Intensity-modulated radiotherapy reduces gastrointestinal toxicity in pelvic radiation therapy with moderate dose. PLoS One. 2017;12:e0183339. doi:10.1371/journal.pone.0183339
24. Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi:10.1001/jama.294.4.433
25. Kupelian PA, Mahadevan A, Reddy CA, et al. Use of different definitions of biochemical failure after external beam radiotherapy changes conclusions about relative treatment efficacy for localized prostate cancer. Urology. 2006;68:593–598. doi:10.1016/j.urology.2006.03.075
26. Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3478 consecutive patients: long-term results. J Urol. 2004;172:910–914. doi:10.1097/01.ju.0000134888.22332.bb
27. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi:10.1056/NEJMoa1702900
28. Shah R, Botteman M, Waldeck R. Treatment characteristics for nonmetastatic castration-resistant prostate cancer in the United States, Europe and Japan. Future Oncol. 2019;15:4069–4081. doi:10.2217/fon-2019-0563
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
