Back to Journals » Patient Preference and Adherence » Volume 10
Patients’ perspectives and preferences in the choice of inhalers: the case for Respimat® or HandiHaler®
Authors Dekhuijzen R, Lavorini F , Usmani OS
Received 26 May 2016
Accepted for publication 7 July 2016
Published 18 August 2016 Volume 2016:10 Pages 1561—1572
DOI https://doi.org/10.2147/PPA.S82857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Pieter Nicolaas Richard Dekhuijzen,1 Federico Lavorini,2 Omar S Usmani3
1Department of Pulmonary Diseases, Radboud University Medical Center, Nijmegen, the Netherlands; 2Department of Experimental and Clinical Medicine, Careggi University Hospital, Florence, Italy; 3National Heart and Lung Institute, Imperial College London and Royal Brompton Hospital, London, UK
Abstract: Poor inhaler technique hampers the efficacy of drug therapy in asthma and chronic obstructive pulmonary disease. Not only does this affect individual patient care, but it also impacts on the wider health care economics associated with these conditions. Treatment guidelines recommend a systematic approach to drug class selection; however, standardization of inhaler selection is currently difficult owing to the complexity of the interaction between the inhaler device and the patient. Specifically, individual patient preference can influence how successful a treatment is overall. This article reviews inhaler devices from the patient perspective, with a particular focus on the dry powder inhaler HandiHaler® and Respimat® Soft Mist™ Inhaler. It discusses factors that influence device preference and treatment compliance and reviews tools that can aid health care professionals to better match inhaler devices to individual patients’ needs.
Keywords: asthma, chronic obstructive pulmonary disease, inhaler technique, Soft Mist™ Inhaler, tiotropium Respimat®, tiotropium HandiHaler®
Introduction
Inhaled therapy remains the cornerstone of treatment for pulmonary disorders such as asthma and chronic obstructive pulmonary disease (COPD). It offers advantages over oral tablet administration, such as effective targeting of treatment to the lungs at a lower dose with a rapid onset of action.1–5
Despite the introduction of new pharmacological treatments and the availability of a range of different types of inhaler devices,1,2,5 asthma and COPD often remain suboptimally controlled in terms of symptom relief and reduction in exacerbation risk.6,7 Incorrect use of inhaler devices is common – real-world studies estimate that up to 94% of patients do not use their inhalers properly.6,8,9 This can lead to inadequate drug dosing, which, in turn, contributes to a worsening of quality of life and suboptimal disease control.8–11
With over 200 different drug–inhaler combinations available,2 matching patient characteristics and preferences to the most appropriate drug treatment and inhaler device remains challenging. Clinical respiratory practice in the majority of patients is to first select the class of medication, followed by the specific agent, and then the inhaler device itself. However, the importance of inhaler selection as part of the treatment decision is becoming increasingly recognized,2,9,12–14 and this article proposes that careful consideration of inhaler selection warrants greater priority among prescribers.
This review examines the key considerations for selecting devices most suited to patients’ needs; it also discusses the importance of correct inhaler technique for optimizing treatment outcomes and evaluates patients’ perspectives and preferences when switched from a dry powder inhaler (DPI) to the Respimat® Soft Mist™ Inhaler (SMI; Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany).
The case for monitoring inhaler suitability
Treatment guidelines emphasize that good symptom control with inhaled maintenance drugs is fundamental to the management of asthma and COPD.3,4 Nevertheless, guidelines have lacked clarity on specific guidance regarding appropriate inhaler selection for achieving optimum symptom control.2,14 It is encouraging, however, to see that the latest Global Initiative for Asthma and Global Initiative for Chronic Obstructive Lung Disease strategy documents both state the importance of inhaler device selection in day-to-day practice.3,4
It is well established that poor inhalation technique can severely impact the clinical efficacy of medications, resulting in impaired disease control, worsening quality of life, increased exacerbation and mortality risk, increased hospitalizations (Figure 1), and, in turn, increased health care expenditure.8–11 Although incorrect inhaler use may be due, in part, to a patient’s impaired dexterity (particularly with elderly COPD patients), evidence suggests that many patients receive inadequate or no training in the use of their device.2,8,9,15,16 In a systematic literature review of patients evaluated about their use of DPIs for either asthma or COPD, one-quarter of the patients had received no verbal instruction on inhaler technique.8 For those patients who had received instruction, the quality and duration of the instruction and review of technique were considered inadequate.8 Perhaps more concerning are the findings from several studies that many medical and nursing personnel do not have a good understanding of correct inhaler technique, despite their awareness of the importance of correct technique on treatment outcomes.9,15,17–19 Educational efforts therefore need to be focused on primary prescribers of inhaler devices so as to limit or avoid the consequences of poor inhaler technique by their patients. Written instruction is known to be ineffective as a training method; verbal instruction, technique assessment, and reassessment are all essential for achieving correct inhaler use.8 Reflecting this need for improved education, a call to action for improving education of inhaler technique at a policy level was published by the International Primary Care Respiratory Group13 and the Aerosol Drug Management Improvement Team.15
  | Figure 1 Association between asthma and COPD disease control and at least one critical inhaler error. |
A meta-analysis of 59 randomized trials assessing respiratory treatments delivered by a metered dose inhaler (MDI), nebulizer, or DPI has suggested that when inhalers are used correctly, there is little difference in clinical efficacy between devices.20 Although inhaler selection remains an essential component of tailored treatment, the results from this meta-analysis highlight that considerable improvements in disease management are possible by investing in training of correct inhaler use.
Ongoing monitoring of inhaler technique should be an integral component of routine management of COPD and asthma patients. An algorithm proposed by the Aerosol Drug Management Improvement Team gives prescribers a practical tool for assessing whether a change in device type is needed or a step-up in therapy is required (Figure 2).2,15 Although initially developed for asthma patients, this algorithm could also be adapted for the management of patients with COPD.2
  | Figure 2 ADMIT asthma therapy adjustment flow chart. |
Overview of handheld inhaler devices
Handheld inhalers are available as either pressurized MDIs (pMDIs), DPIs, or SMI (Respimat®, Boehringer Ingelheim, Germany; currently, the only SMI marketed).5 Nebulizers are beyond the scope of this review; however, the articles by Ibrahim et al21 and Lavorini et al5 provide detailed discussions of nebulizer technology. A description of the currently available handheld devices is provided, and a summary of the advantages and disadvantages of each type is given in Table 1.
  | Table 1 Major components, advantages, and disadvantages of inhaler devices |
pMDIs
Introduced in the 1950s,22 pMDIs were the first of the handheld delivery devices. Technological developments in pMDIs have occurred over the past 60 years, but common components of all MDIs are a pressurized canister of drug in solution or suspension, a chamber for producing an aerosol, and a mouthpiece through which the aerosol is inhaled.
Older pMDIs created a high-velocity spray of relatively large droplets.23,24 The change in propellants from chlorofluorocarbon to hydrofluoroalkane has led to a reduced-velocity spray that delivers a smaller particle size. These properties lead to reduced oropharyngeal deposition and improved distribution of drug particles throughout the airways with newer hydrofluoroalkane pMDIs compared with chlorofluorocarbon MDIs.23–27
In terms of operational design, two generations of pMDI device now exist in practice. First-generation pMDIs are simple, push-activated, pressure-powered aerosols that require precise coordination and inhalation by the patient. A valve-holding chamber or spacer with a one-way valve allows the aerosol to be inhaled with several intakes and can be added to reduce dependency on coordination. Although spacers are essential for children and those patients with very severe cognitive and physical impairment, they are inconvenient to carry and need to be cleaned between uses.16,28
Second-generation pMDIs require breath actuation rather than push activation to reduce dependency on the patient’s coordination of inhalation and actuation. Currently in development is a new generation of “intelligent” pMDIs that have electronic computation of delivery to compensate for inadequate inhalation and provide more reliable feedback on delivery.29
DPIs
DPIs rely on air drawn through the device to pick up powder from a container and carry it into the lungs within the same airstream.24 Some devices require insertion of drug capsules designed to release all the powdered content into the airstream. Other devices contain multiple doses taken from a reservoir or multiple prefilled blisters/cartridges of drug powder.24 These do not require the user to carry packets of separate drug capsules, but function is otherwise similar to single-capsule devices.
Inhalation flow rate through the device is critical to the successful operation of all DPIs, and the inspiratory effort required to deliver the drug to the lungs depends on the device. Not all patients are capable of creating sufficiently controlled inspiratory effort every time, especially during an exacerbation.30 New DPI devices are beginning to address this important issue, including the dose protector of the NEXThaler® (Chiesi Limited, Manchester, UK), which prevents release of the dose until sufficient flow rate is achieved,31 and the electronic power-assisted DPIs (active DPIs) currently in development.5
Slow-moving SMIs
The SMI was introduced in 2007 by Boehringer Ingelheim as Respimat® SMI. It comprises design elements from the pMDI, but was designed to solve some disadvantages associated with pMDIs and DPIs, such as deposition of drug in the oropharynx.32 Respimat® uses spring power (rather than a pressurized container) to generate a low-velocity vapor cloud into the mouth from a liquid formulation of the drug, which is pushed through a specially designed nozzle. The mist generation is sustained for approximately 1.5 seconds but still requires a degree of coordination of inhalation and actuation (although less so than for pMDIs).32 Compared with pMDI devices, lung deposition with Respimat® is up to 50% higher and oropharyngeal deposition is lower.5 Typical scintigraphic images for Respimat® (Boehringer Ingelheim), Turbuhaler® (AstraZeneca, Lund, Sweden) DPI at slow and fast inhaled flow rates, and pMDI devices32,33 are shown in Figure 3. Respimat® also has the benefit of a dose indicator that provides the user with an estimate of doses remaining in the cartridge.
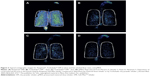  | Figure 3 Typical scintigraphic images for Respimat®, Turbuhaler® DPI at slow and fast inhaled flow rates, and pMDI. |
Inhaler techniques
Each type of device requires a different inhalation technique to achieve optimal drug delivery to the lungs (Table 2). Furthermore, specific instructions vary between devices. This can cause confusion among patients34 and highlights why the prescription of multiple inhaler types to one patient, or switching to different inhaler types, requires careful management and discussion with the patient.
  | Table 2 Correct techniques for using pMDI, DPI (specifically HandiHaler®), and SMI (Respimat®) devices |
Requirements of the “ideal” inhaler
Characteristics of inhaler devices that affect patients’ perceptions of their COPD therapy include perceived efficacy, ease and convenience of use, how they will feel about using the device in public, their physician’s preference, availability of the drug or device preparations, loyalty to the brand of inhaler, cost, time it takes to learn how to use the device, device appearance (size, weight, etc), how to clean the device, and disposability/environmental issues.35 The ideal inhaler would be small and breath-activated; it would also deliver flow-independent drug deposition in the lung and be suitable for use in patients who have low inspiratory airflow.36 It should be lightweight and require no accessories, external assistance, or power source to enable its use. In addition, it should have the capability to count the number of delivered and/or residual doses; not allow degradation of the active ingredient; contain no additives, propellants, or excipients; and be able to quickly aerosolize the drug.37
The following are some of the factors associated with inhaler design and use that can influence how patients interact with their inhalers.
Aerosolizing
Conversion of the drug from a compacted to an inhalable form requires energy, which is either stored within the device (eg, pressurized canister, electronics, or spring) or provided by the airflow from the inhalation process. Drug delivery is affected by the inspiratory flow rate required to generate drug particles of a suitable size for inhalation (eg, 1–5.8 μm) and the fine particle fraction of the aerosol (defined as particles ≤5.8 μm).32,38 These factors vary between inhaler devices (Table 1).
Aerosolizing by pMDIs and the SMI is less dependent on patient inspiratory effort than for DPIs that require a high inspiratory flow rate to ensure correct inhalation and deposition of the drug into the lungs.22 This has advantages for patients with impaired lung function. However, inspiratory flow rates vary between DPIs, depending on the internal resistance of the device, eg, the HandiHaler® (Boehringer Ingelheim International GmbH) is a flow-limiting device, with target flow between 20–30 L/min, compared with reported mean flow rates of 82 mL for the Turbuhaler® DPI (AstraZeneca) and 117 mL for the Diskhaler® DPI (GlaxoSmithKline plc, London, UK; reflecting higher internal resistance).39 To produce fine aerosol particles that are inhalable and maximize drug deposition in the lung,32,38 the target minimum inspiratory flow rates are <50 L/min for a high-resistance DPI, compared with 50–60 mL for a medium-resistance DPI and 90 L/min for a low-resistance DPI.5
Inhaler design also affects the extent to which the aerosol contains fine particles. A high fine particle fraction allows more of the drug dose to be deposited in the lung, rather than in the oropharynx.32 It has been shown that the fine particle fraction of the aerosol generated using the SMI is approximately 75%, which is more than twice the value reported for pMDIs or DPIs.38 However, other new generation inhalers, such as the NEXThaler® DPI (Chiesi Limited), have evolved to deliver extrafine drug particles that can increase drug deposition throughout the bronchial tree.5,40
All methods of aerosolizing require the use of carriers and excipients, whether they are gaseous, liquid, or solid. Although they are pharmacologically inert, they can produce a cold sensation that may cause the patient to stop inhaling.24 SMIs do not require propellants,41 and excipients are minimized. The delivery of the drug as a mist aerosol avoids the cool sensation from evaporating gases that can be experienced with pMDIs.16 This may be beneficial for some patients.
Delivery
After the patient aerosolizes the drug into their mouth, their inhalation flow must carry the aerosolized drug into the lungs – a process highly influenced by the patient. Failure of the drug to be effectively delivered into the lungs can result in a diminished dose as well as the potential for local side effects (eg, sore throat) from oropharyngeal deposition of the drug.42,43 In addition, drug deposited in the oropharynx may be swallowed, leading to gastrointestinal absorption, which may contribute to systemic side effects. Ideally, drug reaching the lungs is targeted to the airways, where bronchodilatory and/or anti-inflammatory effects will provide the greatest benefit, with minimal alveolar deposition, since this is the main site of systemic absorption in the lungs.44
Advancements in inhaler design have greatly improved drug delivery and have allowed lower nominal doses of drug to be used to achieve control in asthma and COPD, thus minimizing the risk of overdosing.33 For example, the SMI Respimat® delivers a slow-moving spray cloud that is of a longer duration and smaller droplet size than the spray from a pMDI, which results in deposition of a higher proportion of the drug in the lungs compared with pMDIs and some DPIs.33
Dependability
Frequent cleaning of devices is often necessary16 since the drug can be deposited on the device and accumulation is inevitable. Nozzles can easily become blocked without regular maintenance. Microbial contamination is also possible within inhalers and spacer devices.45,46 Cleaning regimens must be as simple as possible to encourage compliance with this important component of inhaler use and to ensure operational reliability of the device.
Perceived efficacy of an inhaler is an important factor influencing patients’ device preferences.35 Reassurance from device feedback interfaces such as dose counters has been shown to be important in improving treatment adherence, reminding patients when to replace cartridges, and preventing patients from using their inhalers beyond the recommended number of doses.5,11
Matching inhalers to the patient’s physical ability
The authors of this review and others have previously proposed an algorithm for the selection of a particular inhaler device based on the patient’s physical abilities (Figure 4).12 The algorithm considers three questions: 1) Is the patient capable of conscious inhalation? 2) Is the patient likely to reliably generate and control sufficient inspiratory flow? 3) Is the patient capable of hand–inhalation coordination?
  | Figure 4 Algorithm for choosing inhaler device according to the patient’s inspiratory flow and ability to coordinate inhaler actuation and inspiration. |
Using this algorithm, patients evaluated as having sufficient inspiratory flow with good coordination would be suited to a pMDI (with or without spacer), DPI, or SMI (ie, Respimat®, Boehringer Ingelheim); those with poor coordination may require a breath-actuated pMDI with spacer, DPI, or SMI.12 For patients with insufficient inspiratory flow but adequate coordination (eg, patients with severe COPD or asthma and recurrent exacerbations), a pMDI (with or without spacer) or SMI would be suitable.12 Options for patients with both insufficient inspiratory flow and poor inhaler coordination include a breath-actuated pMDI (with spacer), SMI, or nebulizer.12 According to the algorithm, the attributes of the SMI make it an appropriate choice for all patients who are capable of conscious inhalation, irrespective of their hand–inhalation coordination or ability to generate sufficient inspiratory flow.
Components of patient preference and satisfaction with inhalers
As with all treatment decisions, it is important that the patient be involved in the choice of inhaler device. Patients are more likely to use a device effectively if they are comfortable with it and can use it even when they are incapable of achieving high inspiratory flow rates.30
Many studies have investigated patients’ preferences for different inhaler types.31,35,41,47–50 While acknowledging that many preference studies are sponsored by the device or respiratory drug companies, such studies can provide valuable insights into the features that patients consider to be most important in a device. These include simplicity and convenience (eg, size, durability), and experience of use (eg, taste, side effects).9,35
Simplicity and convenience
Ease of use is an important factor in adherence with any device, but it is particularly important with drug delivery devices for maintenance treatment.5 Use of electronics and other automations can reduce dependency on the patient for accurate technique, but it adds to the cost and complexity in terms of potential faults and possible cleaning issues.
In several surveys of device preference, ease of use has been ranked by patients as one of the most important features;47,48,51,52 however, perception of ease of use varies among patients. Patient perception of the level of convenience that is offered by the device might take into account its size, the instructions for use, its durability, the ease of cleaning the device, how comfortable it is to use (eg, for arthritic hands), and its portability.48
Patients want procedures to be as simple as possible, and one study found that 18% of patients stopped inhaled therapy spontaneously due to perceived complexity.53 Complexity and confusion between required techniques may ensue when mixed types of inhaler devices are prescribed for the delivery of different drug therapies.34
Experience
Overall experience with an inhaler may be influenced by aspects unrelated to its physical form or functionality. Side effects from the drug, excipient, or inhaler use can affect the patient’s experience and willingness to continue using the device. Common issues with inhalers are the presence of an aftertaste, an effect on the throat (such as cold, dry, or sore), and cough.
Poor delivery and/or slow onset of action can reinforce a patient’s decision to voluntarily discontinue their regular medication. Indeed, lack of perceived benefit has been reported to account for 30% of patients with COPD intentionally discontinuing their therapy.11
Apprehension regarding switching device
Patients may be apprehensive when switching to a new inhaler device because they may need to learn new skills and put these into practice with only limited training.16
Experience with SMI versus other inhaler types
A number of studies comparing the patients’ experience of using SMI with other inhaler devices illustrate the influence of some of the factors already described in this section.
Hodder and Price35 have reviewed patient preference studies of Respimat®. The device was shown to be consistently well accepted and preferred by patients with COPD and asthma, with almost three-quarters of patients expressing a preference for Respimat® over a pMDI or Turbuhaler® DPI (AstraZeneca; Figure 5).35 In another trial of device switching in patients with COPD, 21 of 29 patients (72%) considered Respimat® to be easier to use than HandiHaler®; no patient thought HandiHaler® was easier to use than Respimat®.41
  | Figure 5 Proportions of patients indicating preference for Respimat® SMI versus alternative inhaler devices in three studies using the Patient Satisfaction and Preference Questionnaire. |
Features relating to ease of handling (irrespective of patient age) are commonly reported as reasons for patients preferring Respimat® over other devices.35 In the two aforementioned studies in which patients rated their satisfaction with Respimat® compared with a pMDI or Turbuhaler® DPI, Respimat® consistently scored significantly higher on ease of handling during use. Durability of the device and numerous aspects of device performance were also rated better with Respimat® than with pMDI or Turbuhaler®. Size was the only parameter on which Respimat® scored significantly worse than pMDI or Turbuhaler® in these studies; however, ease of carrying the device was not rated significantly worse, and there was no negative impact of size on ease of handling, which, as noted above, was rated higher for Respimat® in both studies.48,54
One survey of patients with COPD suggested that Respimat® had a significantly milder aftertaste than HandiHaler®, but there were no significant differences between the incidence of surveyed adverse events, including dry mouth and throat-related side effects.55 In another survey, Respimat® was associated with increased incidence of cough within the first 4 weeks of switching from HandiHaler®; however, this was transient and most patients overcame having a cough as they got used to using Respimat®.41
Satisfaction with performance of tiotropium Respimat®, including feeling of inhalation and reliable working of the inhaler, was shown in a patient preference study to be consistently better than that of a DPI (Turbuhaler®, AstraZeneca).48
In a survey of patients with COPD that evaluated their handling experiences and preferences 8 weeks after switching from tiotropium HandiHaler® to Respimat®, 46% of patients stated that they preferred Respimat®.55 In a follow-up study, 2–3 years later, the proportion of patients who expressed a preference for Respimat® had significantly increased to 80% (P<0.0001).55 The different responses obtained during the first and second surveys may suggest that the change in inhaler device was stressful for the patients. This highlights the importance of involving patients in their treatment decisions to ensure that they are comfortable with, and understand the need for, any change in treatment.
Identifying the noncompliant patient
Improved compliance and adherence to therapy is a quest that is common to many chronic conditions. It is particularly important among patients with COPD in order to slow disease progression, maintain quality of life, and reduce mortality. Tools such as patient questionnaires and decision algorithms that can be easily adapted and implemented within daily practice may help physicians to select the devices most suited to their patients’ needs and abilities; they may also help to identify patients who have the potential for poor compliance or incorrect inhalation technique.
Involving the patient in their treatment decision-making process can change the physician–patient relationship from one of noncompliance to one of participative adherence to treatment.47 But how can clinicians identify patients who are likely to be noncompliant with their inhaler therapy?
Identification of patients most likely to be noncompliant with therapy may enable more effective targeting of educational and behavioral resources, including continued assessment of inhaler technique, to foster improved compliance and maximize treatment efficacy. Sanduzzi et al11 evaluated several methods that can assess whether a patient will be compliant with their prescribed inhaler; they proposed that simple, brief questionnaires based on the Morisky scale56 were most effective (Table 3).11 These questionnaires address patients’ attitudes toward their medications and their health, the regularity of their medical check-ups, and their home life situation.11 Similar preference questionnaires could be refined for ease of use in consultation rooms to gain a better understanding of individual patient preference and prescribing needs.
  | Table 3 Proposed psychosocioeconomic questions to ask patients with COPD to estimate compliance and to assess adherence phenotype and status |
Conclusion
This review highlights the importance of understanding patient capabilities and preferences, and matching these to the most appropriate type of inhaler device; it also stresses the need to adopt ongoing training and monitoring of inhaler technique. With the large array of devices currently available to the prescribing physician, it is increasingly important to understand the factors that influence a patient’s preference and the likelihood of the inhaler being used correctly (eg, ease of use and convenience). Respimat® represents a novel class of inhaler (SMI) that overcomes some of the drawbacks associated with pMDIs and DPIs, such as nontargeted deposition of drug. It might be expected that SMIs in development will perform in a similar maner to Respimat® with regards to what benefits patients.
Patient satisfaction and preference studies have consistently shown Respimat® to be well accepted by patients, with higher levels of satisfaction and preference versus HandiHaler® and other device types. Whether the observed preference for Respimat® ultimately translates into improved adherence to therapy and improved outcomes remains to be evaluated in clinical studies.
Acknowledgment
Scientific writing and editorial support were provided by Godfrey Lisk, PhD, at PAREXEL, which were funded by Boehringer Ingelheim Pharma GmbH.
Disclosure
In the last 3 years, Pieter Nicolaas Richard Dekhuijzen and/or his department received research grants, unrestricted educational grants, and/or fees for lectures and advisory board meetings from Almirall, AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma, Novartis, Takeda, and Teva.
In the last 3 years, Federico Lavorini received fees for lectures and advisory board meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, Teva, and Cipla.
Omar S Usmani is supported by the National Institute for Health Research (NIHR) Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London, UK. He has also received grant funding to his institution and financial assistance to attend advisory boards and present at symposia from Aerocrine, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Edmond Pharma, GlaxoSmithKline, Micro-Dose Therapeutx, Mundipharma, NAPP, Novartis, Pfizer, Philips-Respironics, Pieris-AG, Prosonix, Roche, Sandoz, Takeda, UCB, and Zentiva. The authors report no other conflicts of interest in this work.
References
Laube BL. The expanding role of aerosols in systemic drug delivery, gene therapy and vaccination: an update. Transl Respir Med. 2014;2:3. | ||
Lavorini F. Inhaled drug delivery in the hands of the patient. J Aerosol Med Pulm Drug Deliv. 2014;27(6):414–418. | ||
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease, 2015. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed 22 July 2016. | ||
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Available from: http://ginasthma.org/2016-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed July 22 2016. | ||
Lavorini F, Fontana GA, Usmani OS. New inhaler devices – the good, the bad and the ugly. Respiration. 2014;88(1):3–15. | ||
Ramsey SD. Suboptimal medical therapy in COPD: exploring the causes and consequences. Chest. 2000;117(2 Suppl):33S–37S. | ||
Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. | ||
Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102(4):593–604. | ||
Price D, Bosnic-Anticevich S, Briggs A, et al. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med. 2013;107(1):37–46. | ||
Rau JL. Practical problems with aerosol therapy in COPD. Respir Care. 2006;51(2):158–172. | ||
Sanduzzi A, Balbo P, Candoli P, et al. COPD: adherence to therapy. Multidiscip Respir Med. 2014;9(1):60. | ||
Dekhuijzen PN, Vincken W, Virchow JC, et al. Prescription of inhalers in asthma and COPD: towards a rational, rapid and effective approach. Respir Med. 2013;107(12):1817–1821. | ||
Papi A, Haughney J, Virchow JC, Roche N, Palkonen S, Price D. Inhaler devices for asthma: a call for action in a neglected field. Eur Respir J. 2011;37(5):982–985. | ||
Dekhuijzen PN, Bjermer L, Lavorini F, Ninane V, Molimard M, Haughney J. Guidance on handheld inhalers in asthma and COPD guidelines. Respir Med. 2014;108(5):694–700. | ||
Crompton GK, Barnes PJ, Broeders M, et al. The need to improve inhalation technique in Europe: a report from the Aerosol Drug Management Improvement Team. Respir Med. 2006;100(9):1479–1494. | ||
Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50(10):1360–1374. | ||
Lee-Wong M, Mayo PH. Results of a programme to improve house staff use of metered dose inhalers and spacers. Postgrad Med J. 2003;79(930):221–225. | ||
Press VG, Pincavage AT, Pappalardo AA, et al. The Chicago Breathe Project: a regional approach to improving education on asthma inhalers for resident physicians and minority patients. J Natl Med Assoc. 2010;102(7):548–555. | ||
Self TH, Arnold LB, Czosnowski LM, Swanson JM, Swanson H. Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma. 2007;44(8):593–598. | ||
Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371. | ||
Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl). 2015;8:131–139. | ||
Lavorini F. The challenge of delivering therapeutic aerosols to asthma patients. ISRN Allergy. 2013;2013:102418. | ||
Leach CL, Davidson PJ, Boudreau RJ. Improved airway targeting with the CFC-free HFA-beclomethasone metered-dose inhaler compared with CFC-beclomethasone. Eur Respir J. 1998;12(6):1346–1353. | ||
Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):600–612. | ||
Acerbi D, Brambilla G, Kottakis I. Advances in asthma and COPD management: delivering CFC-free inhaled therapy using Modulite technology. Pulm Pharmacol Ther. 2007;20(3):290–303. | ||
Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172(12):1497–1504. | ||
Usmani OS. Treating the small airways. Respiration. 2012;84(6):441–453. | ||
Ari A, Fink JB. Guidelines for aerosol devices in infants, children and adults: which to choose, why and how to achieve effective aerosol therapy. Expert Rev Respir Med. 2011;5(4):561–572. | ||
Julius SM, Sherman JM, Hendeles L. Accuracy of three electronic monitors for metered-dose inhalers. Chest. 2002;121(3):871–876. | ||
Vincken W, Dekhuijzen PR, Barnes P. The ADMIT series – issues in inhalation therapy. 4) How to choose inhaler devices for the treatment of COPD. Prim Care Respir J. 2010;19(1):10–20. | ||
Voshaar T, Spinola M, Linnane P, et al. Comparing usability of NEXThaler® with other inhaled corticosteroid/long-acting beta2-agonist fixed combination dry powder inhalers in asthma patients. J Aerosol Med Pulm Drug Deliv. 2014;27(5):363–370. | ||
Anderson P. Use of Respimat Soft Mist inhaler in COPD patients. Int J Chron Obstruct Pulmon Dis. 2006;1(3):251–259. | ||
Pitcairn G, Reader S, Pavia D, Newman S. Deposition of corticosteroid aerosol in the human lung by Respimat Soft Mist inhaler compared to deposition by metered dose inhaler or by Turbuhaler dry powder inhaler. J Aerosol Med. 2005;18(3):264–272. | ||
Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184–191. | ||
Hodder R, Price D. Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat Soft Mist inhaler. Int J Chron Obstruct Pulmon Dis. 2009;4:381–390. | ||
Virchow JC. What plays a role in the choice of inhaler device for asthma therapy? Curr Med Res Opin. 2005;21 Suppl 4:S19–S25. | ||
Melani AS. Inhalatory therapy training: a priority challenge for the physician. Acta Biomed. 2007;78(3):233–245. | ||
Dalby RN, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl). 2011;4:145–155. | ||
Chodosh S, Flanders JS, Kesten S, Serby CW, Hochrainer D, Witek TJ Jr. Effective delivery of particles with the HandiHaler dry powder inhalation system over a range of chronic obstructive pulmonary disease severity. J Aerosol Med. 2001;14(3):309–315. | ||
Scichilone N, Spatafora M, Battaglia S, Arrigo R, Benfante A, Bellia V. Lung penetration and patient adherence considerations in the management of asthma: role of extra-fine formulations. J Asthma Allergy. 2013;6:11–21. | ||
Asakura Y, Nishimura N, Maezawa K, Terajima T, Kizu J, Chohnabayashi N. Effect of switching tiotropium HandiHaler® to Respimat® Soft Mist™ Inhaler in patients with COPD: the difference of adverse events and usability between inhaler devices. J Aerosol Med Pulm Drug Deliv. 2013;26(1):41–45. | ||
Nave R, Mueller H. From inhaler to lung: clinical implications of the formulations of ciclesonide and other inhaled corticosteroids. Int J Gen Med. 2013;6:99–107. | ||
Jackson C, Lipworth B. Optimizing inhaled drug delivery in patients with asthma. Br J Gen Pract. 1995;45(401):683–687. | ||
Weers JG, Bell J, Chan HK, et al. Pulmonary formulations: what remains to be done? J Aerosol Med Pulm Drug Deliv. 2010;23(Suppl 2):S5–S23. | ||
Borovina LR, Tremellen KE, Walker MP, et al. The microbial contamination of pressurised metered-dose inhalers anonymously sourced from the South-East Queensland Australia community population. Int J Pharm Pract. 2012;20(2):129–133. | ||
de Vries TW, Rottier BL, Visserman H, Wilffert B, Weel J. The influence of inhaled corticosteroids and spacer devices on the growth of respiratory pathogenic microorganisms. Am J Infect Control. 2009;37(3):237–240. | ||
Lenney J, Innes JA, Crompton GK. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. EDICI. Respir Med. 2000;94(5):496–500. | ||
Hodder R, Reese PR, Slaton T. Asthma patients prefer Respimat Soft Mist Inhaler to Turbuhaler. Int J Chron Obstruct Pulmon Dis. 2009;4:225–232. | ||
Freytag F, Rau-Berger H, Glaab T, Wolf K. Respimat® Soft Mist™ inhaler preferred to Diskus by patients with COPD and/or asthma [abstract]. Am J Respir Crit Care Med. 2007;175:639. | ||
Chorao P, Pereira AM, Fonseca JA. Inhaler devices in asthma and COPD – an assessment of inhaler technique and patient preferences. Respir Med. 2014;108(7):968–975. | ||
Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv. 2015;28(3):219–228. | ||
Moore AC, Stone S. Meeting the needs of patients with COPD: patients’ preference for the Diskus inhaler compared with the Handihaler. Int J Clin Pract. 2004;58(5):444–450. | ||
Santus P, Picciolo S, Proietto A, et al. Doctor-patient relationship: a resource to improve respiratory diseases management. Eur J Intern Med. 2012;23(5):442–446. | ||
Schurmann W, Schmidtmann S, Moroni P, Massey D, Qidan M. Respimat Soft Mist inhaler versus hydrofluoroalkane metered dose inhaler: patient preference and satisfaction. Treat Respir Med. 2005;4(1):53–61. | ||
Hanada S, Wada S, Ohno T, Sawaguchi H, Muraki M, Tohda Y. Questionnaire on switching from the tiotropium HandiHaler to the Respimat inhaler in patients with chronic obstructive pulmonary disease: changes in handling and preferences immediately and several years after the switch. Int J Chron Obstruct Pulmon Dis. 2015;10:69–77. | ||
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. | ||
Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–938. | ||
Boehringer Ingelheim Ltd. SPIRIVA® HandiHaler® Summary of Product Characteristics. Boehringer Ingelheim Ltd. Available from: http://www.medicines.org.uk/emc/medicine/10039/SPC/. Accessed 2012. | ||
Boehringer Ingelheim Pharma GmbH & Co. KG. Summary of Product Characteristics – SPIRIVA® Respimat® 2.5 Microgram, Inhalation Solution. Ingelheim, Germany: Boehringer Ingelheim Pharma GmbH & Co. KG; 2013. Available from: http://products.boehringer-ingelheim.com/products/prescription_medicines/respiratory/copd/spiolto/public/assets/spiriva_respimat_spc_published.pdf. Accessed 2014. | ||
Newman S. Improving inhaler technique, adherence to therapy and the precision of dosing: major challenges for pulmonary drug delivery. Expert Opin Drug Deliv. 2014;11(3):365–378. | ||
Newman SP. Inhaler treatment options in COPD. Eur Respir Rev. 2005;14:102–108. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
