Back to Journals » Patient Preference and Adherence » Volume 14
Patients’ Perceptions and Preferences Regarding Two Different Forms of Methotrexate Autoinjectors for Moderate to Severe Rheumatoid Arthritis: A European Crossover Survey
Authors Zeitoun JD, Morvan Y
Received 30 June 2020
Accepted for publication 29 September 2020
Published 3 November 2020 Volume 2020:14 Pages 2177—2185
DOI https://doi.org/10.2147/PPA.S269575
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Jean-David Zeitoun,1 Yves Morvan2
1Département de Gastroentérologie et Nutrition, Hôpital Saint-Antoine, Assistance Publique Hôpitaux de Paris, Paris 75012, France; 2IPSOS Healthcare Department, Paris 75013, France
Correspondence: Yves Morvan
IPSOS Healthcare Department, 35 rue du Val de Marne, Paris 75013, France
Tel +33 1 41 98 93 44
Fax +33 1 41 98 99 99
Email [email protected]
Purpose: Treatment adherence is crucial in patients with rheumatoid arthritis (RA). The device used by the patients for self-injections may influence adherence to methotrexate (MTX) treatment. A MTX-autoinjector has been recently marketed in Europe. This crossover survey compared this MTX-autoinjector (MTX-autoinjector A) and an already existing MTX-autoinjector (MTX-autoinjector B) from the patients’ perspective.
Patients and Methods: A total of 100 patients with moderate to severe RA using MTX-autoinjector A (N=35) or MTX-autoinjector B (N=65) were interviewed by an independent Global Market Research Company. Face-to-face interviews were performed using a computer-assisted personal interview system. Evaluation of the unfamiliar MTX-autoinjector was performed once the patients had received information, seen a demonstration, and performed a virtual testing.
Results: A substantial advantage in favor of the MTX-autoinjector A was found with respect to all surveyed indicators. Respectively, 95% and 55% of the users of MTX-autoinjectors A and B claimed to be very or totally satisfied with their familiar MTX-autoinjector. With respect to several specific characteristics, 91% and 60% of the users of MTX-autoinjectors A and B were very or totally satisfied with their familiar MTX-autoinjector, 29% and 77% found the unfamiliar MTX-autoinjector better, and 26% and 73% were interested in trying the unfamiliar MTX-autoinjector. Injection mode (with no push button) and end-of-injection recognition system (with audible signal) were identified as key features explaining a stronger preference for MTX-autoinjector A.
Conclusion: Even though deserving further studying, these findings are expected to guide clinicians when prescribing or renewing prescription of MTX-autoinjector, in particular in poor or non-compliant patients. In a context of growing interest in shared decision-making, the objective would be to choose with each patient the best suited MTX-autoinjector, and ultimately, to obtain a better treatment adherence.
Keywords: decision making, shared, methotrexate, patient satisfaction, rheumatoid arthritis, self-injection device
Plain Language Summary
Self-administration of methotrexate (MTX) using autoinjectors is fairly common in the treatment of rheumatoid arthritis (RA). An MTX-autoinjector has been recently marketed in Europe. To the best of our knowledge, the present crossover survey was the first to compare this MTX-autoinjector with an already existing MTX-autoinjector, from the patients’ perspective. Each patient (N=100) evaluated both his/her familiar MTX-autoinjector and the unfamiliar MTX-autoinjector. Evaluation of the unfamiliar MTX-autoinjector was performed once the patient had received information, seen a demonstration, and performed a virtual testing. Although deserving further studying, this survey showed a substantial advantage in favor of the recently marketed MTX-autoinjector with respect to all surveyed indicators; it identified several characteristics, such as the button-free injection mode and the end-of-injection signal, as key features explaining a stronger preference for the recently marketed MTX-autoinjector. In a context of shared decision-making, these findings may improve the knowledge of clinicians on patients’ preferences, by helping them to open the dialogue with their patients when prescribing MTX self-administration or renewing prescription, especially in poor or non-compliant patients, and ultimately, may improve treatment adherence.
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory joint disease. It affects 0.5% to 1% of adult population in developed countries, with a two to three times higher frequency in women as compared to men, regardless of age.1 To date, no cure exists yet many treatments available worldwide reduce progression of joint damage in up to 90% of patients.2
Despite regulatory authorizations of an increasing number of novel disease-modifying antirheumatic drugs (DMARDs), methotrexate (MTX) which is classified as a conventional synthetic DMARD remains the first-line treatment of choice.3 It has demonstrated tangible clinical benefits, leading to remission or low disease activity in 25% to 50% of treated patients, in particular in early RA.4
Whereas good adherence to treatment is crucial to limit disease progression, and non-adherence to MTX was shown to be a predictor of disease activity,5 gaps in MTX adherence persist in clinical practice, precluding optimal management. Non-adherence exposes patients to poorer-than-expected outcomes.6
In RA patients, self-injections favor a greater autonomy, improve quality of life, and directly decrease healthcare costs.7 It was shown that RA patients who self-injected a given DMARD could achieve higher response rates than others.8,9 To date, several devices for MTX self-injections are available on most healthcare markets. An MTX-autoinjector was launched in 2017 as an alternative product to already marketed forms in adult RA patients.
As defined in the MeSH (2018), medication adherence and compliance (both encompassed under the term “adherence” in the present article) are defined as the extent to which patients take prescribed medications as recommended by their physician. Adherence implies active responsibility shared by both patients and healthcare providers, and is influenced by several factors. Solving issues related to these factors may improve adherence.
As autoinjectors may influence patients’ compliance and persistence, the present survey aimed to compare the recently marketed MTX-autoinjector with an already existing MTX-autoinjector. The comparison was based on patients’ perceptions and preferences. To the best of our knowledge, no comparison of this MTX-autoinjector with any other MTX-autoinjector has been performed to date from a patient’s perspective, possibly because there were only a few devices considered MTX-autoinjectors available at the time of the survey. Moreover, in a context of growing interest in shared decision-making,3,10 a better knowledge by the clinicians of the perceptions and preferences of the patients regarding their MTX-autoinjector would lead to open the dialogue with the patient and conjointly choose the best suited MTX-autoinjector for each patient.
Materials and Methods
Survey Design and Ethical Statement
This was a crossover survey on patients’ perceptions and preferences regarding two forms of MTX-autoinjectors: the recently marketed autoinjector (MTX-autoinjector A) and an already existing MTX-autoinjector (MTX-autoinjector B).
The survey was conducted in four European countries: France, Ireland, Spain, and the United Kingdom (UK). A total of 25 participants were recruited in each country.
For the purpose of the survey, it was decided to align patients’ treatment with market shares: ie, the ratio of participants using the MTX-autoinjector A versus the MTX-autoinjector B was similar to that calculated using sales data in each country.
The survey was conducted in accordance with clinical research guidelines, including the European privacy legislation, known as General Data Protection Regulation [https://eugdpr.org]. No research ethics approval was required in France, Ireland, Spain, and the UK, when performing a survey. All participants provided written informed consent and received a compensation (ie, 40 to 60 € depending on the country).
Participants
Participants were recruited through different means, according to each country’s specificities and opportunities: patient support groups, leaflets in rheumatologic clinics and general practitioners’ offices, pharmacies, social media.
Participants were patients with moderate to severe RA (as determined at the discretion of the physician) who had been treated with MTX for at least one month at the time of the recruitment. The only exclusion criterion was a refusal from the RA patient to participate.
MTX-Autoinjectors
MTX-autoinjector A was a disposable, fixed, single-dose autoinjector with 25 mg/mL of MTX. The product was first marketed in March 2017 in France (NORDIMET®, Nordic Group B.V., Baarn, The Netherlands). MTX-autoinjector A is activated by pressing down the device perpendicularly against the injection site (button-free activation system). The device features a system of audible click and gentle vibration at the start and at the end of the injection (audible signal). Patients can also monitor injection progress through a viewing window. MTX-autoinjector B was a disposable, fixed, single-dose autoinjector with 50 mg/mL of MTX. The product was released on the European market earlier (Metoject®, Medac GmbH, Wedel, Germany). MTX-autoinjector B is activated by pressing a button on the top of the device (push button). Patients can monitor injection through a large viewing window. Both MTX-autoinjectors had similar costs.
All the patients routinely used one of the MTX-autoinjectors (ie, MTX-autoinjectors A or B) prior to the inclusion in the survey. It was thus assumed that participants knew how to administrate their own medication (ie, the familiar MTX-autoinjector) and only needed clarification and demonstration of how to use the other MTX-autoinjector (ie, unfamiliar MTX-autoinjector). Therefore, because this was a crossover survey, after having used their familiar MTX-autoinjectors, participants received information and a demonstration of how to use the unfamiliar MTX-autoinjector, either MTX-autoinjector A or B, and virtually tested the unfamiliar MTX-autoinjector (simulated tests were performed on skin-like blocks).
Data Collection
The interviews were performed by an independent Global Market Research Company (IPSOS Healthcare).
Following acceptance of survey participation, each participant was scheduled for a face-to-face interview using a computer-assisted personal interview (CAPI) system.
The questionnaire used during the interviews was elaborated by the members of an advisory board (cf. Acknowledgements), based on their clinical experience and previously published literature. It included questions related to participants’ demographic and general characteristics, and medical history in particular with respect to the RA, as well as questions about their perception and preferences of the MTX-autoinjector they were familiar with and the alternative one (ie, satisfaction questionnaire).
When completing the questionnaire, participants rated and ranked both MTX-autoinjectors according to a certain number of characteristics (eg, ease of use or gripping). Scores ranged from 1 (Not satisfied at all/Not at all different/Much worse/Not at all relevant) to 5 (Totally satisfied/Completely different/Much better/Totally relevant) for items evaluating satisfaction against each MTX-autoinjector, and from 1 (Worse than the familiar MTX-autoinjector) to 3 (Better than the familiar MTX-autoinjector) for items comparing the two MTX-autoinjectors.
Interviews were designed to last 30 minutes on average even if there was no time constraint.
Outcomes
The main outcome was the global satisfaction of the participants regarding their familiar MTX-autoinjector. It was assessed by the first question of the questionnaire.
Secondary outcomes were the satisfaction of participants with respect to a certain number of characteristics of their familiar MTX-autoinjector (in-depth direct evaluation) and of their unfamiliar MTX-autoinjector (in-depth crossover evaluation). Satisfaction was provided question per question and overall.
Finally, direct comparison between the two MTX-autoinjectors with respect to several characteristics and interest for using the unfamiliar MTX-autoinjector in the future were assessed.
Data Analysis
Data of all questionnaires from the four countries were gathered into a single data set. Descriptive statistical analysis was performed using COSI software (General Electric, Boston, United States of America).
As each group included at least 30 patients (n=35 or n=65), given α=0.05, and assuming that the probability of success of the question (p) follows a binomial law, the 95% confidence interval for p can be calculated using the reduced normal centered law and as follows:
I = [f ± 1.96 × √(f x (1-f)): (√n)]
Univariate analysis (Student’s t-test) was used to compare answers from MTX-autoinjector A and B users (quantitative values, only). The significance threshold was set at 0.05.
Results
From November 20th 2018 through January 21st 2019, 100 RA patients were interviewed. Data regarding demographic characteristics and medical history of the participants are presented in Table 1. Participants were mainly women (82%). Mean age of participants was 52 years. Average disease duration at the time of the survey was 8.2 years. RA was considered severe for 28% of the participants.
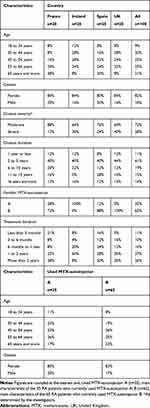 |
Table 1 Demographic and Main Characteristics of Participants (n=100) |
MTX-autoinjector A was used by 35% of the participants (from 0% in the UK to 100% in Ireland) and MTX-autoinjector B by 65% of the participants (from 0% in Ireland to 100% in the UK). Overall, 11% of the participants used their MTX-autoinjector for less than 3 months (from 0% in the UK to 21% in France), and 63% for more than 1 year (from 48% in Spain to 72% in the UK). Patients using MTX-autoinjectors A and B had similar age and gender characteristics.
Global Specific Satisfaction
Ninety-five percent of MTX-autoinjector A users and 55% of MTX-autoinjector B users claimed to be very or totally satisfied with their familiar MTX-autoinjector. The mean rating for MTX-autoinjector A was statistically significantly higher than that for MTX-autoinjector B: 4.6/5 versus 3.7/5 (p=0,05).
Evaluation of the Familiar MTX-Autoinjector
Regarding the features of their familiar MTX-autoinjector during the in-depth direct evaluation (Table 2), 71% of MTX-autoinjector A users and 22% of the MTX-autoinjector B users were totally satisfied by their familiar MTX-autoinjector (specific satisfaction). The end-of-recognition system (with audible signal), the convenient size/format, and the ease of use made the difference in favor of the MTX-autoinjector A. The weakness of MTX-autoinjector B was its injection mode (push button).
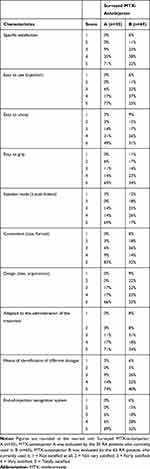 |
Table 2 Detailed Evaluation Regarding Main Features of Each MTX-Autoinjector by the Users of the Autoinjector |
Evaluation of the Unfamiliar MTX-Autoinjector
When MTX-autoinjector A users (n=35) were asked about MTX-autoinjector B, 11% and 23% judged it different or completely different, respectively (mean score: 3.2/5), and when MTX-autoinjector B users (n=65) were surveyed about MTX-autoinjector A, 31% and 28% of them judged the latter different or completely different, respectively (mean score: 3.7/5)
Regarding the features of their unfamiliar MTX-autoinjector after cross-testing, 29% of MTX-autoinjector A users found the MTX-autoinjector B better (relative satisfaction) versus 77% of MTX-autoinjector B users who found the MTX-autoinjector A better (Table 3). Among surveyed characteristics, the injection mode (with no push button for MTX-autoinjector A) and the ease of use made the difference in favor of MTX-autoinjector A. The weaknesses of MTX-autoinjector B were its design (ergonomics) and the end-of-injection recognition system (no audible signal).
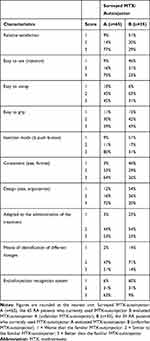 |
Table 3 Detailed Evaluation Regarding Main Features of Both MTX-Autoinjectors After Cross-Testing |
Direct Comparison of Both Forms of MTX-Autoinjectors
When asked about direct comparison of the two MTX-autoinjectors, participants systematically responded in favor of MTX-autoinjector A (Table 4). The end-of-injection recognition system and the injection mode with no push button made the difference in favor of MTX-autoinjector A: respectively, 86% and 73% of patients who used MTX-autoinjectors A and B judged the feedback at the end of injection better for MTX-autoinjector A and 77% and 87%, its injection mode more convenient.
 |
Table 4 Direct Comparison of Both MTX-Autoinjectors by All Participants After Cross-Testing |
Finally, among users of MTX-autoinjector A, 26% (9/35) reported being interested in trying the alternative MTX-autoinjector, comparatively, 73% (47/65) of the users of the MTX-autoinjector B replied positively to the same question.
Discussion
The present European survey of RA patients under MTX found significant differences in terms of satisfaction, and after crossover testing, between the two MTX-autoinjectors: MTX-autoinjector A (NORDiMET®) and MTX-autoinjector B (Metoject®). Those differences were systematically in favor of the MTX-autoinjector A. The survey also identified several key characteristics underlying patients’ preferences.
Whether considering basic rates following cross-testing, satisfaction evaluation, or final comparison between both MTX-autoinjectors, MTX-autoinjector A systematically performed better than MTX-autoinjector B. Following virtual cross-testing, 43% of the users of MTX-autoinjector A replied that MTX-autoinjector B seemed to be better or much better whereas 61% of the users of MTX-autoinjector B judged MTX-autoinjector A better or much better. Then, the level of dissatisfaction at the start of the evaluation was more than 7 times higher among users of the MTX-autoinjector B than among patients treated with MTX-autoinjector A. Finally, and perhaps more importantly, direct comparative rating at the end of the survey showed that patients from both groups gave large preference to MTX-autoinjector A regarding all tested characteristics: the number of users of MTX-autoinjector B willing to try MTX-autoinjector A was almost 3 times higher than that of users of MTX-autoinjector A willing to try MTX-autoinjector B. These results associated with those from a recent randomized, open-label, parallel group study by Saraux et al clearly confirm the interest for MTX-autoinjector A.11
The present survey also identified several key characteristics underlying preferences of a majority of patients that help to better understand optimal design of MTX-autoinjectors in patients with RA and raises several issues worth discussing about MTX-autoinjectors in RA. The button-free injection mode (no push button), the end-of-injection recognition system (audible click), the ease-of-use feature, and to a lesser extent the convenient size, format, and design of the device were the key characteristics underlying the preferences of a majority of patients for MTX-autoinjector A. These results confirmed the choices made when developing MTX-autoinjector A. MTX-autoinjector A was designed as a prefilled pen favoring ease of use for patients whose manual dexterity is potentially disabled by their inflammatory condition. It features an ergonomic design and a button-free activation system. These results also confirmed those of other studies performed with biosimilar-autoinjectors that showed the preference of RA patients for injection mode with no push button and/or system with feedback after completion of injection.12–14
Autoinjectors are designed to improve adherence, but self-injection is associated with a number of challenges including needle phobia, deformity and pain in the hands and fingers that can limit grip strength and severely impact the capacity of the patient to hold the device confidently and/or activate the injection correctly.15 In clinical practice, although most patients prefer an autoinjector with no push button and appreciate to be informed of the end-of-injection (as supported by the present survey and other studies), each patient would present with their own challenges. In a context of growing interest in shared clinical decision-making, discussing with the patients, taking into account their opinion, belief, routine, and the challenge they have to face, would contribute to choosing the best suited MTX-autoinjector for each patient. These findings would therefore alert clinicians on the need to provide a portfolio of MTX-autoinjectors to patients, and to check patients’ satisfaction a few months after prescription, in particular when adherence to treatment seems to be poor. Identifying the difficulties encountered by each patient would also help clinicians to better serve patients, helping them to increase their capacity to manage their disease, to take greater responsibility for their own health, and therefore to be more adherent to MTX treatment.
This survey has some limitations. Firstly, this was a crossover survey and not a clinical study. A clinical study would have provided a higher level of evidence. However, all the indicators gave preference to MTX-autoinjector A and the results have been obtained after simulated tests performed on skin-like blocks. Secondly, the intermediate number of patients and their geographic origin preclude definitive extrapolation of our findings to other settings. More extensive evaluation with patients from other countries may allow to generalize the clear advantage that we identified for MTX-autoinjector A. Thirdly, the unbalanced survey sample with respect to usual treatment may be considered as a bias. Moreover, the proportion of French, Irish, Spanish, and British users of MTX-autoinjector A or MTX-autoinjector B differed, ranging from 0% to 100%, and may be considered as a bias. It should be noted that MTX-autoinjector A was not available in the UK nor was MTX-injector B available in Ireland at the time of the survey. Indeed, we chose to align patients’ treatment with market shares (similar ratio of participants using MTX-autoinjector A versus MTX-autoinjector B in the study and according to the sales data in each country). Despite these differences, both groups proved to be similar with respect to age or gender, making comparisons possible. Lastly, only a virtual testing regarding the unfamiliar MTX-autoinjector of patients was performed. Further assessment with patients involved in true switch between both MTX-autoinjectors would be needed for confirmation.
This survey also has several strengths. Firstly, it was a European survey gathering 100 participants thought to be representative of Western European patients treated by MTX. The sample was constructed so as to include the same number of patients in each country, independently of the used MTX-autoinjector. Secondly, patient assessment of MTX-autoinjectors was made in only one precise indication, namely moderate to severe RA, which enhanced the internal validity of their evaluation, in particular since their condition often generates impaired manual dexterity directly impacting autoinjector manipulation. Thirdly, we proceeded through physical interviews for each participant, thereby excluding a substantial number of possible biases inherent to online surveys for instance. Lastly, our methods allowed a broad and diverse range of evaluations, with basic satisfaction assessment, virtual cross-testing through the use of skin-like blocks, and final direct comparison of both products.
Conclusion
This European crossover survey of two forms of MTX-autoinjectors in patients with RA found a substantial advantage in favor of MTX-autoinjector A with respect to all surveyed indicators. Mainly, 95% of its users versus 55% of the users of the other MTX-autoinjector claimed to be very or totally satisfied by their MTX-autoinjector. In addition, this survey identified several characteristics as key features explaining a stronger preference by the patients. Even though deserving further studying, in particular through actual cross-testing of both treatments, our findings indicate a likely superiority of MTX-autoinjector A as perceived by the patients. These findings may improve the knowledge of clinicians, by helping them to open the dialogue with their RA patients (in particular poor or non-compliant patients) when prescribing MTX self-injection or renewing the prescription, their objective being to choose the best suited MTX-autoinjector, and ultimately, to obtain a better treatment adherence.
Abbreviations
DMARD, disease-modifying antirheumatic drugs; MTX, methotrexate; RA, rheumatoid arthritis.
Acknowledgments
The authors want to thank all the patients who participated in the present survey. They also want to thank the members of the advisory board. Finally, they thank Matthieu Chanard and Fabienne Péretz (Abelia Science, Saint-Georges-sur-Baulche, France) for their writing assistance. Advisory board: Anissa Allal (Nordic Group), Yves Morvan (IPSOS Healthcare), Jean-David Zeitoun, MD, PhD.
Disclosure
Survey conducted by IPSOS HealthCare Global Market Research Company and funded by Nordic Group. JDZ reports personal fees from Ipsos, during the conduct of the study; personal fees from Ferring, Pierre Fabre, Boehringer Ingelheim, AbbVie, Janssen, and Astra Zeneca, outside the submitted work; being an advisor for several consulting firms in link with pharmaceutical industry (Cepton, Oliver Wyman, Roland Berger, TBWA, Havas). He also reports speaking fees from manufacturers’ professional association, consulting fees from IQVIA, Ferring, Pierre Fabre, AbbVie, Astra Zeneca, Biogen, Boehringer Ingelheim, and Johnson & Johnson. He is a personal investor in approximately 20 digital companies, medtech companies or biotech companies, and as a limited partner in an investment fund. He is also a shareholder and advisory board member in several medtech companies. He reports being cofounder and shareholder of Inato, a digital company involved in clinical research and whose customers are pharmaceutical companies. YM is an employee of IPSOS HealthCare and reports non-financial support from IPSOS HealthCare Global Market Research Company and grants from Nordic Group, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi:10.1016/S0140-6736(16)30173-8
2. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–1372. doi:10.1001/jama.2018.13103
3. Taylor PC, Balsa Criado A, Mongey AB, Avouac J, Marotte H, Mueller RB. How to get the most from methotrexate (MTX) treatment for your rheumatoid arthritis patient?-MTX in the treat-to-target strategy. J Clin Med. 2019;8(4):515. doi:10.3390/jcm8040515
4. Braun J, Kästner P, Flaxenberg P, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, Phase IV trial. Arthritis Rheum. 2008;58(1):73–81.
5. Nam JL, Villeneuve E, Hensor EM, et al. Remission induction comparing infliximab and high-dose intravenous steroid, followed by treat-to-target: a double-blind, randomised, controlled trial in new-onset, treatment-naive, rheumatoid arthritis (the IDEA study). Ann Rheum Dis. 2014;73(1):75–85. doi:10.1136/annrheumdis-2013-203440
6. Pasma A, Schenk CV, Timman R, et al. Non-adherence to disease-modifying antirheumatic drugs is associated with higher disease activity in early arthritis patients in the first year of the disease. Arthritis Res Ther. 2015;17:281. doi:10.1186/s13075-015-0801-4
7. Hope HF, Bluett J, Barton A, Hyrich KL, Cordingley L, Verstappen SM. Psychological factors predict adherence to methotrexate in rheumatoid arthritis; findings from a systematic review of rates, predictors and associations with patient-reported and clinical outcomes. RMD Open. 2016;2(1):e000171. doi:10.1136/rmdopen-2015-000171
8. van den Bemt BJF, Gettings L, Domańska B, Bruggraber R, Mountian I, Kristensen LE. A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Deliv. 2019;26(1):384–392. doi:10.1080/10717544.2019.1587043
9. Schulze-Koops H, Giacomelli R, Samborski W, et al. Factors influencing the patient evaluation of injection experience with the SmartJect autoinjector in rheumatoid arthritis. Clin Exp Rheumatol. 2015;33(2):201–208.
10. Daien C, Hua C, Gaujoux-Viala C, et al. Update of French society for rheumatology recommendations for managing rheumatoid arthritis. Joint Bone Spine. 2019;86(2):135–150. doi:10.1016/j.jbspin.2018.10.002
11. Saraux A, Hudry C, Zinovieva E, Herman-Demars H; Self-I Investigators group. Use of auto-injector for methotrexate subcutaneous self-injections: high satisfaction level and good compliance in SELF-I study, a randomized, open-label, parallel group study. Rheumatol Ther. 2019;6(1):47–60. doi:10.1007/s40744-018-0134-2
12. Fenwick S, Thakur K, Munro D. Nurse and patient perceptions and preferences for subcutaneous autoinjectors for inflammatory joint or bowel disease: findings from a European survey. Rheumatol Ther. 2019;6(2):195–206. doi:10.1007/s40744-019-0144-8
13. Tischer B, Mehl A. Patients’ and nurses’ preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Prefer Adherence. 2018;12:1413–1424.
14. Thakur K, Biberger A, Handrich A, Rezk MF. Patient perceptions and preferences of two etanercept autoinjectors for rheumatoid arthritis: findings from a patient survey in Europe. Rheumatol Ther. 2016;3(2):245–256. doi:10.1007/s40744-016-0048-9
15. Domańska B, VanLunen B, Peterson L, Mountian I, Schiff M. Comparative usability study for a certolizumab pegol autoinjection device in patients with rheumatoid arthritis [published correction appears in Expert Opin Drug Deliv]. Expert Opin Drug Deliv. 2017;14(1):15–22. doi:10.1080/17425247.2016.1256283
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
