Back to Journals » Patient Preference and Adherence » Volume 15
Patients’ Experiences and Perspectives of Receiving Written Medicine Information About Medicines: A Qualitative Study
Authors Wongtaweepkij K , Corlett S , Krska J , Pongwecharak J, Jarernsiripornkul N
Received 22 December 2020
Accepted for publication 18 February 2021
Published 9 March 2021 Volume 2021:15 Pages 569—580
DOI https://doi.org/10.2147/PPA.S298563
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Kamonphat Wongtaweepkij,1 Sarah Corlett,2 Janet Krska,2 Juraporn Pongwecharak,3 Narumol Jarernsiripornkul1
1Division of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand; 2Medway School of Pharmacy, The Universities of Greenwich and Kent, Kent, UK; 3Pharmacy Practice and Management Research Unit, Division of Pharmaceutical Care, Faculty of Pharmacy, Rangsit Center, Thammasat University, Pathumthani, Thailand
Correspondence: Narumol Jarernsiripornkul
Division of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, 40002, Thailand
Email [email protected]
Sarah Corlett
Medway School of Pharmacy, The Universities of Greenwich and Kent, Chatham, Maritime, Kent, UK
Email [email protected]
Purpose: Written medicine information informs patients about the benefits and risks of medicines and supports their safe and effective use. In Thailand, patient information leaflets (PILs) are not obligatory and therefore not routinely supplied. This study aimed to explore the experiences and information needs of patients, their views on PILs and the likely impact of PILs on their knowledge, perceptions and behaviors towards medicines. These factors are important to establish the value of PILs.
Methods: Semi-structured interviews with outpatients who received simvastatin or atorvastatin were conducted exploring their experiences of receiving medicine information, their views on the utility of and need for PILs, the impact of PILs on their behaviors, and recommendations for how PILs could be improved. All interviews were audio-recorded, transcribed verbatim, and analyzed using a framework approach.
Results: Thirty interviews were conducted from which four themes emerged: experience of receiving medicine information, views of package inserts and PILs, impact of PILs on knowledge, perceptions and behaviors, and patients’ need for medicine information. Most participants received verbal information from healthcare professionals, as well as written information. Verbal information was perceived as being particularly useful to inform about changes to medicine regimens or the long-term adverse effects of medicines. Patients perceived that the PILs had influenced their knowledge about medicines, and also their behaviors including safety awareness, adherence, and engagement with healthcare professionals. Participants suggested that the information in electronic format could provide an additional resource. Some changes to improve the content and general format of the PIL were identified.
Conclusion: PILs are perceived as useful by patients and met their information needs, although they were viewed as an adjunct to verbal advice provided by healthcare professionals. PILs influenced patients’ medicine taking behaviors and encouraged sharing of information with their physicians.
Keywords: patient information leaflets, patients’ experience, needs, perceptions, qualitative research
Introduction
Receiving sufficient information facilitates optimal benefit and safe use of medicines.1 Previous studies have found that patients not only need to receive general information such as the benefits of medicines, how to use them, and the duration of treatment but also safety-related information such as potential adverse drug reactions (ADRs) and drug interactions.2,3 Written medicine information (WMI) can improve patient knowledge, satisfaction, attitudes towards, and use of medicines.4–6 However, informing patients about the risks of medicines increases their perception of the likelihood of experiencing adverse effects7 and the type and severity of these are negatively associated with their willingness to adhere.8 The descriptions of risk commonly included in WMI lead some patients to seek reassurance by consulting their healthcare professionals (HPs), a medical dictionary or the internet.9
In Thailand, patients primarily receive information about medicines from their HPs.10 Written information, in the form of a package insert (PI), is often also enclosed in the medicine container. However, not all patients receive a PI with each medicine supply. Those who receive a small number of tablets or a few strips of medicines at one time will typically only receive the dosage information on the label. The PI contains information about the medicine that is suitable for the prescriber or HP, not the patient. Medical terminologies are used with unattractive layout and designs making PIs challenging to read and understand for lay people.11 Patient information leaflets (PILs) in contrast are targeted at patients and are developed using concise, clear, and user-friendly text to increase patients’ understanding of their medications.12 It is not a legal requirement to supply PILs in Thailand with all medicines. Whilst the number of PILs in Thailand has increased within the last decade, the proportion of medicines supplied with a PIL is still low in number compared to the number of available medicines. A study conducted in 2013, determining the proportion of non-steroidal anti-inflammatory drugs in Thailand that contained WMI found that there is only 4% of products contained PILs, with the remainder containing PIs.13
Previously we have reported that Thai people considered PILs as a useful source of medicine information and that they had positive attitudes towards them.3 These findings are consistent with another survey conducted in outpatients who realized the importance and potential value of receiving PILs in addition to the HPs’ advice.14 However, to date, no qualitative studies have explored the views of Thai patients on PILs. This study sought the views of patients receiving a statin since this group of drugs is widely prescribed in Thailand. Statins, a group of lipid-lowering therapies, are used first-line to reduce cholesterol, morbidity and mortality of cardiovascular events following the Thai Atherosclerosis Society.15,16 Due to a large proportion of patients taking statins, medicine information is required to help patients to take the medicines as intended. Although statins are generally well tolerated by most, severe adverse events do occur in some patients.17
The aims of this study were to (1) determine patients’ experiences of receiving information about simvastatin or atorvastatin, (2) explore their views on statin PILs including their usefulness and the need for them, the format of leaflets, and ease of understanding of information included, (3) to explore the impact of statin PILs on patients’ knowledge of, perceptions towards and behaviors relating to their medicine, and (4) to identify needs for medicine information in general.
Methods
Study Design and Settings
This qualitative research study used semi-structured interviews using a topic guide. The study was conducted at Srinagarind Hospital, Queen Sirikit Heart Centre, and a Primary Care Unit in North-East Thailand between May and August 2019.
Participants
Outpatients aged 18 years or over, who currently used or were first prescribed simvastatin or atorvastatin were eligible for the study. Participants were excluded if they had any problems with communication. Those eligible were given information about the study and gave their verbal consent to take part. The study was conducted in accordance with the Declaration of Helsinki Ethical Principles. The participants were provided informed consent for publication of anonymised responses. The research team provided statin-PILs; simvastatin or atorvastatin-PIL (see in Supplementary Materials) depending on which statin they were taking, and recorded the participants’ telephone number. These were needed to arrange the interviews which were conducted at a later date. The participant’s age, educational level, number of their underlying diseases and medicines and prior experience of receiving PILs were recorded. These factors have been demonstrated to affect knowledge and perception about medicine information in previous studies14,18 (Table 1).
 |
Table 1 Factors That Might Influence Knowledge and Perceptions About Medicine Information |
Study Procedures
Questions in the interview guide were drawn from the published literature on patients’ experiences of receiving information about medicines, views of PILs, impact of PILs, and needs for receiving information about medicines.9,19–21 The interview guide was reviewed by two academic experts and one pharmacist; two Thai and one English.
All participants who were invited to join the study received a copy of the standard PIL for either simvastatin or atorvastatin, developed by Thai Food and Drug Administration (FDA)22 Both PILs contained six sections: indications, precautions before taking the medicines, how to take, actions that patients should do while taking the medicines, storage, and adverse effects. The latter was listed as symptoms where patients should stop taking the medicines and symptoms that meant that they could continue with treatment. Information about adverse effects did not include the frequency of these occurring. The PILs were 1–2 pages of A4 in length and did not include any images. The sections included headings and subheadings, and all details were written using bullet points and short sentences.
The researcher (KW) contacted participants for an interview 1–2 weeks later by phone, at a mutually convenient time and date. The interviews were conducted by KW, in an informal environment, such as patients’ home, café, and public places near patients’ home, and were audio-recorded. Written consent was obtained. All interviews were transcribed verbatim in Thai and translated into English by KW. To ensure validity of the translation, the first five Thai transcripts were independently translated by another translator, and both translations were reviewed by an independent native English-speaker to check for agreement (SC). If there was any disagreement, the research team discussed this difference and the translated interviews were re-worked and rechecked until there was agreement. Learning from the early transcripts was then applied to subsequent translations.
Data Analysis
The transcripts were read and re-read before a framework was developed and used to guide data analysis.23 The framework method is a systematic approach to analyze qualitative data containing six general steps including transcription, familiarization with the interview, coding, developing an analytical framework, applying the framework, and interpreting the data.23 The framework was utilized at all three stages of the study, including the first 5 interviews, the next 10 and then the remaining 15 interviews. The first two were independently analyzed by JK, SC, and KW. The third was analyzed by SC and KW to create the initial framework. The coding frame was discussed and refined as necessary. New codes and themes were created if they did not fit within the framework. The codes and framework from the second stage of interviews (interviews 6–15) were again modified by grouping and regrouping of similar themes until no additional codes or themes emerged. The remaining 15 interviews were analyzed using the agreed framework. Software program Atlas.ti version 7.0 was used to collect, organize and support the qualitative data from all interviews by highlighting quotations and creating codes.
Ethical Approval
The study was approved by the Khon Kaen University Ethics Committee for Human Research (Number HE611500). Written informed consent was obtained from all participants before the start of the interviews.
Results
Demographic Data
Of sixty-eight patients who were eligible and expressed interest in the study, thirty participants were interviewed. The average duration of interviews was 53.48 ± 13.15 minutes. Half of the participants were male, and average age was 53.27 ± 15.90 years. Over half (n=18, 60%) held a bachelor degree or higher. Just over half (n=16, 53%) were taking atorvastatin. The majority of participants (n=24, 80%) were taking at least two medicines for their underlying diseases and two-thirds (n=20, 67%) had not received any PILs for any of their other medicines until they received the one from the researcher (Table 2).
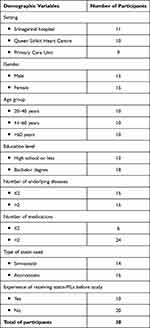 |
Table 2 Summary of Demographic Characteristics of Participants (n=30) |
Four main themes were identified. These themes were classified as experience of receiving medicine information, views of PIs and PILs, impact of PILs on knowledge, behaviors, and emotions and patients’ needs for medicine information.
Theme 1: Experiences of Receiving Medicine Information
Participants described receiving both verbal and written information, although verbal information was most commonly experienced.
Subtheme 1: Verbal Information
Almost every participant mentioned verbal information. Most stated that HPs were their main source of medicine information with two mentioning that their friends and relatives had provided information. About half of participants received information about the indication for their medicine. Physicians were more often described as providing information about adverse effects, what to do if a dose was missed and the importance of continuing with treatment, whereas pharmacists were more frequently mentioned as providing information on how to take the medicine.
The doctor told me this medicine is for reducing blood cholesterol, and also told me that it has side effects that I have to tell the doctor if I have dark urine or muscle pain. [case 8, Male, 52, Bachelor degree, atorvastatin]
The pharmacist quickly explained me that I should take it at bedtime and how many tablets. [case 16, Female, 54, Bachelor degree, simvastatin]
Subtheme 2: Written Information
Half the participants described receiving written information about their medicines (simvastatin/atorvastatin). This included the brief information written on the actual drug envelopes, information from package inserts (PIs) and also from electronic sources. About one-third of the participants had received statin-PILs prior to the study. Participants stated that they received information about the indication and how to take the medicine from the drug envelope, and adverse effects from the PIs.
Some participants had searched for information on-line, when they had some questions about their medicines including adverse effects and what to do if a dose was missed, by using search engines and hospital websites. However, others expressed concerns about accessing material on-line either because they struggled with the technical aspects of doing so or because the material was too detailed, there was too much of it, or they had concerns about the reliability and trustworthiness of the source.
Websites and online-forms should not be so long. Some patients would read only the first line and then stop reading it because it’s too long. [case 3, Male, 64, Bachelor degree, atorvastatin]
I am concerned about the reliability of the information. It is absolutely not like the information I receive from the doctors. [case 8, Male, 52, Bachelor degree, atorvastatin]
However, others found that accessing medicine information via websites, mobile applications, and social media provided an easy means of obtaining the information that they needed.
I believe that everyone has a mobile phone and can also use mobile applications. I can say that. For 60-year-old people or less, most of them know how to use mobiles. [case 28, Male, 33, Bachelor degree, atorvastatin]
Theme 2: Views of PIs and PILs
Subtheme 1: Views of PIs
Although not asked specifically to provide their views on the PIs, a number of participants did so. Issues relating to content, (superfluous information that was not useful or needed by the patient including the mechanism of action of a medicine or a list of its active ingredients was highlighted as examples), format and language used were raised. PIs were criticized for being written in English and medical language making them difficult to understand. Furthermore, the font size was too small causing difficulties in searching for and identifying specific information.
The language in this one (PI) is too complex, like doctor’s language. I used to receive a leaflet like this in the past, and I could not finish reading it because I felt dizzy. [case 1, Male, 78, High school, simvastatin]
Subtheme 2: Views of PILs
As the participants read the PILs of simvastatin or atorvastatin, they had both positive and negative views about them.
Over two-thirds of participants agreed that PILs contained essential content that was appropriate for patients to help them to use medicines safely such as information about drug–drug interactions and precautions for use. Some participants also stated that the PILs provided information about actions to be taken when a dose of medication is missed which they also viewed as helpful. Several participants referred to valuing the PIL as a reference source that they could refer to when any abnormal symptoms happened or when they had free time.
Precaution section would be useful for new patients and for those patients taking the medicine for a while without any concerns. For example, some patients may be taking the medicine with herbal supplements, they would get new knowledge and stop taking the herbal supplements. [case 6, Male, 60, Bachelor degree, atorvastatin]
The PIL allowed me to know what to do if I forgot the medicine. The outcome of treatment may be not good if I take the drug inconsistently … I would read it again when I have abnormal symptoms such as chest tightness and difficulty breathing … [case 26, Male, 21, High school, atorvastatin]
Participants considered that the statin-PILs were written in simple language and over half talked about format of the PILs. PILs were regarded as easy to read due to the font size and good layout. Some also pointed out that related content was grouped together and bullet lists were used to present and summarize complex information. They also said that the headings and subheadings enabled participants to read and find information easily. PILs were identified as helping people to remember information.
The headings are clear. Having subheadings make me focus the details that I want to read. I stopped to read the information about contraindication in pregnancy after I found the heading “contraindication,” and I understand more after I read this topic carefully. [case 27, Female, 37, Bachelor degree, atorvastatin]
Despite the many positive views on PILs, over three-quarters of participants were also able to identify ways that they could be improved. Specific details of adverse effects, why the adverse effects occur, as well as what to do when adverse effects occur, were repeatedly mentioned. A few participants also needed to know more about drug–herb and drug–food interactions which were mentioned in the PILs without description of which herbs or foods were relevant. A few participants suggested that more should be added about the benefits of taking medicine to encourage patients to adhere.
It still lacks of information about what to do after the symptoms happen. I need to know basic support before going to the doctors. [case 25, Female, 24, High school, atorvastatin]
The benefits of the medicine should be explained more to motivate patients. This leaflet provides benefit information less than side effects. For example, there are only two benefits but there are many bad effects mentioned on here. You should add outstanding advantages. [case 21, Female, 40, Bachelor degree, atorvastatin]
A few participants commented that content about non-serious adverse effects was not necessary for patients, and precautions before taking the medicine were too long because they thought the physicians were supposed to check about underlying diseases and concomitant medicines before prescribing the medicine. Some precautions such as drug–food interaction and using the medicine during pregnancy, however, were singled out as being useful.
The precautions and when I should not use the drug are too much. Just give me the information that doctors are not concerned about. For example, the doctor might not know if I drink grapefruit juice. These situations are out of the doctor’s control and he/she might be unaware of that important information. [case 16, Female, 54, Bachelor degree, simvastatin]
Ideas for improving the design of PILs were also suggested. Some participants commented that the headings and important information of serious adverse effects should be discriminated from general information by using different colors. Many participants also agreed that using more colors would enable patients to read and remember. Folding the PILs like brochures and improving the quality of paper would make it easier to store the PILs. Some participants also gave suggestions of how PILs could be more interesting by adding related pictures and adjusting the font size.
The important information should be bolded, underlined, and created into headings with hyphens, bullets or numbering, that make it different from general information. [case 3, Male, 64, Bachelor degree, atorvastatin]
Theme 3: Impacts of PILs on Knowledge, Perceptions, and Behaviors
Subtheme 1: Impacts on Knowledge Regarding Taking Medications
Most participants agreed that the statin-PILs increased their general knowledge about their medicine and specifically their awareness of the medicine’s side-effects. Some participants also stated that they had more awareness after reading the PIL as to what to do if those symptoms occurred than they had had before. A few participants also said that they knew more about what to do if they missed a dose. One participant also mentioned that the PIL had given him information about how to correctly store the medicine.
I saw the information that I should take it immediately when I forgot the dose. In the old days, I didn’t know about that. Now I would say I can take it as soon as I remember unless my next dose is due in less than 12 hours. [case 5, Female, 67, Bachelor degree, simvastatin]
Subtheme 2: Impacts on Perceptions
Worries about side-effects, prompted by reading the list of possible adverse effects within the PIL, were raised as a potential cause of anxiety by one in five participants. Some said that they did not want to take this medicine because they were afraid of having any serious adverse effects listed on the PIL. Whilst others claimed that it was a large number of adverse effects including serious and non-serious adverse effects which triggered their anxieties. However, most participants said that they were not worried about adverse effects and diseases that are mentioned in precaution section of the PILs because they had not experienced them after checking their symptoms against the PIL.
I was frightened that it had a lot of side effects. I would be scared if I experienced symptoms that I saw in the leaflet. [case 15, Male, 60, Bachelor degree, atorvastatin]
Three participants said that reading the indication section, as well as benefits of taking this medicine boosted their confidence that they were receiving the right medicine for treating hypercholesterolemia.
I think it is the indication of this medicine that is for reducing blood cholesterol. I read it and felt comfortable that I got the right medicine for reducing the lipid levels in my brain vessels. [case 4, Female, 78, High school, atorvastatin]
Subtheme 3: Impacts on Behaviors
Nineteen participants claimed that they had taken action to improve the safety of the medicine after reading the PILs. Some stated that they observed and compared their symptoms to the adverse symptoms mentioned on the PILs. One participant also checked the drug name mentioned in the precaution section with her current medicines to ensure that she was not taking any medicines that she should avoid. Another participant said that she stopped taking herbs after reading about cautions within the PIL relating to taking the medicine with herbs.
I read and thought about myself [and] if I had any symptoms like those mentioned on the leaflet. When I found some information related to my conditions, I stopped to think of them. [case 4, Female, 78, High school, atorvastatin]
Some participants thought about monitoring their symptoms and looking to check if new medicines they received were safe to take with their statin. One participant also stated that he would not take the medicine with alcoholic beverages after reading precaution section.
I might have liver disease in the future or I will check the drug names when I am prescribed antifungals. [case 18, Male, 60, High school, atorvastatin]
There was one participant who stated that he had a consultation with a physician after he read about the side effect information and found that he had muscle pain. Consequently, his medicine (simvastatin) had changed to a new medicine. Many participants stated that reading about potential drug interactions or side-effects on the PIL would prompt them to discuss their medicines with the doctor to confirm that the medicines that they had been prescribed could be used concomitantly or were safe for them. Concerns relating to precautions in using the medicine in pregnancy were also given by participants as a prompt to referring themselves to a HP.
I am planning to have a baby and I should consult the doctor because it (PIL) said patients planning to have a baby should stop this medication. [case 27, Female, 37, Bachelor degree, atorvastatin]
There was one participant who reported that she had stopped taking the medicine immediately after she suspected she had a side effect that was listed on the PIL. A few participants also said that they might stop taking this medicine if they had any side effects.
I would stop taking this medicine. I would check the symptom, if I feel better, that symptom would be caused by the medicine. [case 2, Male, 72, Bachelor degree, simvastatin]
Theme 4: Patients’ Needs for Medicine Information
Participants expressed their needs for medicine information both verbal and written.
Subtheme 1: Verbal Information from Healthcare Professionals
Most participants talked about wanting to know more from the HP than the information considered as routine practice, such as how to take the medicine. Some would like HPs to explain the reason why their medicine had been changed and how the benefits of a new medicine outweighed the old one. One participant suggested that there should be access to an expert, perhaps on-line or by telephone, who could answer patients’ queries relating to symptoms that they are experiencing with their medicines or concerns relating to the long-term adverse effects of taking medicine.
It should have experts who answer patients calling to ask about side effects. For example, I have this symptom after taking the medicine and I think it’s abnormal. I would like to ask them whether it is dangerous or not, whether I should continue to take it, or I should go to see the doctor. [case 21, Female, 40, Bachelor degree, atorvastatin]
Subtheme 2: Written Information
Participants mentioned two main types of written information (PILs and electronic information) when discussing their need for information.
PILs were the preferred source of information with the main reasons given being that they could read the PILs themselves, without internet connection, and that the information was current and regularly updated. Almost one-half of the participants would like to receive PILs along with HP’s advice. Some described that receiving PILs from the HPs had influenced their behaviors to read these documents more often so that they were now more likely to read PILs that they picked up themselves either from public place or other health centers. Some participants thought that it would be good to receive PILs from specific health centers and pharmacies so that patients could read them while waiting to see the physicians.
I would like to receive PIL from pharmacists because they can explain to me further information if I have any questions after reading them. [case 26, Male, 21, High school, atorvastatin]
It should be provided in the right setting. For example, if you provided leaflets of anti-diabetic drugs in the Heart Centre, patients might not be interested as much as leaflet of drugs for heart diseases or antithrombotic drugs. [case 18, Male, 60, High school, atorvastatin]
One-third of participants expected PILs to be provided with the medicine container. They reported that they would like to see them replacing the current leaflets (PIs) that they perceived as unhelpful. One participant suggested that PILs should be designed for putting inside the drug envelopes because some patients are not supplied medicines in the original pack. This particularly applies to those who receive less than three months supply.
Some patients don’t receive the whole packages, so they could not know the information about their medicines. The leaflet like this (PIL) should be provided inside the envelope so that all patients could access it. [case 6, Male, 60, Bachelor degree, atorvastatin]
Regarding how frequently the PILs should be distributed, most participants agreed that it would be beneficial to receive a PIL the first time that a new medicine was supplied, and when any changes or new directions were given. Only four participants said they should receive PILs every time a medicine was supplied.
One in five participants thought that a PIL should be provided with every medicine used within Thailand. In contrast, the majority proposed that PILs should be provided with specific categories of medicines that they perceived as either being widely used or higher risk. These included medicines for chronic diseases, antibiotics and medicines that could cause serious adverse effects.
It could be for medicines which are at high risk of causing allergy and antibiotics. For instance, patients who have allergies when they meet pharmacists, they might forget to tell them about their allergy. [case 2, Male, 72, Bachelor degree, simvastatin]
Electronic Information About Medicines
Electronic information was mentioned by about half of the participants. Accessing electronic information was thought to be very convenient for every age group. Regarding the types of electronic information, preferred sources were varied, including the internet, mobile applications, chat programs, Facebook, and receiving information using the QR code. Some participants stated that they would like to receive information in both the paper format and the electronic version. Concerns relating to the barrier of accessing electronic information for elderly patients were commonly mentioned. Four participants suggested that information about medicines was created as digital media that could be accessed on the internet, television and also on the hospital’s communication channels.
QR code would be better to scan and save into the mobile to read at home. I suggest putting a QR code on the drug envelope is a good idea. I believe that everyone has a mobile phone and can also use mobile application. For older patients, they come with their children. They can have their children scan the QR code. For younger people, most of them know how to use mobiles. [case 28, Male, 33, Bachelor degree, atorvastatin]
The younger generation likes to read it online, but old patients, who aren’t able to use any technologies, the online medicine information is not convenient for them. They might feel the paper-form is better. [case 5, Female, 67, Bachelor degree, simvastatin]
Discussion
The findings of our study have shown that patients most often have experience of receiving medicine information verbally. However, WMI was perceived as being useful even though two-thirds had not received a PIL prior to their participation in this study; their experience of WMI therefore related mostly to PIs. When provided with a PI, most participants read it, particularly the safety advice, including particular precautions for use, adverse effects and drug interactions. Furthermore, most patients stated that they liked to have WMI when they received a new medicine. Participants claimed that access to WMI improved their knowledge about medicines, raised their awareness and enabled them to check whether they were experiencing any side effects, as well as encouraging them to discuss their questions about medicines with their HP.
Most participants in our study were not provided with a leaflet every time they received their medicines and where they did, as already discussed, this was a PI. Most interviewees thought that the PIs contained excessive information and were difficult to understand. This finding is in agreement with another study that most consumers thought that PIs contain too much detail which was difficult to remember.24 The reading level of PIs is known to be considerably higher than the recommended reading level for consumers causing difficulties in understanding.25–28 The design of the PIs has also been described as unsuitable for patients due to poor font size, lack of prominence for important warnings, low quality of paper and lack of pictorial aids.25,26 It is clear that written information with simple language and patient-friendly format should be accessible for all medicine users. Older people in Thailand have been reported as having limited literacy,29 although most Thai patients had moderate health literacy levels, education being the only factor associated with health literacy.30
Good information should be designed in large font size with optimal spacing, using bullet points to enhance readability, using bold, lower-case text for emphasis and using conversational tone of voice.31,32 Although all of the participants in this study had negative views regarding the current PIs, they thought that the statin-PILs were well designed and included appropriate content. The PILs were regarded as easy to understand and keep for future reference. However, most participants agreed that more information about adverse effects should be added. Studies in Australia and England have shown that describing adverse effects could affect patients’ expectations of adverse effects that could influence patients’ decision whether to take the medicine.7,33 A cross-sectional survey demonstrated that verbal descriptions could lead to an overestimate of side-effect expectations.33 A qualitative study also found that some patients perceived that risks of side-effects outweigh the benefits of the medicine.7 Our participants agreed that statin-PILs contained only few details about what to do to prevent and manage possible side effects; hence, statin-PILs should provide more information about this.
Our results support finding from a survey that PILs are perceived as a useful source of medicine information.3 Participants in our study used the information to improve their awareness of medicines, such as compatibility of the statin with other medicines, foods or herbal products. Reading the PILs has been shown to help participants to judge the occurrence of side effects, but does not lead to experiences of suspected ADRs.34 Moreover, receiving a PIL prompted patients to consult their HP, as our finding showed one participant would like to consult a HP after reading that it was contraindicated in pregnancy, and many participants would like to discuss side-effects with their HPs. This patient behavior has been reported by other studies where those who receiving PILs were more aware of when to contact the physician, and sought guidance and support from the HPs.9 Therefore, providing WMI may facilitate opportunities for HPs to further educate patients about medications, which could contribute to both improving health literacy and adherence.
Some participants felt less anxious about taking medicines by being informed about the medicine, and being able to confirm for themselves that they did not have any of the precautions associated with its use, gave them confidence. In contrast, a few participants who suspected that they were experiencing an ADR felt anxious and one participant stopped taking the medicine without consultation. These findings illustrate the importance of access to additional verbal information from HPs. Previous studies have shown mixed results – some suggest that receiving written information about risk of medicines could increase anxiety in some patients and lead some to discontinue their medicines,9,10,35 whilst others suggest written information provision did not increase anxiety.36 Hence, it is important that HPs also inform patients about medicine safety and advise patients what to do if they suspect an adverse event, as well as providing PILs. Pharmacists should routinely provide both verbal advice along with written information about medicines.37 Other professions could also contribute to patient education about medicines, for example, physicians could provide side-effect information to patients more frequently.38 The participants in our study would like to receive PILs with the medicine containers, therefore the Thai FDA should promote the need for PILs and ensure they are widely distributed with marketed drugs in the country.
Patients normally read the written information when they receive the medicine for the first time,3 but are not likely to read the PILs again when the same medicines are repeated.19 Providing PILs directly from HPs could influence patients to follow their advice and to have more interest in reading the leaflets for themselves. In recent years, patients can access health information in many ways, such as via websites, mobile applications, and multimedia. Many patients have positive attitudes towards and are ready to use electronic medicine information.3,21 However, some patients still do not trust online health information due to uncertainty of its reliability.39 From our study, electronic medicine information was considered to be an alternative source of information for patients who were able to use electronic devices and younger patients. Further research is needed to explore how PILs can be used more widely and to develop other formats such as using electronic medicine information that consumers could easily access and read via tablets or smartphones. Electronic PILs could be a useful alternative source of medicine information in Thailand.
Strengths and Limitations
Our study is the first qualitative study in Thailand, allowing patients to explain their experiences, their views of PILs, and needs of receiving medicine information. Although we included patients with varied characteristics, most of them were taking more than two medicines and had college and university education. The interviews were originally transcribed in Thai and translated to English. Only five Thai transcripts were translated by another translator and reviewed by another native-English speaker to check for agreement. To increase the validity of transcriptions, more of the transcriptions could have been compared.
Conclusion
The findings of our qualitative study have shown that patients need both verbal and written information about medicines. Patients perceived that the content of PILs was useful and that the information within PILs did affect their awareness of side effects, could affect their adherence, and could encourage some patients to want to share information with their HPs. Pharmacists should provide PILs during dispensing and should encourage patients to read these, particularly in those who start a new medicine. Whilst participants had positive views on the content of PILs, some content and general format of the PILs needed to be improved. Electronic information sources were used by some participants and should be developed to enable written medicine information to be more accessible to a wider variety of people.
Acknowledgments
We thank the Royal Golden Jubilee Ph.D. Programme by Thailand Research Fund for supporting this research (Grant No. PHD/0117/2559). We also thank Ms. Supawinee Pongpunna, Queen Sirikit Heart Centre, Khon Kaen University, Thailand for validating the interview guide, outpatients from the Primary Care Unit, Srinagarind Hospital and Queen Sirikit Heart Centre who participated in the interview and to all staff who provided help in data collection.
Funding
This research was financially supported by the Royal Golden Jubilee Ph.D. Programme Scholarship (Grant No. PHD/0117/2559) by Thailand Research Fund.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gibbs S, Waters W, George C. The benefits of prescription information leaflets. Br J Clin Pharmacol. 1989;28(3):345–351. doi:10.1111/j.1365-2125.1989.tb05436.x
2. Nair K, Dolovich L, Cassels A, et al. What patients want to know about their medications. Focus group study of patient and clinician perspectives. Can Fam Physician. 2002;48:104–110.
3. Wongtaweepkij K, Krska J, Pongwecharak J, Jarernsiripornkul N. Experiences and views of medicine information among the general public in Thailand. Patient Prefer Adherence. 2020;14:1073–1082. doi:10.2147/PPA.S257454
4. Mai A, Aslani P. Impact of vietnamese written and verbal medicine information on vietnamese-speaking Australians’ knowledge and satisfaction. Br J Clin Pharmacol. 2007;64(4):527–535. doi:10.1111/j.1365-2125.2007.02968.x
5. Adepu R, Swamy M. Development and evaluation of patient information leaflets (PIL) usefulness. Indian J Pharm Sci. 2012;74(2):174. doi:10.4103/0250-474X.103857
6. Akour A, Bardaweel S, Awwad O, Al-Muhaissen S, Hussein R. Impact of a pharmacist-provided information booklet on knowledge and attitudes towards oral contraception among Jordanian women: an interventional study. Eur J Contracept Reprod Health Care. 2017;22(6):459–464. doi:10.1080/13625187.2017.1412425
7. Tong V, Raynor DK, Blalock SJ, Aslani P. Consumer interpretation of ramipril and clopidogrel medication risk information – implications for risk communication strategies. Patient Prefer Adherence. 2015;9:983–988. doi:10.2147/PPA.S86414
8. Caughey GE, Tait K, Vitry AI, Shakib S. Influence of medication risks and benefits on treatment preferences in older patients with multimorbidity. Patient Prefer Adherence. 2017;11:131–140. doi:10.2147/PPA.S118836
9. Herber OR, Gies V, Schwappach D, Thürmann P, Wilm S. Patient information leaflets: informing or frightening? A focus group study exploring patients’ emotional reactions and subsequent behavior towards package leaflets of commonly prescribed medications in family practices. BMC Fam Pract. 2014;15(1):163. doi:10.1186/1471-2296-15-163
10. Jarernsiripornkul N, Phueanpinit P, Pongwecharak J, Krska J. Experiences of and attitudes towards receiving information about non-steroidal anti-inflammatory drugs: a cross-sectional survey of patients in Thailand. Expert Opin Drug Saf. 2016;15(4):417–426. doi:10.1517/14740338.2016.1139571
11. Tong V, Raynor DK, Aslani P. Design and comprehensibility of over-the-counter product labels and leaflets: a narrative review. Int J Clin Pharm. 2014;36(5):865–872. doi:10.1007/s11096-014-9975-0
12. The Medicines and Healthcare Products Regulatory Agency. Best practice guidance on patient information best practice guidance on patient [Internet]. 2012. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/328405/Best_practice_guidance_on_patient_information_leaflets.pdf.
13. Phueanpinit P, Pongwecharak J, Krska J, Jarernsiripornkul N. Medicine information leaflets for non-steroidal anti-inflammatory drugs in Thailand. Int J Clin Pharm. 2016;38(1):25–29. doi:10.1007/s11096-015-0220-2
14. Pongpunna S, Pratipanawatr T, Jarernsiripornkul N. Survey of outpatients’ use and needs of patient medicine information leaflets in Thailand. Int J Clin Pharm. 2019;41(1):140–150. doi:10.1007/s11096-018-0748-z
15. Wongsalap Y, Jedsadayanmata A. Trends and predictors of high-intensity statin therapy and LDL-C goal achievement among Thai patients with acute coronary syndrome. J Cardiol. 2020;75(3):275–281. doi:10.1016/j.jjcc.2019.08.012
16. Thai Atherosclerosis Society. RCPT clinical practice guideline on pharmacologic therapy of dyslipidemia for atherosclerotic cardiovascular disease prevention [Internet]. 2017. Available from: https://www.thaiathero.org/thaiatherodetail.php?id=102.
17. Jose J. Statins and its hepatic effects: newer data, implications, and changing recommendations. J Pharm Bioallied Sci. 2016;8(1):23–28. doi:10.4103/0975-7406.171699
18. Jarernsiripornkul N, Phueanpinit P, Pongwecharak J, Krska J, Schouten B. Development and evaluation of user-tested Thai patient information leaflets for non-steroidal anti-inflammatory drugs: effect on patients’ knowledge. PLoS One. 2019;14(1):1–15. doi:10.1371/journal.pone.0210395
19. Raynor D, Silcock J, Knapp P, Edmondson H. How do patients use medicine information leaflets in the UK? Int J Pharm Pract. 2007;15(3):209–218. doi:10.1211/ijpp.15.3.0008
20. Hamrosi KK, Aslani P, Raynor DK. Beyond needs and expectations: identifying the barriers and facilitators to written medicine information provision and use in Australia. Health Expect. 2014;17(2):220–231. doi:10.1111/j.1369-7625.2011.00753.x
21. Hammar T, Nilsson A-L, Hovstadius B. Patients’ views on electronic patient information leaflets. Pharm Pract (Granada). 2016;14(2):702. doi:10.18549/PharmPract.2016.02.702
22. Division of Innovative Health Products and Services. Guideline for leaflet development for drug research and innovation [Internet]. 2019.
23. Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13(1):117. doi:10.1186/1471-2288-13-117
24. Bawazir SA, Abou-Auda HS, Gubara OA, Al-Khamis KI, Al-Yamani MJMS. Public attitude toward drug technical package inserts in Saudi Arabia. J Pharm Technol. 2003;19(3):209–218. doi:10.1177/875512250301900302
25. Basara LR, Juergens JP. Patient package insert readability and design. Am Pharm. 1994;34(8):48–53. doi:10.1016/s0160-3450(15)30339-1
26. Wallace LS, Keenum AJ, Roskos SE, Blake GH, Colwell ST, Weiss BD. Suitability and readability of consumer medical information accompanying prescription medication samples. Patient Educ Couns. 2008;70(3):420–425. doi:10.1016/j.pec.2007.11.017
27. Piñero-López MÁ, Modamio P, Lastra CF, Mariño EL. Readability analysis of the package leaflets for biological medicines available on the internet between 2007 and 2013: an analytical longitudinal study. J Med Internet Res. 2016;18(5):e100. doi:10.2196/jmir.5145
28. Zarea Gavgani V, Mirzadeh-Qasabeh S, Hanaee J, Hamishehkar H. Calculating reading ease score of patient package inserts in Iran. Drug Healthc Patient Saf. 2018;10:9–19. doi:10.2147/DHPS.S150428
29. Nilnate W, Hengpraprom S, Hanvoravongchai P. Level of health literacy in Thai Elders, Bangkok, Thailand. J Health Res. 2016;30(5):315–321. doi:10.14456/jhr.2016.4
30. Junkhaw T, Munisamy M, Samrongthong R, Taneepanichskul S. Factors associated with health literacy in suburban Bangkok type 2 diabetics (T2DM): a cross-sectional survey. J Med Assoc Thai. 2019;102(7):809–815.
31. Raynor DK, Dickinson D. Key principles to guide development of consumer medicine information–content analysis of information design texts. Ann Pharmacother. 2009;43(4):700–706. doi:10.1345/aph.1L522
32. Dickinson D, Raynor DK, Duman M. Patient information leaflets for medicines: using consumer testing to determine the most effective design. Patient Educ Couns. 2001;43(2):147–159. doi:10.1016/S0738-3991(00)00156-7
33. Webster RK, Weinman J, Rubin GJ. How does the side-effect information in patient information leaflets influence peoples’ side-effect expectations? A cross-sectional national survey of 18- to 65-year-olds in England. Health Expect. 2017;20(6):1411–1420. doi:10.1111/hex.12584
34. Krska J, Morecroft CW. Patients’ use of information about medicine side effects in relation to experiences of suspected adverse drug reactions: a cross-sectional survey in medical in-patients. Drug Saf. 2013;36(8):673–680. doi:10.1007/s40264-013-0065-3
35. Vinker S, Eliyahu V, Yaphe J. The effect of drug information leaflets on patient behavior. Isr Med Assoc J. 2007;9(5):383–386.
36. Garrud P, Wood M, Stainsby L. Impact of risk information in a patient education leaflet. Patient Educ Couns. 2001;43(3):301–304. doi:10.1016/s0738-3991(00)00168-3
37. American Society of Health-System Pharmacis. ASHP guidelines on pharmacist-conducted patient education and counseling. Am J Health Syst Pharm. 1997;54(4):431–434. doi:10.1093/ajhp/54.4.431
38. Phueanpinit P, Pongwecharak J, Sumanont S, Krska J, Jarernsiripornkul N. Physicians’ communication of risks from non-steroidal anti-inflammatory drugs and attitude towards providing adverse drug reaction information to patients. J Eval Clin Pract. 2017;23(6):1387–1394. doi:10.1111/jep.12806
39. Lee K, Hoti K, Hughes JD, Emmerton L. Dr google and the consumer: a qualitative study exploring the navigational needs and online health information-seeking behaviors of consumers with chronic health conditions. J Med Internet Res. 2014;16(12):e262. doi:10.2196/jmir.3706
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
