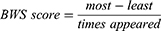Back to Journals » Patient Preference and Adherence » Volume 17
Patient Satisfaction and Sensory Attributes of Nasal Spray Treatments of Olopatadine Hydrochloride/Mometasone Furoate Monohydrate and Azelastine Hydrochloride/Fluticasone Propionate for Allergic Rhinitis in Australia – An Observational Real-World Clinical Study
Authors Fifer S , Toh L, Barkate H, Aggarwal V, Borade D, Gordonsmith RH, Wu W, Morgan C, Young K
Received 14 September 2022
Accepted for publication 10 November 2022
Published 15 January 2023 Volume 2023:17 Pages 141—151
DOI https://doi.org/10.2147/PPA.S389875
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Simon Fifer,1 Lili Toh,1 Hanmant Barkate,2 Vineet Aggarwal,2 Dhammraj Borade,2 Roger Hereward Gordonsmith,3 Wen Wu,3 Claire Morgan,4 Katherine Young4
1Community and Patient Preference Research (CaPPRe) Pty Ltd, Sydney, New South Wales, Australia; 2Global Medical Affairs, Glenmark Pharmaceuticals Ltd, Mumbai, Maharashtra, India; 3Global Medical Affairs, Glenmark Pharmaceuticals Europe Ltd, Watford, Hertfordshire, UK; 4Medical Affairs, Seqirus (Australia) Pty Ltd, Melbourne, Victoria, Australia
Correspondence: Simon Fifer, Community and Patient Preference Research (CaPPRe), Level 20, 25 Bligh Street, Sydney, New South Wales, 2000, Australia, Tel +61 403 862 091, Email [email protected]
Purpose: Combination intranasal corticosteroid and antihistamine sprays are a first-line treatment option for allergic rhinitis (AR), of which Azelastine Hydrochloride and Fluticasone Propionate nasal spray (AZE/FLU; Dymista®), and Olopatadine Hydrochloride and Mometasone Furoate Monohydrate nasal spray (OLO/MOM; Ryaltris®) are currently registered in Australia. As it is not known how patients value treatment attributes of current combination nasal sprays, this observational, real-world clinical study aimed to understand patients’ satisfaction with, and importance of, treatment attributes of OLO/MOM and AZE/FLU using an Anchored Best-Worst Scaling (ABWS) exercise.
Participants and Methods: Four hundred and twenty-six adults in Australia with moderate to severe AR using either OLO/MOM or AZE/FLU completed an online survey incorporating an ABWS with 11 domains: 7 sensory (immediate taste of medication, aftertaste of medication, smell of medication, irritation to your nose, urge to sneeze, dripping out your nose/down your throat, dryness of your nose/throat) and 4 treatment-related (convenience, fast acting, duration of effect, and AR symptom control). The ABWS involved rescaling individual BWS scores using anchored ratings (0– 10) for most and least satisfied/important domains to create a total satisfaction index (TSI) (0– 100) to be compared across groups. Statistical comparisons were completed using ANOVA (TSI) and MANOVA (individual domains).
Results: Participants using OLO/MOM (M = 68.26, SE = 1.39) had significantly higher TSI than participants using AZE/FLU (M=62.78, SE = 0.70) (p < 0.001), significantly higher satisfaction on 7 of 11 domains and regarded 8 of 11 domains as significantly more important compared to participants using AZE/FLU (all p < 0.05). Preferred domains were predominantly sensory attributes.
Conclusion: Current findings showed that participants using OLO/MOM were more satisfied with their overall treatment compared to participants using AZE/FLU, particularly with sensory attributes, thus highlighting the suitability of OLO/MOM for people with AR who value sensory attributes. Prescribers of AR treatments are encouraged to discuss treatment attributes with patients to facilitate shared decision-making.
Keywords: hay fever, patient preference, treatment satisfaction, best-worst scaling
A Letter to the Editor has been published for this article.
A Response to Letter by Professor Scadding has been published for this article.
Background/Introduction
Allergic rhinitis (AR) is a highly prevalent disease, affecting more than 4.6 million Australians.1 AR can be classified into two types: seasonal allergic rhinitis (SAR) which is triggered by seasonal allergens (eg, pollen); and perennial allergic rhinitis (PAR) which occurs throughout the year and is triggered by indoor allergens (eg, house dust mite).2 Controlling symptoms of AR can improve quality of life (QoL) in patients with AR.3–5
Combination intranasal corticosteroid and antihistamine sprays are a first-line treatment option for AR2 with two products currently registered in Australia: Azelastine Hydrochloride/Fluticasone Propionate nasal spray (AZE/FLU; Dymista®), and Olopatadine Hydrochloride/Mometasone Furoate Monohydrate nasal spray (OLO/MOM; Ryaltris®).6
Evidence suggests that sensory attributes of AR treatments have an effect on patient preference and adherence,7–9 and treatments with greater satisfaction on sensory attributes also had greater likelihood of extended use.10 Understanding patient preferences for sensory attributes of combination intranasal corticosteroid and antihistamine treatments could assist shared decision-making to support improved adherence to treatment, and in turn patients’ QoL.
While previous studies have examined patient preference or satisfaction on sensory attributes of several intranasal corticosteroid treatments7–9, less is known about patient preference or satisfaction with treatment attributes and overall satisfaction for combination nasal spray treatments such as OLO/MOM. The current observational real-world study examined patient’s satisfaction with treatment attributes for OLO/MOM and AZE/FLU and explored the importance of these attributes for a more comprehensive evaluation of overall satisfaction with these treatments. Key research objectives can be elaborated as i) understanding patient satisfaction with various treatment attributes of OLO/MOM and AZE/FLU, ii) exploring the importance of these attributes, and iii) comparing overall treatment satisfaction scores with OLO/MOM and AZE/FLU. To investigate these objectives, participants with AR using either OLO/MOM or AZE/FLU were asked to complete an online survey with an Anchored Best-Worst scaling (ABWS) task, and their rescaled Best-Worst Scaling (BWS) and TSI scores were compared.
Materials and Methods
Participants
Participants were recruited via online panel companies specialising in patient population and health-care provider samples. Interested panel members were directed to the online survey link hosted on Forsta platform (survey software)16 where they answered a series of screening questions determining their eligibility before being directed to a participant information sheet and consent form, followed by the main survey. Participants were asked to read through the participant information sheet and consent form, then check a box to indicate they had read the information and agreed to participate. The study was reviewed and approved by The Bellberry Human Research Ethics Committee (Ref No: 2021–09-1062) in accordance with the National Statement on Ethical Conduct in Human Research. The study complied with the Declaration of Helsinki. In the routine management of participants by online panel companies, genuine participants who completed the survey received an incentive of approximately AU$43 for their time.
The inclusion criteria for participants were a) adults in Australia with moderate to severe AR with or without conjunctivitis and b) self-identified as using OLO/MOM or AZE/FLU in the last 12 months. The exclusion criteria were those who a) had experienced loss of smell and/or taste in the last 12 months and b) were employed by a pharmaceutical or vaccine company.
Treatment Background Information
Participants were asked whether their allergic rhinitis is seasonal (SAR), perennial (PAR) or both, as well as how long they have been using their current treatment (OLO/MOM or AZE/FLU), and who recommended their current treatment.
Assessing Satisfaction and Importance Using Best-Worst Scaling (BWS)
Best-Worst Scaling (BWS) is a survey technique developed in the 1980s to overcome measurement issues faced in traditional scale-based research.11,12 BWS utilises an individual’s ability to reliably identify extremes (“best” and “worst”) in a set of three or more items with respect to a continuum such as satisfaction. A BWS task involves participants being shown a series of scenarios each listing a subset of attributes from a master list where a Balanced Incomplete Block Design (BIBD) ensures all domains are shown an equal number of times. Participants are asked to review scenarios and choose what they are most and least satisfied with.
BWS is preferred over traditional methods (rating scales, ranking, paired comparisons) since it is a) simple and intuitive for participants to complete, b) no scale biases, c) able to handle a large number of items, d) scores demonstrate greater discrimination among items and between groups of respondents compared to traditional scale methods and, e) scores can be estimated for individual respondents. A wealth of studies have routinely used BWS tasks to identify patient preferences and prioritisation (e.g.,13,14).
BWS scores range from −1 to 1 (negative scores indicate the domain was chosen as least satisfied/important more often than most satisfied/important, and vice versa for positive scores) and are a relative measure that cannot be used to compare between individuals. Researchers recently extended the BWS methodology with an index score that can be compared between subgroups (eg, patients), referred to as an anchored best-worst scaling (ABWS).15 Building the index involves having 2 continuums in a BWS task (satisfaction and importance). BWS scores for each domain are calculated for each participant by finding the difference between the number of times it was chosen as most and least (satisfied/important), and then dividing it by the total number of times it appeared throughout the exercise.
Satisfaction and importance BWS scores are rescaled based on absolute anchor points (eg, 0–10) rated for most satisfied/important and least satisfied/important domains. Importance BWS scores are exponentiated to create importance weights. Rescaled satisfaction scores are then weighted by importance weights, resulting in an index score ranging from 0 to 100. The index score in the current study will be referred to as the Treatment Satisfaction Index (TSI) score.
ABWS Task
Domains were adapted from a questionnaire measuring patient preferences for intranasal corticosteroids treating AR.17 These included 7 sensory attributes: immediate taste of the medication, aftertaste of the medication, smell of the medication, irritation to your nose, urge to sneeze, dripping out your nose/down your throat, dryness of your nose or throat, and 4 treatment-related attributes: convenience (how easy it is to administer), fast acting (how quickly it works), duration of effect (long lasting), and efficacy (how well it controls your AR symptoms). We emphasise that the “efficacy” domain does not measure treatment efficacy as no efficacy outcomes were measured. Rather, it asks participants to assess the importance and level of satisfaction with regards AR symptom control. These results are therefore not indicative of how efficacious these treatments are. This domain will henceforth be referred to as “AR symptom control”.
Each of the 11 scenarios displayed 6 of the 11 domains, and participants were asked to select: i) which domains they were most satisfied with and least satisfied with, ii) which domains were most important and least important to them (see Figure 1 for an example scenario).
 |
Figure 1 Example BWS scenario. |
Rescaling Satisfaction and Importance
After the BWS task, participants were shown their individual results on which domain(s) they nominated as most and least important, identified based on the maximum and minimum BWS importance scores. They were then asked to rate how important these most and least important domains were on a scale of 0 (“Not important at all”) to 10 (“Extremely important”) (see Figure 2). Their BWS scores were then rescaled based on how important they rated their most and least important domains (ie, their raw BWS importance scores were transformed to range between their minimum and maximum importance ratings). Similarly, they were asked to rate the domain(s) they nominated as most and least satisfied on a scale of 0 (“Not satisfied at all”) to 10 (“Completely satisfied”), and BWS scores were then rescaled. Calculations for rescaling, importance weights and TSI were programmed and performed in Forsta.
 |
Figure 2 Example survey page asking participants to rate how important their most and least important domains are based on preceding BWS task. |
Comparing TSI Scores and Rescaled Satisfaction and Importance Scores
A one-way analysis of variance (ANOVA) was used to compare TSI scores between participants using OLO/MOM and AZE/FLU. A multiple analysis of variance (MANOVA) was used to compare differences on all rescaled scores between participants using OLO/MOM and those using AZE/FLU.
Results
Demographic Information
The study recruited 426 adults between October 2021 and March 2022 (n = 98 using OLO/MOM, n = 328 using AZE/FLU). Overall about two-thirds of participants were female (n = 293, 69%) and the majority (n = 283, 66%) were between 18 and 40 years of age. The median age group is the same for both participants using OLO/MOM and participants using AZE/FLU at 31–40 years old. Participant demographics are reported in Table 1.
Treatment Background
Overall, 38% (n = 162) of participants reported having seasonal AR only, while 33% (n = 141) had perennial AR only and 28% (n = 117) had both. Around half of participants using OLO/MOM (n = 48, 49%) and three quarters of participants using AZE/FLU (n = 249, 76%) reported using 1 or more previous treatments over the past year. Most participants had been using their current treatment for less than one year (n = 222, 52%) with slightly higher proportion in OLO/MOM group (n = 58, 59%) than in the AZE/FLU group (n = 164, 50%) Table 2.
TSI Scores for OLO/MOM and AZE/FLU
Participants using OLO/MOM reported a higher TSI (TSI M = 68.26, SE = 1.39) than participants using AZE/FLU (TSI M = 62.78, SE = 0.70). A one-way ANOVA revealed a significant difference in TSI between OLO/MOM and AZE/FLU (F(1424) = 13.70, p < 0.001).
Rescaled Importance and Satisfaction Scores for Treatment Attributes of OLO/MOM and AZE/FLU
The ABWS generated rescaled importance and satisfaction scores for each domain are shown in Table 3 for each of the two participant groups. MANOVA on the 11 rescaled satisfaction scores and 11 rescaled importance scores showed a statistically significant difference between OLO/MOM and AZE/FLU (F (22, 403) = 5.84, p < 0.001; Wilk’s Λ = 0.758).
Participants using OLO/MOM had significantly higher satisfaction scores than participants using AZE/FLU for 7 of the 11 domains: immediate taste of medication, aftertaste of medication, smell of medication, irritation to nose, urge to sneeze, dripping out nose/down throat, and dryness of nose or throat. Participants using AZE/FLU had higher satisfaction scores on AR symptom control but there were no significant differences for convenience, fast acting and duration of effect.
Participants using AZE/FLU had higher importance scores on two domains, duration of effect and AR symptom control, while participants using OLO/MOM had higher importance scores on 8 domains; immediate taste of medication, aftertaste of medication, smell of medication, irritation to nose, urge to sneeze, dripping out nose/down throat, dryness of nose or throat, convenience. There was no significant difference for the fast acting domain. Figure 3 illustrates the comparison of OLO/MOM and AZE/FLU on importance and satisfaction scores.
Gap Analysis: Satisfaction in Relation to Importance
To investigate how satisfaction relates to importance in each of these domains, we examined the gaps between rescaled satisfaction and importance scores for each domain. A positive gap indicates greater importance than satisfaction, while a negative gap indicates greater satisfaction than importance. Figure 4 illustrates the gaps in importance and satisfaction scores for participants using OLO/MOM and participants using AZE/FLU.
Domains with a positive gap for OLO/MOM include AR symptom control, dripping out nose/down throat, irritation to nose, duration of effect while domains with a positive gap for AZE/FLU include AR symptom control, dripping out nose/down throat, irritation to nose, duration of effect, fast acting, and aftertaste of medication. Where positive gaps were identified in both treatment groups, participants using AZE/FLU had a larger gap on all identified domains compared to participants using OLO/MOM. These were AR symptom control, dripping out nose/down throat, irritation to nose and duration of effect.
For participants using OLO/MOM, we observed negative gaps for domains such as urge to sneeze, smell of medication, immediate taste of medication, dryness of nose or throat, convenience and aftertaste of medication. For participants using AZE/FLU, the negative gaps were observed for domains such as urge to sneeze, smell of medication, immediate taste of medication, dryness of nose or throat and convenience.
Differences in Subgroups
Some interesting effects were observed when segmenting the full sample into subgroups. We report the trends in our observations; no statistical analyses were performed for these subgroups due to the small number of participants within some groups. We found that among participants who used 1 treatment only in the past 12 months (n = 129; 38.76% OLO/MOM, 61.24% AZE/FLU), participants using OLO/MOM (TSI M = 75.35, SE = 1.82) had a higher treatment satisfaction index than participants using AZE/FLU (TSI M = 62.92, SE = 1.46). Although this effect has a similar pattern to that observed in the whole sample, it appears to be more pronounced. Information on whether participants were treatment naïve before their current treatment was not available, therefore number of treatments used previously in the last 12 months was used as a proxy.
In addition, among participants with SAR only (n = 162; 36.7% of OLO/MOM, 38.4% of AZE/FLU), participants using OLO/MOM had a higher treatment satisfaction index (TSI M = 67.87, SE = 1.69) than participants using AZE/FLU (TSI M = 65.75, SE = 1.07). Similarly, among participants with PAR only (n = 141; 23.5% of OLO/MOM, 36.0% of AZE/FLU), participants using OLO/MOM (TSI M = 62.02, SE = 2.72) had a higher treatment satisfaction index than participants using AZE/FLU (TSI M = 61.68, SE = 1.20). These patterns of results were similar to the full sample, albeit to a smaller extent which may be attributed to the smaller OLO/MOM sample size. For participants with both SAR and PAR (n = 111; 28.83% OLO/MOM, 71.17% AZE/FLU), the same pattern was seen (OLO/MOM TSI M = 69.34, SE = 2.28; AZE/FLU TSI M = 60.19, SE = 1.40).
Discussion
To the best of our knowledge, the current study was the first to compare satisfaction of treatment attributes of two different combination products of intranasal corticosteroid and antihistamine sprays using an ABWS. We found that the overall TSI was higher for participants using OLO/MOM than AZE/FLU, and that participants using OLO/MOM had a higher satisfaction on nearly all treatment attributes compared to participants using AZE/FLU. In addition, sensory attributes were more important to participants with OLO/MOM than those using AZE/FLU. This aligns with findings from previous studies demonstrating that sensory attributes such as scent/odour, immediate taste and aftertaste are important factors for patient choice in AR treatments8 and complying with instructed use.8,9 Our findings, together with previous studies, highlight the importance of satisfaction in sensory attributes for AR nasal spray treatments.
Comparing Satisfaction and Importance of Domains Between Treatments
Taking into account TSI scores and the number of treatment attributes participants found important and were satisfied with, participants using OLO/MOM were more satisfied with their treatment overall compared to participants using AZE/FLU. Participants using OLO/MOM were more satisfied and held greater importance for sensory attributes which highlights the suitability of OLO/MOM for participants with AR who value sensory attributes.
For participants using AZE/FLU, they were more satisfied and held greater importance for AR symptom control (AR symptom control) compared to participants using OLO/MOM, although both treatments were regarded highly on satisfaction and importance for this domain.
Gaps Between Satisfaction and Importance for Each Treatment Attribute
We focus on the interpretation of positive gaps since they highlight which treatment attributes participants found important but fell short in terms of satisfaction (ie, identifying areas where an alternative treatment choice may be more suitable for the patient).
In our sample, we found that both groups of participants using OLO/MOM and AZE/FLU regarded AR symptom control, dripping out nose/down throat, irritation to nose, and duration of effect as important attributes that had lower satisfaction ratings. Additionally, participants using AZE/FLU regarded fast acting and aftertaste of medication to be important treatment attributes that they were not as satisfied with. In all domains where positive gaps were identified in both groups, participants using OLO/MOM had smaller positive gaps compared to participants using AZE/FLU. This suggests that participants using AZE/FLU perceived the treatment to fall short on satisfaction for these domains to a larger extent than participants using OLO/MOM. These domains were: AR symptom control, dripping out nose/down throat, irritation to nose, and duration of effect. While participants using AZE/FLU may have rated higher satisfaction and importance for AR symptom control, the positive gap was larger – indicating that AR symptom control fell short on satisfaction to a greater extent than OLO/MOM. These results corroborate our main finding that participants using OLO/MOM are more satisfied overall with their treatment compared to participants using AZE/FLU.
Limitations and Future Directions
As with all studies, our findings should be interpreted in light of the current study’s limitations. First, there was unequal sampling of participants using OLO/MOM compared to AZE/FLU due to OLO/MOM being relatively new to the market at the time of conducting the survey. Future research should aim to recruit equal numbers of participants using OLO/MOM and AZE/FLU for a more robust analysis. Second, as information on whether participants were treatment naïve before their current treatment was not available, previous treatments used in the past 12 months was used as a proxy to segment subgroups. Future research exploring patient satisfaction in these subgroups can collect data on participants’ full treatment history for more nuanced results.
Third, there seems to be a difference in the representation of gender and number of previous treatments when comparing between treatment groups which could be due to the uneven and small sample size. Although women are typically more likely to be diagnosed with AR than men based on the Australian national data,18,19 there were substantially more women than men in our sample, particularly in the AZE/FLU group. However, there is no evidence to our knowledge that suggests gender influences AR treatment preferences and is therefore unlikely to confound results of this study. Additionally, we did observe that the pattern of age groups in our sample (predominantly more adults in the 18–40 years range), is similar to the national data and similar across treatment groups18,19 For previous treatments, we noticed there was a much higher proportion of participants who had more than one previous treatment in the last 12 months compared to less than one previous treatment among participants using AZE/FLU, while this was relatively even for participants using OLO/MOM. We are not certain what to make of this unexpected finding. The subgroup assessment suggests that participants using OLO/MOM had higher overall satisfaction irrespective of whether they had used one or more treatments in the past 12 months. Future research should recruit larger numbers for a more representative sample. Lastly, a small number of participants using OLO/MOM (n = 9) indicated they were on their current treatment for more than 3 years. These participants may have misunderstood or misinterpreted the question as OLO/MOM has not been in the market for that length of time. These participants were not excluded as they still met the eligibility criteria.
Practical Implications
The current study closes the knowledge gap on patient preference with differential AR combination nasal spray treatments, especially on relatively new treatments such as OLO/MOM. Prescribers of AR treatments are encouraged to discuss with their patients what attributes they value in treatments to facilitate shared decision-making. Participants with AR who value sensory attributes of their treatment could benefit from using OLO/MOM nasal spray, since they are likely to be more satisfied with their treatment which may subsequently increase adherence.
Conclusion
Through an ABWS exercise, this study found that participants using OLO/MOM were more satisfied with their overall treatment, and had greater satisfaction with and importance on sensory attributes of the treatment, compared to participants using AZE/FLU. This pattern of results held true even among subgroups such as those who had not had previous treatments in the last 12 months, and those with SAR only, PAR only or both SAR and PAR. This demonstrates the suitability of OLO/MOM for a variety of participants who value sensory attributes in their treatment. It is recommended that prescribers of AR treatments discuss treatment options with their patients to discern what treatment attributes they value. This patient engagement may improve patient’s treatment satisfaction and subsequently enhance treatment adherence and patient outcomes.
Abbreviations
AR, Allergic Rhinitis; SAR, Seasonal Allergic Rhinitis; PAR, Perennial Allergic Rhinitis; QoL, Quality of Life; AZE/FLU, Azelastine Hydrochloride and Fluticasone Propionate nasal spray; Dymista®; OLO/MOM, Olopatadine Hydrochloride and Mometasone Furoate Monohydrate; Ryaltris®; BWS, Best-Worst Scaling; BIBD, Balanced Incomplete Block Design; ABWS, Anchored Best-Worst Scaling; TSI, Treatment Satisfaction Index; ANOVA, Analysis of Variance; MANOVA, Multivariate Analysis of Variance.
Acknowledgments
The authors would like to thank Declan, J. Monro, Jennifer, M. Ross & Brett Yang for technical help.
Funding
The study was funded by Seqirus (Australia) Pty Ltd who were not part of the overall study design. Members of companies were involved in final discussion of results and dissemination. Funding bodies had no specific role in data collection, or data analysis.
Disclosure
KY and CM are both employees of Seqirus (Australia) Pty Ltd and HB, VA, DB, RHG, and WW are employees of Glenmark Pharmaceuticals. SF and LT are from CaPPRe who were contracted by Seqirus (Australia) Pty Ltd to design the experiment, provide data management, conduct the analysis and write the manuscript. The authors report no other conflicts of interest in this work.
References
1. Australian Institute of Health and Welfare (AIHW). Allergic rhinitis (‘hay fever’); 2019. Available from: https://www.aihw.gov.au/reports/chronic-respiratory-conditions/allergic-rhinitis-hay-fever/contents/allergic-rhinitis.
2. Australasian Society of Clinical Immunology and Allergy (ASCIA). Allergic rhinitis clinical update; 2020.
3. Meltzer EO. Quality of life in adults and children with allergic rhinitis. J Allergy Clin Immunol. 2001;108(1):S45–S53. doi:10.1067/mai.2001.115566
4. Speth MM, Hoehle LP, Phillips KM, Caradonna DS, Gray ST, Sedaghat AR. Treatment history and association between allergic rhinitis symptoms and quality of life. Ir J Med Sci. 2019;188(2):703–710. doi:10.1007/s11845-018-1866-2
5. Camelo-Nunes IC, Solé D. Allergic rhinitis: indicators of quality of life. J Bras Pneumol. 2010;36:124–133. doi:10.1590/S1806-37132010000100017
6. National Asthma Council Australia (NACA). Allergic rhinitis treatments chart; 2021. Available from: https://www.nationalasthma.org.au/living-with-asthma/resources/health-professionals/charts/allergic-rhinitis-treatments-chart.
7. Meltzer EO, Stahlman JE, Leflein J, et al. Preferences of adult patients with allergic rhinitis for the sensory attributes of fluticasone furoate versus fluticasone propionate nasal sprays: a randomized, multicenter, double-blind, single-dose, crossover study. Clin Ther. 2008;30(2):271–279. doi:10.1016/j.clinthera.2008.02.005
8. Meltzer EO, Bardelas J, Goldsobel A, Kaiser H. A preference evaluation study comparing the sensory attributes of mometasone furoate and fluticasone propionate nasal sprays by patients with allergic rhinitis. Treat Respir Med. 2005;4(4):289–296. doi:10.2165/00151829-200504040-00007
9. Mahadevia PJ, Shah S, Leibman C, Kleinman L, O’Dowd L. Patient preferences for sensory attributes of intranasal corticosteroids and willingness to adhere to prescribed therapy for allergic rhinitis: a conjoint analysis. Ann Allergy Asthma Immunol. 2004;93(4):345–350. doi:10.1016/S1081-1206(10)61393-2
10. Meltzer EO, Garadi R, LaForce C, et al. Comparative study of sensory attributes of two antihistamine nasal sprays: olopatadine 0.6% and azelastine 0.1%. In: Allergy and Asthma Proceedings. Vol. 29. OceanSide Publications, Inc; 2008:659–668.
11. Finn A, Louviere JJ. Determining the appropriate response to evidence of public concern: the case of food safety. J Public Policy Mark. 1992;11(2):12–25. doi:10.1177/074391569201100202
12. Louviere JJ, Woodworth GG. Best-worst scaling: a model for the largest difference judgments. Faculty of Business. University of Sydney; 1990.
13. Lancsar E, Louviere J, Donaldson C, Currie G, Burgess L. Best worst discrete choice experiments in health: methods and an application. Soc Sci Med. 2013;76:74–82. doi:10.1016/j.socscimed.2012.10.007
14. Mühlbacher AC, Kaczynski A, Zweifel P, Johnson FR. Experimental measurement of preferences in health and healthcare using best-worst scaling: an overview. Health Econ Rev. 2016;6(1):1–14. doi:10.1186/s13561-016-0080-z
15. Burke PF, Rose JM, Fifer S, Masters D, Kuegler S, Cabrera A. A new subjective well-being index using anchored best-worst scaling; 2022. Social Science Research. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4322054.
16. Forsta. Forsta plus survey platform; 2022. Available from: https://www.forsta.com/platform/survey-design/.
17. Meltzer EO, Hadley J, Blaiss M, et al. Development of questionnaires to measure patient preferences for intranasal corticosteroids in patients with allergic rhinitis. Otolaryngology. 2005;132(2):197–207. doi:10.1016/j.otohns.2004.10.010
18. Australian Institute of Health and Welfare (AIHW). Allergic rhinitis (‘hay fever’); 2020. Available from: https://www.aihw.gov.au/reports/chronic-respiratory-conditions/allergic-rhinitis-hay-fever.
19. Australian Institute of Health and Welfare (AIHW). Allergic rhinitis (‘hay fever’) in Australia; 2011. Available from: https://www.aihw.gov.au/getmedia/f155276c-b1c1-4bb9-94de-e7e09555bce4/13567.pdf.aspx?inline=true.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.