Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Patient risk factors for developing a drug-related problem in a cardiology ward
Authors Urbina O, Ferrández O, Luque S, Grau S, Mojal S, Pellicer R, Riu M, Salas E, Comin J
Received 25 July 2014
Accepted for publication 11 September 2014
Published 17 December 2014 Volume 2015:11 Pages 9—15
DOI https://doi.org/10.2147/TCRM.S71749
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Olatz Urbina,1 Olivia Ferrández,1 Sònia Luque,1 Santiago Grau,1,2 Sergi Mojal,3 Rosa Pellicer,1 Marta Riu,4 Esther Salas,1 Josep Comin-Colet5
1Pharmacy Department, Hospital Universitari del Mar, Barcelona, Spain; 2Universitat Autònoma de Barcelona, Barcelona, Spain; 3Department of Statistics, Institut Hospital del Mar d’Investigacions Mèdiques, Barcelona, Spain; 4Department of Epidemiology and Health Services Evaluation, CIBER de Epidemiología y Salud Pública (CIBERESP), Hospital Universitari del Mar, Barcelona, Spain; 5Heart Failure Unit, Cardiology Department, Hospital Universitari del Mar, Barcelona, Spain
Background: Because of the high incidence of drug-related problems (DRPs) among hospitalized patients with cardiovascular diseases and their potential impact on morbidity and mortality, it is important to identify the most susceptible patients, who therefore require closer monitoring of drug therapy.
Purpose: To identify the profile of patients at higher risk of developing at least one DRP during hospitalization in a cardiology ward.
Method: We consecutively included all patients hospitalized in the cardiology ward of a teaching hospital in 2009. DRPs were identified through a computerized warning system designed by the pharmacy department and integrated into the electronic medical record.
Results: A total of 964 admissions were included, and at least one DRP was detected in 29.8%. The variables associated with a higher risk of these events were polypharmacy (odds ratio [OR]=1.228; 95% confidence interval [CI]=1.153–1.308), female sex (OR=1.496; 95% CI=1.026–2.180), and first admission (OR=1.494; 95% CI=1.005–2.221).
Conclusion: Monitoring patients through a computerized warning system allowed the detection of at least one DRP in one-third of the patients. Knowledge of the risk factors for developing these problems in patients admitted to hospital for cardiovascular problems helps in identifying the most susceptible patients.
Keywords: cardiovascular diseases, patient safety, drug therapy monitoring, computerized provider order entry, clinical pharmacist, pharmacy warning system
Introduction
In the last few years, the use of polypharmacy has increased in patients with cardiovascular diseases, mainly because of the higher number of associated comorbidities in this patient group.1,2 Specifically, heart failure entails the management of multiple medical conditions, requiring a significant increase in the mean number of drugs from admission to discharge.2,3
A study in 62,376 patients with heart failure aged 65 years or older reported that the mean number and daily dose of drugs increased from 6.8 and 10.1 from April 1998 to March 1999 to 7.5 and 11.1 from July 2000 to June 2001, respectively.4 These increases are an inevitable consequence of the optimization of heart failure management. The huge complexity of drug therapy for some cardiovascular diseases and its high prevalence highlight the importance of adopting efficient strategies to closely monitor these patients.
A drug-related problem (DRP) is defined as an event or circumstance involving drug therapy that actually or potentially interferes with the desired health outcome.5 In patients with heart failure and other cardiovascular diseases, the frequency of DRPs has been reported to be as high as 69%6 and 78%,7 respectively. In addition, the presence of a DRP has been related to negative clinical outcomes.6,7 These findings demonstrate the need for strategies that would allow an exhaustive review of drug therapy in patients with cardiovascular diseases in order to detect potential DRP that could trigger a health problem.
Computerized provider order entry (CPOE) is an electronic system that health care professionals can use to enter drugs, treatments, and test orders, and transmit the orders directly to the department responsible for fulfilling the order.8 The recent availability of these programs, which allow physicians to introduce prescriptions in the electronic medical record (EMR), and their use in routine drug therapy monitoring, together with pharmacy warning systems (PWS) that enhance safety in hospitalized patients, have helped in identifying DRP and their causes,9,10 thus improving the medication process. Nevertheless, there are patients with certain clinical and/or demographic characteristics who are at higher risk for developing a DRP and whose treatment needs to be more closely monitored.11,12
Some studies have identified different risk factors for developing at least one DRP in patients with cardiovascular diseases3,7 and the use of multiple medications is one of the variables most commonly identified.
However, most of these experiences have been developed in the community setting rather than in the acute clinical setting and have mainly focused on patients with chronic heart failure. To our knowledge, none have used a specific computerized warning system to detect potential DRPs.12 The objective of this study was to identify the risk factors for the development of at least one DRP in patients admitted to a cardiology ward.
Materials and method
This is a prospective observational study developed from January to December 2009, which included patients admitted to the cardiology ward of a teaching hospital with 413 conventional beds, 18 beds for critically ill patients, and a catchment area of 300,000 inhabitants.13 The cardiology ward has approximately 30 hospital beds. We excluded direct admissions to the coronary unit and patients younger than 18 years.
Prescriptions are issued through a CPOE developed by a multidisciplinary team in the hospital. Likewise, the system employed to detect potential DRP was designed by the pharmacy department. DRPs were classified according to the Pharmaceutical Care Network Europe (PCNE) classification for DRP version 6.2.5
The two computer applications are integrated within the EMR, which contain all the demographic and clinical data of the patient. Each time a prescription is written, the application generates a series of alerts (causes of potential DRPs), which are based on drug information and each patient’s characteristics. All the alerts and prescriptions are reviewed by a team of clinical pharmacists. The functioning of these tools and data collection, diagnosis-related group, readmission, and anatomic therapeutic classification have been described in a previous study analyzing the risk factors for developing a DRP in patients admitted to an acute-care hospital.12
Specific definition of incorrect use of the CPOE in the cardiology ward
Prescription errors can result from incorrect use of the CPOE. An example of such errors would be the use of the free text comment associated with a prescription line to indicate the correct drug prescription by the prescriber. For example, acenocoumarol 4 mg/day may be prescribed, and the free text comment may specify that only half a tablet be administered on Mondays, Wednesdays, and Fridays. Acenocoumarol may then incorrectly appear in the nursing activities chart as 4 mg once-daily administration, which could lead to a DRP. This kind of problem is due to a lack of knowledge of the CPOE, which enables medication to be prescribed at intervals other than once-daily administration.
Statistical analysis
For the descriptive analysis of the sample, categorical variables are expressed as absolute and relative frequencies and quantitative variables as mean and standard deviation.
A bivariate analysis of the data was performed with binary logistic regression, showing the odds ratio (OR) (95% confidence interval [CI]), to confirm or exclude an association between the presence of at least one DRP during hospital admission with respect to each of the variables analyzed.
Subsequently, a multivariate saturated model was calculated, introducing all the variables independently of their statistical significance in order to avoid any confounding factors.
The model’s discriminatory ability was verified through the receiver operating characteristic curve, and the Hosmer–Lemeshow test was employed to check the calibration of model.
Statistical significance was set at P<0.05. The statistical analysis was carried out using the SPSS 18.0 statistical package (IBM Corp, New York, USA).
Results
There were 16,485 admissions during the study period, of which 1,233 (7.48%) were admissions to the cardiology ward. Of these, 266 (21.57%) were direct admissions to the coronary unit and three admissions (0.24%) involved patients younger than 18 years. After application of the exclusion criteria, 964 (78.18%) admissions were included in this study.
The 964 admissions corresponded to 842 patients (1.14 admissions/patient) (range: 1–4). The mean age was 68.7 years (SD: 13.5) (range: 20–95), and 588 (61.0%) were men. At least one DRP was identified in 287 admissions (29.8%); in these admissions, the mean number of DRPs was 1.87 (SD: 1.19).
When the 842 patients were considered, at least one DRP was detected in 259 of them (30.8%).
A total of 8,923 drug prescriptions were issued during the study period, with a mean of 9.25 (SD: 4.99) prescriptions/admission. One or more DRP was identified in 516 drug prescriptions (5.78%). Among these, the mean of prescriptions with a DRP per admission was 1.80 (SD: 1.08).
In all, 448 DRPs were detected, mainly involving drug–drug or drug–food interactions, prescription errors due to inadequate knowledge of the use of the CPOE, prescription of an inappropriate dose for a specific drug, inappropriate dosing schedule, or dose adjustment due to a change in renal function (Table 1).
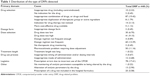 
|
Table 1 Distribution of the type of DRPs detected |
The distribution of the main DRP according to the cardiac disease present in admitted patients is shown in Table 2.
 
|
Table 2 Distribution of the main causes of DRPs by type of heart disease |
The bivariate and multivariate analyses of the demographic and clinical characteristics of the admitted patients with one or more DRP compared with patients free of these events are shown in Table 3.
None of the patients had underlying liver failure or cachexia. The mean length of hospital stay was 9.58 days (SD: 8.21) in patients with at least one DRP versus 5.03 days (SD: 4.96) in those without DRP (P<0.001). Among patients with at least one DRP, seven died (2.70%) versus eight (1.37%) among those with no DRP (P=0.256).
In the logistic regression analysis, the factors significantly associated with the occurrence of at least one DRP were each additional drug received (OR=1.228; 95% CI=1.153–1.308), female sex (OR=1.496; 95% CI=1.026–2.180), and first admission (OR=1.494; 95% CI=1.005–2.221).
In the multivariate model, the area under the curve (AUC) value of the receiver operating characteristic curve was 0.788 (95% CI=0.756–0.820).
Calibration of the model by the Hosmer–Lemeshow test showed no statistical significance (P=0.458), indicating that the model was correctly calibrated.
Discussion
This study aimed to determine the factors associated with an increased risk of developing one or more DRP during hospital admission in patients with cardiovascular disease. We found that one of the variables associated with a higher risk of having at least one DRP was the number of drugs prescribed during hospital admission, with each additional drug representing a 22% increase in risk. This finding has been previously reported. A study of 91 patients attending the emergency department with a primary diagnosis of heart failure found a significant correlation between polypharmacy and medication errors, as well as between the number of drugs prescribed and drug–drug interactions, interactions due to renal insufficiency, liver failure, or both.3 Another study of 97 patients with heart failure found a positive correlation between the number of drugs and the number of DRPs.7 Other studies, although not specifically related to cardiovascular disease, have found a significant correlation between the number of drugs prescribed and the risk of DRP in hospitalized patients14 and the risk of adverse drug reactions in elderly in-hospital patients.11
In the present study, certain cardiovascular diseases, such as heart failure and ischemic heart disease, were associated with a higher risk of developing at least one DRP in the bivariate analysis. Nevertheless, this association was not maintained in the multivariate analysis. In contrast, another study identified an association between heart failure and a higher risk of adverse drug reactions in older patients (OR=1.79; 95% CI=1.39–2.30).11 However, that study did not compare the risk of developing DRPs among distinct cardiovascular diseases.
Among demographic variables in our study, only female sex was associated with a higher risk of developing at least one DRP. Similarly, a study of 1,857 patients with chronic heart failure reported that female patients were less frequently treated with required drugs, such as angiotensin converting-enzyme inhibitors or angiotensin II receptor blockers, and were less frequently prescribed adequate doses of β-blockers.15 In contrast, other studies have found no relationship between age or sex and the frequency of DRP in heart transplant recipients,16 or between demographic variables and a higher number of drugs in patients with heart failure.3
Another variable associated with DRP in our study was not having had a prior admission. A possible explanation for this finding is that patients with prior admissions might have undergone an exhaustive medication review, which could have prevented some DRPs.17 Clarification of the role of medication reviews in preventing DRPs may be provided by a controlled clinical trial aiming to evaluate the effects of a medication review and cognitive behavioral therapy carried out by community pharmacists on the DRP rate in elderly patients discharged from hospital, but the results of this study are still pending.17
In the present study, we found no association between the risk of DRP and administration of drugs in certain therapeutic groups. Nevertheless, a prior study evaluated adverse drug events in patients admitted to two tertiary hospitals. In a cohort analysis of patients admitted to one of the hospitals, risk factors for DRPs were administration of diuretics (OR=1.7; 95% CI=1.0–2.6) and electrolyte concentrates (OR=1.7; 95% CI=1.1–2.5). In a case–control analysis of patients admitted to both hospitals, exposure to psychoactive drugs was identified as a risk factor (OR=2.1; 95% CI=1.3–3.6).18 Although administration of cardiovascular drugs was an independent predictor of serious adverse events in the case–control analysis (OR=2.4; 95% CI=1.3–4.5), the authors considered this to be a chance finding for two possible reasons: this therapeutic class was an infrequent cause of events and their administration could be a marker of an underlying condition.
Numerous studies have demonstrated that multidisciplinary management reduces admission rates and overall mortality in patients with chronic cardiovascular diseases such as heart failure.19–24 In our study, the mean length of hospital stay was more than 4 days longer in patients with at least one DRP than in those without. Because of the economic impact of prolonged hospital stays,25–27 this topic should be studied in greater depth.
In the present study, at least one DRP was detected in approximately 30% of admissions. This percentage is lower than that reported in other studies in patients with cardiovascular diseases.6,7 In one of these studies, 69% of 85 outpatients with cardiovascular disease had at least one DRP.6 In another study, 78% of 97 patients with heart failure managed in an outpatient clinic had a drug-related negative outcome or showed a risk of a drug-related negative outcome.7 However, both studies were performed in a non-hospital outpatient setting, where the medication was checked by a pharmacist daily. Another study reported that 40% (19 of 48) of readmissions in patients who had previously received a heart transplant were caused by DRPs and that 58% (11 of 19) of these were preventable.16 These values are closer to those observed in our study.
The most frequent types of DRP identified in this study were interactions, prescription errors caused by inadequate knowledge of the CPOE, prescription of an inappropriate dose or frequency of administration, and dose adjustment according to renal function. A study of 97 patients with heart failure found that one of the most frequent DRP detected (22%) involved inadequate dose, regimen, or duration of a drug,7 while a study of 19 heart transplant recipients who were readmitted for a DRP found that the most common cause was an inappropriate dose (47.4%).16 In our study, dose adjustment according to renal function was more frequent in patients with heart failure than in those with other cardiovascular diseases. In contrast, prescription errors caused by inadequate knowledge of the CPOE were less frequent in patients with heart failure than in those with other cardiovascular diseases.
Among the limitations of this study is the impossibility of determining the association between the DRP detected by the PWS designed by the pharmacy department and health outcomes. In addition, we did not evaluate the effect of the DRP rate due to admission of patients with cardiovascular diseases in units other than the cardiology ward. Finally, when the study was being performed, the pharmacy application contained information on 82.3% of the active ingredients available in the hospital, which could have led to nondetection of DRPs related to drugs not included in the information in the system. Nevertheless, this percentage of drugs represented >99% of the prescriptions issued during the study period.
A strength of this study is that the DRP warning system contained data on a large number of drugs as well as information on diagnostic and laboratory tests. Unlike other studies, in addition to including renal function, this study also included analysis of other physiological conditions that could alter the pharmacokinetics of the drugs used in cardiovascular disease, such as liver failure, cachexia, and obesity. Moreover, we included 964 patients admitted to hospital with cardiovascular disease, a sample that is much larger than that included in other studies.
Several authors have highlighted the need to equip prescription and medication review systems with utilities to integrate all the valuable information (demographic, clinical, and pharmacological conditions).28 Identifying the risk factors for DRP in patients with cardiovascular disease in an acute setting could facilitate the design and implementation of a specific predictive model in our EMR to rapidly detect the profile of these patients. Because daily medication reviews of all hospitalized patients is extremely time consuming, an efficient screening tool to identify patients at highest risk of DRP would enable clinical pharmacists to prioritize and optimize their work flow.
In conclusion, this study identified the number of drugs, female sex, and first admission as the risk predictors for developing DRPs in patients with cardiovascular diseases in a cardiology ward.
As proposed by other authors,28 early identification of patients most at risk for DRPs during admission could aid closer monitoring, which could in turn lead to improved clinical, economic, and humanistic outcomes.
Acknowledgment
This study is a part of a PhD program in medicine of the Universitat Autònoma de Barcelona.
Disclosure
The authors report no conflict of interests in this work.
References
Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011;124(2):136–143. | ||
Masoudi FA, Krumholz HM. Polypharmacy and comorbidity in heart failure. BMJ. 2003;327:513–514. | ||
Ledwidge M, Travers B, Ryder M, Ryan E, McDonald K. Specialist care of heart failure improves appropriate pharmacotherapy at the expense of greater polypharmacy and drug-interactions. Eur J Heart Fail. 2004;6:235–243. | ||
Masoudi FA, Baillie CA, Wang Y, et al. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998–2001. Arch Intern Med. 2005;165:2069–2076. | ||
Pharmaceutical Care Network Europe [homepage on the Internet]. The PCNE Classification for drug-related problems V 6.2. [updated January 14, 2010]. Available from: http://www.pcne.org/sig/drp/documents/PCNE%20classification%20V6-2.pdf. Accessed September 1, 2014. | ||
Niquille A, Bugnon O. Relationship between drug-related problems and health outcomes: a cross-sectional study among cardiovascular patients. Pharm World Sci. 2010;32:512–519. | ||
Gastelurrutia P, Benrimoj SI, Espejo J, Tuneu L, Mangues MA, Bayes-Genis A. Negative clinical outcomes associated with drug-related problems in heart failure (HF) outpatients: impact of a pharmacist in a multidisciplinary HF clinic. J Card Fail. 2011;17(3):217–223. | ||
ASHP Section of Pharmacy Informatics and Development. ASHP guidelines on pharmacy planning for implementation of computerized provider-order-entry systems in hospitals and health systems. Am J Health Syst Pharm. 2011;68:e9–e31. | ||
Bedouch P, Allenet B, Grass A, et al. Drug-related problems in medical wards with a computerized physician order entry system. J Clin Pharm Ther. 2009;34:187–195. | ||
Roten I, Marty S, Beney J. Electronic screening of medical records to detect inpatients at risk of drug-related problems. Pharm World Sci. 2010;32:103–107. | ||
Onder G, Petrovic M, Tangiisuran B, et al. Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: the GerontoNet ADR risk score. Arch Intern Med. 2010;170(13):1142–1148. | ||
Urbina O, Ferrández O, Grau S, et al. Design of a score to identify hospitalized patients at risk of drug-related problem. Pharmacoepidemiol Drug Saf. 2014;23(9):923–932. | ||
Estudis d’Economia de la salut (volum III). Planificació i Avaluació. Departament de Salut. Generalitat de Catalunya; 2010. Available from: http://www.saveva.com/domamPlus/pub/depsalut/html/ca/dir505/estecosalutiii.pdf. Accessed February 14, 2014. | ||
Blix HS, Viktil KK, Reikvam A, et al. The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals. Eur J Clin Pharmacol. 2004;60:651–658. | ||
Baumhakel M, Muller U, Bohm M. Influence of gender of physicians and patients on guideline-recommended treatment of chronic heart failure in a cross-sectional study. Eur J Heart Fail. 2009;11:299–303. | ||
Repp KL, Hayes C, Woods TM, Allen KB, Kennedy K, Borkon MA. Drug-related problems and hospital admissions in cardiac transplant recipients. Ann Pharmacother. 2012;46:1299–1307. | ||
Ahmad A, Hugtenburg J, Welschen LM, Dekker JM, Nijpels G. Effect of medication review and cognitive behaviour treatment by community pharmacists of patients discharged from the hospital on drug related problems and compliance: design of a randomized controlled trial. BMC Public Health. 2010;10:133. | ||
Bates DW, Miller EB, Cullen DJ, et al. Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med. 1999;159:2553–2560. | ||
Peterson ED, Albert NM, Amin A, Patterson JH, Fonarow GC. Implementing critical pathways and a multidisciplinary team approach to cardiovascular disease management. Am J Cardiol. 2008;102(5A):47G–56G. | ||
Altowaijri A, Phillips CJ, Fitzsimmons D. A systematic review of the clinical and economic effectiveness of clinical pharmacist intervention in secondary prevention of cardiovascular disease. J Manag Care Pharm. 2013;19(5):408–416. | ||
Milfred-Laforest SK, Chow SL, Didomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. Pharmacotherapy. 2013;33(5):529–548. | ||
Berra K, Fletcher B, Hayman LL, Miller NH. Global cardiovascular disease prevention: a call to action for nursing executive summary. J Cardiovasc Nurs. 2013;28(6):505–513. | ||
McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44(4):810–819. | ||
Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91:899–906. | ||
Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997;277(4):307–311. | ||
Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;27(4):301–306. | ||
Schneider PJ, Gift MG, Lee YP, Rothermich EA, Sill BE. Cost of medication-related problems at a university hospital. Am J Health Syst Pharm. 1995;52(21):2415–2418. | ||
Onder G, van der Cammen T, Petrovic M, Somers A, Rajkumar C. Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing. 2013;42:284–291. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

