Back to Journals » Clinical Ophthalmology » Volume 13
Patient-reported severity of dry eye and quality of life in diabetes
Authors Yazdani-Ibn-Taz MK, Han MM , Jonuscheit S, Collier A, Nally JE, Hagan S
Received 16 August 2018
Accepted for publication 14 November 2018
Published 25 January 2019 Volume 2019:13 Pages 217—224
DOI https://doi.org/10.2147/OPTH.S184173
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Md Kaosar Yazdani-Ibn-Taz,1 Myint Myint Han,1 Sven Jonuscheit,1 Andrew Collier,1,2 Jane E Nally,1 Suzanne Hagan1
1School of Health and Life Sciences, Glasgow Caledonian University (GCU), Glasgow G4 0BA, UK; 2Diabetes Day Centre, University Hospital Ayr, Ayr KA6 6DX, UK
Purpose: The purpose of the study was to assess the relationship between patient-reported severity of dry eye disease (DED), quality of life (QoL), presence of diabetic retinopathy (DR) and length of disease duration in people with type 1 diabetes mellitus (DM1) and type 2 diabetes mellitus (DM2).
Patients and methods: A survey of 152 people (110 with and 42 without diabetes). All participants completed the Ocular Surface Disease Index (OSDI) and Dry Eye-related Quality of Life Score (DEQS) questionnaires.
Results: Forty-four percent of all diabetic subjects reported dry eye symptoms, compared to 29% in the control group. Patients with DM2 reported dry eye symptoms more frequently than those with DM1 (55% and 27% respectively, P=0.001). Dry eye severity was linked to a significant deterioration in QoL in both types of diabetes (DM1, r=0.609 and P=0.036; DM2, r=0.417 and P=0.011). Irrespective of DR, the presence of DED was significantly higher in DM2 compared to DM1 (with DR, P=0.011; without DR, P=0.018).
Conclusion: Dry eye symptoms are associated with reduced QoL and are more common in people with DM2 than in DM1, irrespective of DR status. Routine clinical screening for severe DED could potentially allow for a timely and more effective treatment and could contribute to mitigating the dry eye-associated reduction in QoL in those with DM2.
Keywords: ocular surface, dry eye, symptoms, retinopathy
Introduction
Diabetes is one of the leading causes of morbidity and mortality worldwide.1 In 2017, 425 million people were diagnosed with diabetes globally, and this figure is expected to exceed 629 million by 2045.2 People with diabetes are more prone to suffer from dry eye disease (DED; either aqueous-deficient or evaporative) than those without diabetes.3–7 DED is a multifactorial disease associated with aging and autoimmune disease.8 Signs and symptoms may vary from patient to patient and include ocular discomfort, pain, blurred vision, corneal ulcers, and in severe cases, blindness.8,9 DED also has a significant impact on patient quality of life (QoL), including physical, social, and psychological; negatively affecting daily activities10–13 and workplace productivity.13,14 DED has a substantial economic impact as a result of these QoL effects.13
As DED has been reported as a symptom-based disease,15 we assessed the severity of patient-reported DED using the Ocular Surface Disease Index (OSDI) and the Dry Eye-related Quality of Life Score (DEQS) questionnaires. Previous studies have suggested there is an association between diabetes and DED,3–6,16 with a recent study suggesting a correlation between the prevalence of DED and glycemic control.17 To date, however, there have been no studies investigating the effects of diabetes-associated DED on patient QoL.
The aims of this study were to assess patient-reported severity of DED and its impact on QoL in people with type 1 and type 2 diabetes mellitus (DM1 and DM2), compared to healthy controls. A secondary aim was to investigate the possible association of DED and QoL with age, gender, duration of diabetes, presence of diabetic retinopathy (DR) status, diabetic maculopathy, diabetic neuropathy, and glycemic control (HbA1c).
Patients and methods
Study design and participants
This study was approved by the Ethics committee of Glasgow Caledonian University (no HLS/LS/A15/047) and was conducted in accordance with Declaration of Helsinki guidelines. Written informed consent was obtained from each subject involved in this project after a full explanation of the procedures involved. The study comprised 110 participants with diabetes (DM1, n=45; DM2, n=65) attending the Diabetes Clinic (University Hospital Ayr) and 42 controls. Patients with Graves’ disease, connective tissue disorders, and chronic kidney disease were excluded. Patient demographic characteristics (age and sex) and related medical information (ie, presence of DR, duration of diabetes, use of medications, etc) were obtained from SCI-Diabetes.
Data collection
Patient-reported dry eye symptoms and health-related QoL
Each participant was asked to complete two questionnaires: 1) the OSDI (Allergan, Inc, Irvine, CA, USA) for DED severity assessment,18 and 2) the DEQS (Santen Pharmaceutical Co. Ltd. and Dry Eye Society in Japan), to allow for assessment of QoL. Both the questionnaires provide a score of 0–100, with higher scores indicating greater symptom severity and greater reduction of QoL, respectively.18,19
OSDI
OSDI is the most commonly used DED questionnaire for clinical trials, measuring symptom frequency and severity. The OSDI questionnaire consists of 12 questions grouped into three sections: ocular symptoms, vision-related function, and environmental factors.18 It is designed to assess the patients’ symptoms18,20 and the impact of these symptoms on day-to-day life.18 This questionnaire has been accepted for use in US Food and Drug Administration clinical trials for DED.18,21,22 The OSDI represents DED symptoms of the previous 7 days and yields scores ranging from 0 (least symptoms) to 100 (worst symptoms). Each item is graded on a scale of 0–4, where 0 indicates none of the time; 1, some of the time; 2, half of the time; and 4, all of the time.18,23,24 DED severity is determined based on the score calculated with the OSDI formula, that is, the sum of the score is multiplied by 25 and then is divided by the number of questions answered.23 A score of 12 is considered the cutoff for normal, 13–22 for mild, 23–32 for moderate, and ≥33 for severe DED.18,23,24
DEQS
The DEQS is a self-evaluating method, which provides assessment of the effect of the symptoms of DED on QoL in general, including the mental health of the patient. The DEQS includes 15 questions and two sub-categories: impact on daily life and bothersome ocular symptoms. Each questionnaire was scored on a 4-point Likert scale ranging from 1 to 4, with a larger number indicating a greater burden. The final score is calculated using the DEQS formula, that is, multiplying the sum of the score by 25 and then dividing the total by the number of questions answered. The summary score for the DEQS ranges from 0 to 100, where the higher the score, the greater the disability. This questionnaire has been validated and is often used in clinical trials.19
Statistical analyses
IBM SPSS software (Version 24; IBM Corporation, Armonk, NY, USA) was used for all analyses. Descriptive statistics were generated. Frequency tables were used to assess DED severity. Pearson’s chi-squared test, Spearman’s correlation (r) test were used to determine any differences and association between variables. Scatter plots were generated to assess the impact of DED on patients’ QoL. A P-value of <0.05 was considered statistically significant.
Results
Participant demographics
The mean duration of diabetes was significantly longer for those with DM1 (22±13 years) than with DM2 (12±7 years; P<0.001). All subjects with diabetes had a significantly higher mean age (55±16 years) than the controls (43±9 years; P<0.001). Although there was a significant difference in the male:female ratio between DM1 and controls (P=0.002), the presence of more female participants did not influence DED presence in the controls (P=0.554). The demographic characteristics of the two diabetic groups and the controls are shown in Table 1.
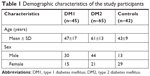  | Table 1 Demographic characteristics of the study participants |
Participant-reported DED
DED in the diabetes groups (OSDI)
DED symptoms were reported by 44% (n=48) of all subjects with diabetes. The presence of DED symptoms was significantly higher in those with DM2 compared to DM1 (P=0.001). Just over one quarter of all DM1 subjects reported DED symptoms (27%; n=12), while 55% of DM2 participants (n=36) reported DED symptoms (Table 2). Among DM1 participants with DED symptoms, five had mild, three had moderate, and four had severe DED symptoms (Table 2). Nearly half of those with DM2 (n=17) reported mild symptoms, eight had moderate, and eleven reported severe symptoms of DED. In the control group, 12 described DED symptoms, with eight having mild symptoms, two with moderate symptoms, and two with severe symptoms. For the mean OSDI score in patients with DED, no significant difference was found between DM2 and DM1 (P=0.198). Mean OSDI scores in patients with DED were, however, significantly higher in both DM2 (P=0.001) and DM1 (P=0.03), compared to controls.
Participant-reported QoL in DED (DEQS)
There was a positive association between DED severity (measured by OSDI) and impact on QoL (measured by DEQS) in participants with diabetes (r=0.487; P<0.001). That is, DED severity of both DM1 (r=0.609; P=0.036) and DM2 (r=0.417; P=0.011) were linked to a significant deterioration in QoL (Figures 1 and 2). For the participants with DM1 and DM2, DED equally affected their QoL; no significant difference was observed between the groups (P=0.346). For the mean DEQS score in those with DED, no significant difference was found between DM2 and DM1 (P=0.198). Likewise, in comparison with controls, the mean DEQS score in those with DED was not different for either DM2 (P=0.131) or DM1 (P=0.834) (Table 2).
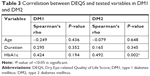
While more severe DED symptoms had a significantly worse impact on QoL in DM2 patients (P=0.013), no such impact was observed in those with DM1 (P=0.053). A significant positive correlation was also found between DEQS and HbA1c in DM2 (r=0.492; P=0.002), indicating that higher HbA1c levels were associated with worse QoL, specifically in DM2. By contrast, no significant association was found between DEQS and HbA1c in DM1 (Table 3).
DED and DR
For those with DM1 and DR, 28% (n=8) had DED, of whom 37.5% (n=3) reported mild, 25% (n=2) moderate, and 37.5% (n=3) reported severe DED symptoms. In those with DM2 and DR, 63% (n=22) suffered DED, of whom 45.5% (n=10) reported mild, 23% (n=5) moderate, and 32% (n=7) reported severe DED symptoms. DED presence was significantly higher in DM2 with DR (63%) than for DM1 with DR (28%; P=0.011). Among DM1 participants without DR, 25% (n=4) reported DED, of whom 50% (n=2) had mild, 25% (n=1) had moderate, and 25% (n=1) had severe symptoms. In DM2 without DR, 46.7% (n=14) had DED, of whom 50% (n=7) had mild, 21% (n=3) had moderate, and 29% (n=4) had severe symptoms. For people without DR, DED presence was significantly higher in DM2 compared to DM1 (P=0.018).
DED, diabetes complications, duration of diabetes, and other health indicators
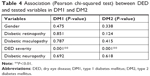
There was no association between DR, diabetic maculopathy, diabetic neuropathy, and DED. Similarly, no significant association was found between DED and age, gender, or glycemic control (P>0.05; Tables 4 and 5). For control subjects, DED was not associated with either age (P=0.676) or gender (P=0.554).
  | Table 5 Correlation between OSDI and tested variables in DM1 and DM2 |
Discussion
Patients with diabetes are known to have an increased prevalence of DED compared to healthy controls,25–27 and the impact of DED on patients’ QoL has been reported as similar to that observed for patients with angina, hip fractures, or those undergoing dialysis.28 Although the prevalence and association of DED with diabetes has been reported previously,3–6,25–27,29 this study demonstrated differences in the proportion of diabetic patients affected by DED and its impact on patients’ QoL, depending on diabetes type. The overall DED presence in the current study (44%) was comparable to previous generic diabetes studies, that is, studies where no distinction was made between DM1 and DM2 patients. These include Fuerst et al,3 Hom and De Land,26 and Ogundo et al27 who reported DED prevalence among diabetics of 52%, 52.9% and 49.8%, respectively, but was higher than that the value of 20.6% reported by Kaiserman et al.5 Our findings differ from that of Kaiserman et al5 as they determined DED by ocular lubrication use; this difference might be because 1) patients from relatively poorer economic backgrounds are less likely to purchase ocular lubricants (despite their need), or 2) through lack of awareness of using ocular lubricants, due to decreased corneal sensitivity that commonly occurs in those with diabetes.5
In the current study, there was a significant association between DED and DM2 (P=0.003) as well as DED and DM1 (P=0.003), which corresponds with earlier studies for DM217,30,31 and for DM1.16 Of particular interest was our finding that there was a higher DED prevalence in those with DM2 (56% by OSDI) than for DM1 (27%). In our study, the prevalence of DM2-associated DED was comparable to other reports on DM2 cohorts, such as Manaviat et al30 (54.3%; determined by tear break-up time [TBUT] and Schirmer test) and Rathnakumar et al31 (53%, TBUT and Schirmer test), but was higher than that reported by Najafi et al4,32 (27.7%, as determined by tear osmolarity). This difference between our findings and Najafi et al4 might be due to the fact that in long-standing diabetes, patients with higher tear osmolarity often experience fewer dry eye symptoms.3 Other studies have shown that DM2 is significantly associated with DED.33,34 The only other study we could find that compared DED in both the diabetes types reported an overall DED prevalence of 52.8% across both the types, with 57% in DM1 and 70% in DM2 patients.16 Between studies, the differences in DED presence for DM2 may be due to the DED definition used, the study populations, and the diagnostic methods, that is, objective vs subjective means. It is unclear why there was a significant difference in DED presence between the two types. The possibility exists that it reflects their different underlying pathophysiologies.
Nevertheless, we have shown that DED is an important clinical entity, which can be found in a large proportion of people with DM2. Interestingly, in our study, DED presence in DM1 (27%) was similar to the controls (29%). Furthermore, our study provides novel information by comparing DED presence and severity in relation to QoL in both DM1 and DM2, using patient-reported outcomes. A strong positive association was observed (P<0.001) between DED severity and impact on QoL of the respondents in DM (both types), irrespective of their DR status.
We did not observe a correlation between DED and other demographic data, such as age, sex, duration of diabetes, or HbA1c. There have been previous reports that suggested that DED is more prevalent in the elderly,7,29,35–37 female sex,4,7,23,27,35–37 higher HbA1c levels,5,17,29 diabetes duration,3,30 DR,3,4 diabetic maculopathy,4,30 and DN.38 However, other studies similarly reported that DED has no correlation with age and gender,3,30 HBA1c value,3,30,39 diabetes duration,4 or DN.4 The lack of correlation may be because of reduced corneal sensitivity with increased severity of DR4,40 or DN41,42 or increasing age36,43–45 and reduced corneal sensitivity cannot be measured by subjective tests.46 Moreover, HbA1c only reflects blood glucose level over the past 3 months, while loss of corneal sensitivity can occur as a result of long-term hyperglycemia.3,40 Therefore, HbA1c levels might not be associated with decreased corneal sensation, which can impact the perception of DED symptoms.3
This study is not without limitations. First, using subjective (patient-reported) methods in diagnosing DED could have created a biased result, due to inter-observer differences in reporting. In this study, there are several reasons for and benefits in using the OSDI and DEQS questionnaires. The OSDI is regarded as a valid and reliable tool for measuring DED presence and severity in research or clinical practice. Most importantly, it has defined cutoff values that can effectively evaluate DED presence and its severity.3,13,15,18,24 It should be noted that clinical DED diagnosis is regarded as difficult at the best of times, due to discrepancies between signs and symptoms.47–49 Furthermore, relatively objective tests such as TBUT, Schirmer test, and lissamine green staining have shown a poor correlation with clinical signs.50,51 Previous population-based studies have assessed the prevalence of DED by symptoms alone50–52 and DED symptom assessment has been commonly reported as the foremost diagnostic tool by both optometrists and ophthalmologists.52–54 A recent cross-sectional survey of optometrists and ophthalmologists in New Zealand reported that 90% of respondents ranked patient symptoms as the most useful diagnostic tool for DED.55 The DEQS is also a validated and reliable method used in routine clinical practice to assess the various effects of DED on the patient’s day-to-day activities.19
Second, this was a cross-sectional study and not a longitudinal one; it was therefore difficult to determine whether DED preceded or followed the diagnosis of diabetes. Thus, it was not possible to state that DED was solely a consequence of diabetes. Other risk factors, such as use of medications (which may cause tear hyposecretion, such as antihistamines, tricyclic antidepressants, or oral contraceptives), contact lens usage, and laser therapy, should be considered as exclusion criteria for future studies. Other limitations of this study were age and sex differences between study participants and controls and the relatively small patient sample cohorts. This is likely to be addressed in future studies. Despite these limitations, there is strong evidence that the study demonstrated a significant association between DED and DM as well as DED and both DM1 and DM2, while DED severity was linked to a significantly reduced QoL for both diabetes types. We also report that the severity of dry eye in those with or without DR is significantly higher in DM2 than in DM1.
To date, few studies have directly compared DED in DM1 and DM2, which have different underlying etio-pathogeneses. Nor have there been any reports on the effects that diabetes-associated DED may have on overall patient QoL. Despite the fact that DED is much more prevalent in diabetes, it is often overlooked in this patient demographic due to the more pressing concerns of DR monitoring. As DED is more prevalent in DM2, adding DED assessment to DR screening could be beneficial in people with DM2. This additional screening might incur an extra cost which need to be evaluated in future studies. However, medical cost for DED treatment can outweigh the loss of productivity and may produce economic benefits.14 Not all patients with diabetic eye disease will have DED, but those with DED tend to experience lower QoL. Thus, improving the QoL of the patients (through relieving the symptoms, improvement of visual acuity, restoration of ocular surface and tear film, and correction of underlying defects) should be the primary aim for DED treatment as it has a wider physical, social, psychological, and economic impact on both individuals and society as a whole.9,13,14
Conclusion
Clinical examination for DED could be beneficial in people with diabetes and should be routinely included when assessing patients for diabetic eye disease, especially for those with DM2.
Acknowledgments
The authors would like to thank the staff at Diabetes Day Center of the University Hospital Ayr for their support, the study participants for taking part in this study, Professor Jon Godwin of GCU and Dr Angus McFadyen of akm-stats.com for their guidance in statistics.
Disclosure
The authors report no conflicts of interest in this work.
References
Papatheodorou K, Banach M, Edmonds M, Papanas N, Papazoglou D. Complications of diabetes. J Diabetes Res. 2015;2015(5):189525. | ||
International Diabetes Federation. IDF Diabetes Atlas – 8th ed; 2017. http://www.diabetesatlas.org/resources/2017-atlas.html. Accessed January 4, 2019. | ||
Fuerst N, Langelier N, Massaro-Giordano M, et al. Tear osmolarity and dry eye symptoms in diabetics. Clin Ophthalmol. 2014;8:507. | ||
Najafi L, Malek M, Valojerdi AE, et al. Dry eye and its correlation to diabetes microvascular complications in people with type 2 diabetes mellitus. J Diabetes Complications. 2013;27(5):459–462. | ||
Kaiserman I, Kaiserman N, Nakar S, Vinker S. Dry eye in diabetic patients. Am J Ophthalmol. 2005;139(3):498–503. | ||
Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes. 2015;6(1):92–108. | ||
Lemp MA. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop (2007). Ocular Surf. 2007;5:75–92. | ||
Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Ärztebl Int. 2015;112:71–82. | ||
Kaštelan S, Tomić M, Salopek-Rabatić J, Novak B. Diagnostic procedures and management of dry eye. Biomed Res Int. 2013;2013:309723. | ||
van Tilborg MM, Murphy PJ, Evans KS. Impact of dry eye symptoms and daily activities in a modern office. Optom Vis Sci. 2017;94(6):688–693. | ||
Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of Meibomian gland dysfunction. Invest Opthalmol Visual Sci. 2011;52(4):2050–2064. | ||
Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–415. | ||
Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1(2):51–57. | ||
Yamada M, Mizuno Y, Shigeyasu C. Impact of dry eye on work productivity. Clinicoecon Outcomes Res. 2012;4:307–312. | ||
Nichols KK. Patient-reported symptoms in dry dye disease. Ocul Surf. 2006;4(3):137–145. | ||
Seifart U, Strempel I. The dry eye and diabetes mellitus. Ophthalmologe. 1994;91(2):235–239. | ||
Zou X, Lu L, Xu Y, et al. Prevalence and clinical characteristics of dry eye disease in community-based type 2 diabetic patients: the Beixinjing eye study. BMC Ophthalmol. 2018;18(1):117. | ||
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. | ||
Sakane Y, Yamaguchi M, Yokoi N, et al. Development and validation of the dry eye-related quality-of-life score questionnaire. JAMA Ophthalmol. 2013;131(10):1331–1338. | ||
Smith JA. The epidemiology of dry eye disease: report of the epidemiology subcommittee of the international dry eye workshop (2007). Ocular Surf. 2007;5(2):93–107. | ||
Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107(4):631–639. | ||
Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin a ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease. Ophthalmology. 2000;107(5):967–974. | ||
Grubbs JR, Tolleson-Rinehart S, Huynh K, Davis RM. A review of quality of life measures in dry eye questionnaires. Cornea. 2014;33(2):215–218. | ||
Miller KL, Mink DR, Mathias SD, Walt JG. Multiple methods to estimate the minimal clinically important difference of the Ocular Surface Disease Index®. Value Health. 2007;10(3):A146. | ||
Demill DL, Hussain M, Pop-Busui R. Ocular surface disease in patients with diabetic peripheral neuropathy. Br J Ophthalmol. 2016;100(7):924–928. | ||
Hom M, De Land P. Self-reported dry eyes and diabetic history. Optometry. 2006;77(11):554–558. | ||
Ogundo C, Ilako D, Maina J. Prevalence of dry eye syndrome in diabetic patients attending Kenyatta National Hospital, Kenya. JOECSA. 2015;19:63–68. | ||
Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412–1419. | ||
Zhang X, Zhao L, Deng S, Sun X, Wang N. Dry eye syndrome in patients with diabetes mellitus: prevalence, etiology, and clinical characteristics. J Ophthalmol. 2016;2016(2):1–7. | ||
Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008;8(1):10. | ||
Rathnakumar K, Ramachandran K, Ramesh V. Prevalence of dry eye disease in Type 2 diabetic patients and its association with retinopathy. Int J Pharm Sci Res. 2017;8(10):4298–4304. | ||
Najafi L, Malek M, Valojerdi AE, Khamseh ME, Aghaei H. Dry eye disease in type 2 diabetes mellitus; comparison of the tear osmolarity test with other common diagnostic tests: a diagnostic accuracy study using STARD standard. J Diabetes Metab Disord. 2015;14(1):39. | ||
Baek J, Doh SH, Chung SK. Assessment of the tear meniscus using optical coherence tomography in patients with type 2 diabetes mellitus. Cornea. 2015;34(12):1534–1540. | ||
Ma A, Mak MS, Shih KC. Association of long-term glycaemic control on tear break-up times and dry eye symptoms in Chinese patients with type 2 diabetes. Clin Exp Ophthalmol. 2018;46(6):608–615. | ||
Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. | ||
Sharma A, Hindman HB. Aging: a predisposition to dry eyes. J Ophthalmol. 2014;2014(1):1–8. | ||
Shah S, Jani H. Prevalence and associated factors of dry eye: our experience in patients above 40 years of age at a tertiary care center. Oman J Ophthalmol. 2015;8(3):151–156. | ||
Achtsidis V, Eleftheriadou I, Kozanidou E, et al. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care. 2014;37(10):e210–e211. | ||
Schein OD, Muñoz B, Tielsch JM, Bandeen-Roche K, West S, et al. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124(6):723–728. | ||
Gao Y, Zhang Y, Ru YS, et al. Ocular surface changes in type II diabetic patients with proliferative diabetic retinopathy. Int J Ophthalmol. 2015;8(2):358. | ||
Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Malik RA. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care. 2007;30(7):1895–1897. | ||
Misra SL, Braatvedt GD, Patel DV. Impact of diabetes mellitus on the ocular surface: a review. Clin Exp Ophthalmol. 2016;44(4):278–288. | ||
Li J, Zheng K, Deng Z, et al. Prevalence and risk factors of dry eye disease among a hospital-based population in southeast China. Eye Contact Lens. 2015;41(1):44–50. | ||
Murphy PJ, Patel S, Kong N, Ryder REJ, Marshall J. Noninvasive assessment of corneal sensitivity in young and elderly diabetic and nondiabetic subjects. Investigative Opthalmology & Visual Science. 2004;45(6):1737–1742. | ||
Roszkowska AM, Colosi P, Ferreri FM, Galasso S. Age-related modifications of corneal sensitivity. Ophthalmologica. 2004;218(5):350–355. | ||
Hashemi H, Khabazkhoob M, Kheirkhah A, et al. Prevalence of dry eye syndrome in an adult population. Clin Exp Ophthalmol. 2014;42(3):242–248. | ||
Begley CG, Chalmers RL, Abetz L, et al. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Investigative Opthalmology & Visual Science. 2003;44(11):4753–4761. | ||
Jie Y, Xu L, Wu YY, Jonas JB. Prevalence of dry eye among adult Chinese in the Beijing eye study. Eye. 2009;23(3):688–693. | ||
Ong ES, Felix ER, Levitt RC, Feuer WJ, Sarantopoulos CD, Galor A. Epidemiology of discordance between symptoms and signs of dry eye. Br J Ophthalmol. 2018;102(5):674–679. | ||
Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: the shihpai eye study. Ophthalmology. 2003;110(6):1096–1101. | ||
Wang Y, Lv H, Liu Y, et al. Characteristics of symptoms experienced by persons with dry eye disease while driving in China. Eye. 2017;31(11):1550–1555. | ||
Lee AJ, Lee J, Saw SM, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002;86(12):1347–1351. | ||
Graham JE, McGilligan VE, Berrar D, et al. Attitudes towards diagnostic tests and therapies for dry eye disease. Ophthalmic Res. 2010;43(1):11–17. | ||
Downie LE, Rumney N, Gad A, Keller PR, Purslow C, Vingrys AJ. Comparing self-reported optometric dry eye clinical practices in Australia and the United Kingdom: is there scope for practice improvement? Ophthalmic Physiol Opt. 2016;36(2):140–151. | ||
Xue AL, Downie LE, Ormonde SE, Craig JP. A comparison of the self-reported dry eye practices of New Zealand optometrists and ophthalmologists. Ophthalmic Physiol Opt. 2017;37(2):191–201. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.