Back to Journals » Patient Related Outcome Measures » Volume 9
Patient-reported outcomes in head and neck cancer: prospective multi-institutional patient-reported toxicity
Authors Peach MS , Trifiletti DM , Vachani C, Arnold-Korzeniowski K, Bach C, Hampshire M, Metz JM, Hill-Kayser CE
Received 12 October 2017
Accepted for publication 12 April 2018
Published 27 July 2018 Volume 2018:9 Pages 245—252
DOI https://doi.org/10.2147/PROM.S153919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Robert Howland
M Sean Peach,1 Daniel M Trifiletti,2 Carolyn Vachani,3 Karen Arnold-Korzeniowski,3 Christina Bach,3 Margaret Hampshire,3 James M Metz,3 Christine E Hill-Kayser3
1Department of Radiation Oncology, University of Virginia, Charlottesville, VA, USA; 2Department of Radiation Oncology, Mayo Clinic, Jacksonville, FL, USA; 3Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA, USA
Purpose: Head and neck cancer is occurring in an increasingly younger patient population, with treatment toxicity that can cause significant morbidity. Using a patient guided, Internet-based survivorship care plan program, we obtained and looked at patterns of patient-reported outcomes data from survivors seeking information after treatment for head and neck cancer.
Methods: The Internet-based OncoLife and LIVESTRONG Care Plan programs were employed, which design unique survivorship care plans based on patient-reported data. Care plans created for survivors of head and neck cancer were used in this evaluation. Demographics, treatment modality, and toxicity were included in this evaluation. Toxicity was further analyzed, grouped into system-based subsets.
Results: A total of 602 care plans were created from self-identified head and neck cancer survivors, from which patient-reported outcome data were attained. A majority of patients were Caucasian (96.2%) with median age at diagnosis of 55 years, living in suburban locations (39.9%), with ~50% receiving care within 20 miles of their residence. There was an equal distribution of education levels from high school only to graduate school. The majority of patients received care through cancer centers (96.7%), with a split between academic and non-academic centers. Ninety-three percent of patients had radiation therapy as part of their treatment modality, with 70.3% having chemotherapy and 60.1% having surgery. The most common system toxicities affected the oropharynx, followed by epithelium (skin/hair/nail), and then general global health. Specifically, the most common side effects were difficulty swallowing (61.5%) and changes in skin color/texture (49.7%). One third of patients experienced hearing/tinnitus/vertigo, xerostomia, loss of tissue flexibility, or fatigue.
Conclusion: The current work demonstrates the ability to obtain patient-reported outcomes of head and neck cancer survivors through an Internet-based survivorship care plan program. For this group dysphagia and dermatitis were the most commonly reported toxicities, as was expected; however, global effects of therapy, such as fatigue, were also significant and should be addressed in future survivorship planning.
Keywords: head and neck radiation, surgery, chemotherapy, patient reported outcomes, survivorship care plan, Internet, patient-reported outcomes
Introduction
Head and neck cancer represents the 6th most common cancer group in the world and comprises a diverse collection of disease sites, histology, and pathogenesis.1 While the incidence of tobacco-related head and neck cancer has decreased over recent decades, cases related to human papillomavirus (oropharyngeal cancer) have increased.3 Significant progress has recently been made in disease management, with a significant portion of patients cured with current practice. This management is often multimodal and not infrequently trimodal, involving surgery, chemotherapy, and radiation therapy. Given the multiple organs/systems in the head and neck region, including the constrictor muscles involved in swallowing, neck muscles, salivary glands, taste receptors, and cranial nerves, head and neck cancer survivors often face substantial late and long-term effects of the disease and its treatments.4 Further, p16-positive oropharyngeal patients may develop disease at a younger age,3 thus creating a population of cancer survivors that will live with potentially significant treatment-related morbidity for decades. The potential severity of toxicity in head and neck cancer survivors highlights the importance of survivorship, defined by the National Cancer Institute as the “focus on the health and life of a person with cancer post treatment until the end of life”.5 A crucial part of general survivorship care is the need for survivorship care plans (SCPs), defined as “comprehensive care summaries and follow-up plans that are clearly and effectively explained” and that include diagnosis/treatment-specific information regarding potential adverse effects, interventions, oncologic/primary follow-up, legal information, and availability of psychosocial services.6
Survivorship and SCPs are guided by an understanding of the types and extent of toxicity expected for specific disease sites and treatment modalities. While these data points are frequently obtained in follow-up and more broadly from clinical trials/studies, there is often discordance between objective physician measurements and the more subjective experiences of the patient.7,8 Further, physician interpretation of patients’ subjective experience often underreports the breadth and intensity of these experiences. For this reason there has been greater emphasis placed on patient-reported outcomes (PROs),7 a concept first emphasized by the US Food and Drug Administration in 2006 and described as “self-report of disease and/or treatment effects in clinical research”. Beyond their value in detailing survivorship, PROs have been shown to be prognostic for survival and correlate with tumor response. Combining PRO information with physician observational endpoints therefore provides a more complete picture of survivorship that can enhance the understanding of the outcomes from different modalities of therapy, and help contribute to management of post-treatment toxicity in SCPs.
In this study, anonymous patients throughout the world utilized an Internet-based program to create SCPs (available at www.OncoLink.org). Longitudinal PROs were obtained from patients who self-identified as head and neck cancer survivors when they created care plans. Items queried in this process included treatment types, toxicity, symptoms, and development of other disease. These characteristics, generated from the survivorship plans of over 600 patients, were compared against delivered treatment modality to determine any patterns of patient-reported toxicity associated with treatment type.
Methods
OncoLink is an online cancer website designed and established at the University of Pennsylvania in 1994, and with survivorship care planning tools included on the site since 2007. Briefly, patients voluntarily using these tools are asked to anonymously provide cancer type, treatments received, and current symptoms which are used to generate customized survivorship care plans. During the self-directed inquiry regarding symptom experiences, questions are created based on previously completed responses with regard to diagnosis and treatment modalities. This logistical strategy allows targeted questioning about late effects that are most likely to be significant to a given patient. Data input is not limited to cancer survivors but was also obtained from relatives, friends, and members of the treatment team including health care professionals creating care plans for patients. The details of how SCPs are created have been previously described.9
Using the care plan databases, all patients identifying as survivors of head and neck cancer were selected for analysis as part of this study. In 2011, an expanded number of PRO questions in regard to long-term toxicity were added to the care plan tool, and data collection for this study began at that time. Collectively, demographics, treatment, and toxicity information gleaned from SCP data were included in this analysis. Patients were surveyed for a diverse collection of post-treatment toxicity. Questions for each particular side effect were answered in a multiple choice format with the options of “yes”, “no”, and “I don’t know”. Only the questions with at least ten individuals responding “yes” were included in the data collection to decrease sampling error. Only the information obtained from cancer survivor, family member, or friend survey responders was used in our analysis. Physician-obtained diagnoses or physician interpretation was not included. The toxicity was grouped into systems to see if there were any unexpected side effect trends.
All data acquisition and research was carried out under an institutional review board-approved protocol at the University of Pennsylvania. Entries were screened by user IP address and data so that duplicate entries could be identified and removed from the data set.
Results
A total of 602 care plans were created from patients self-identified as head and neck survivors out of a total of 17,128 plans created between October 2011 and December 2016. The sample population was mostly Caucasian (86.2%) and predominantly male (65.4%) (Table 1). The level of education was well divided, with a majority (39.9%) residing in suburban locations. Only 7.3% of patients had care directed primarily through a private physician practice, while the remainder of survivors had care split between academic and non-academic cancer centers. Approximately 50% of patients’ care was received within 20 miles of where they lived. Only 10.5% of survivors were previously offered survivorship information.
  | Table 1 Head and neck cancer survivor survivorship care plan demographic information Note: Percentages may not sum to 100.0% because of rounding. |
Nearly all patients (93%) had radiation therapy as part of their care, while 60.1% and 70.3% had surgery and chemotherapy, respectively (Table 2). Most survivors underwent multimodal care, with trimodal therapy representing 35% of queried survivors and chemoradiation 34%. Surgery was combined with only chemotherapy or only radiation therapy in 1% and 19% of survivors, respectively. Only 5% of survivors were treated with surgery alone, 5% radiation therapy alone, and <1% chemotherapy alone.
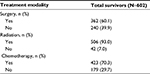  | Table 2 Head and neck cancer survivor survivorship care plan treatment modality information Note: Percentages may not sum to 100.0% because of rounding. |
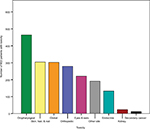
Overall, long-term side effects and the development of treatment-related late effects involved multiple systems. When late and long-term effects were grouped into systems, the majority of reported toxicity was oropharyngeal, followed in decreasing order morbidities within the following categories: epithelial (skin/hair/nail), overall global health (such as fatigue), orthopedic, eyes/ears, endocrine, kidney, and lastly secondary cancers (Figure 1). Specifically, the most commonly reported toxicities were difficulty swallowing (61.5%), and change in skin color/texture (49.7%) (Table 3). Approximately one third of those queried reported decreased hearing/tinnitus/vertigo, xerostomia, loss of flexibility in irradiated region, and fatigue (defined as overwhelming physical, mental, or emotional exhaustion). Slightly less common were dental problems (26.7%) and thyroid disease, with 20.3% of survivors diagnosed with hypo- or hyperthyroidism. One of four patients (25%) was concerned about cognitive changes, such as memory loss, difficulty with short-term memory, concentration, or learning new skills. The remaining side effects/newly diagnosed conditions were experienced by less than 20% of the cohort.
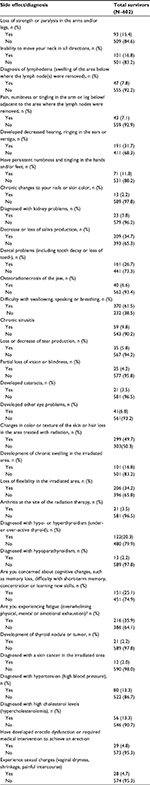  | Table 3 Head and neck cancer survivor survivorship care plan toxicity information Note: Percentages may not sum to 100.0% because of rounding. |
Discussion
The group of head and neck cancer survivors living in the USA is expected to continue to increase: disease incidence is increasing, disease demographics are changing with younger age at diagnosis more common, and cure rates continue to increase. Further, with advances in immunotherapy for head and neck cancer,10 those patients with incurable disease at presentation or recurrence after multimodal therapy may have viable treatment options which will increase overall survival.
With an anticipated increase in cancer survivorship for head and neck cancer, understanding the true impact of therapies has great significance for both the cancer survivor and the newly diagnosed patient. First, greater understanding of the full array and magnitude of toxicity from each treatment modality will allow for more informed decisions for the newly diagnosed patient. For the cancer survivor, this enhanced understanding will be important in shaping new toxicity interventions and post-treatment care, including the SCP. In this regard, PROs will become an essential endpoint, as physicians are only able to capture a limited picture of the cancer survivor experience. This has been demonstrated in more recent prospective cancer trials of multiple sites in which there was significant discordance in patient-reported symptoms and physician interpretation, with physicians most frequently underestimating severity7,11 and breadth of toxicity.12 Regarding head and neck cancer specifically, two studies have observed this relationship for patients treated with chemoradiation.13,14 Most problematic is that the breakdown of those items that have the most discordance between patients and physicians highlights symptoms that have the greatest impact on quality of life.15 Here, we demonstrate that PRO data can be gathered efficiently and from a large number of patients using an Internet-based tool.
Having a better understanding of the patient experience has been shown to enhance physician outlook. For example, one prospective work demonstrated increased concordance with interpretation of lung cancer patient toxicity when PRO was available to the physician verses when not.16 For head and neck cancer in particular, the incorporation of PRO has been appreciated by physicians to enhance their assessment of late symptoms.17 However, knowledge is only one aspect of survivorship, as an actionable plan to maximize post-treatment quality of life and function is the end goal. On the surface SCPs appear a natural offshoot of normal post-oncological treatment follow-up. There are many resources available such as the National Comprehensive Cancer Network clinical practice guidelines that provide advice on post-treatment management such as disease surveillance intervals and screening modalities;18 however, such guidelines do not emphasize toxicity management or psychosocial aspects of survivorship. This is where incorporation of PROs into SCPs can lead to follow-up plans tailored to the survivor.
For the typical oncologic physician there are several practical and logistical barriers to creating comprehensive SCPs for patients which encompass all aspects of survivorship. This was demonstrated by a study that looked at the value and feasibility of a computer-based head and neck cancer PRO and SCP system similar to what is offered by OncoLink.19 Physicians enrolled in the study indicated that it was difficult to develop SCPs on their own due to difficulty detecting symptoms, lack of patients’ perception for the need of supportive care, and lack of time. Given these constraints, it is not surprising that in the current work only 10.5% of patients obtained survivorship plans, a similarly small percentage was found in other works.20 Therefore, more widespread use of programs such as that offered by OncoLink has great potential to expand tailored SCPs for patients in what is a busy clinic environment, using meaningful endpoints to develop these plans from PROs.
Since the emphasis of PROs’ importance in the mid-1990s, they have been incorporated into research, the first large head and neck study utilizing such endpoints in 1999 for combination chemoradiation.21 However, compared to other disease sites the number and breadth has been small in comparison. Most methods for gathering PROs encompass common quality of life surveys or symptom questionnaires that focus on most commonly faced head and neck toxicity such as mucositis, dysphagia, and voice quality. For example, studies have incorporated the MD Anderson Symptom Inventory - Head and Neck Module,23 the MD Anderson Dysphagia Inventory,24 and the European Organisation for Research and Treatment of Head & Neck35 (H&N35) quality of life module27 among others.14,22,25,26,28,29,30 However, using such modules and not obtaining broader PRO may lead to investigators and physicians missing toxicity that may significantly impact quality of life and even survival. For example, utilizing OncoLink PRO of lung cancer patients, it was determined that neurocognitive and musculoskeletal side effects caused a high incidence of morbidity, something that physicians did not focus on for this particular disease site.31 In the study reported here, survivors reported some toxicities, such as dysphagia, that might have been anticipated by providers, but reported others, such as fatigue, that are often minimized by health care providers despite significant impact on quality of life.
Limitations
While there is significant strength to the collection of PROs and incorporation into SCPs, there are limitations to the current implementation in OncoLink and limitations to PROs themselves. First, the study uses a convenience sample frame, and patients are asked to provide relatively limited information regarding treatments that they received. Bias may exist due to the sampling method, although this will be reduced by the large sample size. Although specific patient treatment details are not available, the information garnered from this large population will inform clinicians caring for any cancer patient regarding areas of toxicity that may impact patients after head and neck cancer treatment and warrant up-front attention and, potentially, discussion.32
Another limitation from the current OncoLink implementation is that most of the questions are binary in that symptoms are not qualified but rather, responses are “yes”, “no”, or “I don’t know”. Thus, there could be minor symptoms shared by most survivors that do not significantly impact quality of life, but these will be overrepresented compared to less frequent but more severe symptoms in the generated SCPs. In the future, a scaling to symptoms would add a significant extra dimension to PROs in this system. Again, however, these early data may draw attention to areas of potential late toxicity.
Regarding the participating population, there is a concern that those responders are more technologically advanced, which may make such Internet-based PROs and SCPs unrepresentative of the general population. However, older proportions of the population each year have increasing access to technology. Further, the age of p16-positive head and neck cancer patients is younger than historic head and neck cancer patients. There are also other potential demographics other than age that may lead to bias toward participation. For example, there were barriers to technology based on socioeconomic status, race, and culture. However, with the expanding use of and access to smart technology such as cell phones this is increasingly of less concern.
Another limitation of the current study is that extensive data do not exist to show the efficacy of PRO-based SCPs. A small prospective single institution study was recently performed using digitized PROs during and after head and neck cancer radiotherapy.33 The physicians involved in the study felt that the addition of PROs improved follow-up care and improved communication with the patient and treatment team, essentially resulting in an enhanced SCP. Another study demonstrated that PROs in the form of subjective dysphagia measures were predictive of internal lymphedema,34 a symptom that normally requires endoscopic evaluation to diagnose. This would support weighting of subjective dysphagia in SCPs as a predictor of internal lymphedema. Similarly, PRO data in regard to social effects of post-treatment jaw function precluded decreased maximal interincisal opening distances,35 thus allowing an earlier intervention against trismus to prevent what can be a significantly morbid and permanent condition. Lastly, when PRO data were compared to objective assessments for patients undergoing oropharyngeal radiation therapy, it was determined that patients interpreted xerostomia as dysphagia, thus allowing more appropriate intervention.
Conclusion
These data demonstrate the feasibility of improving understanding of PROs after treatment for cancers of the head and neck using an Internet-based tool for survivorship planning. The population of head and neck cancer survivors is increasing in size, and is a group that remains at high risk for significant late effects that may impact speech, swallowing, and nutrition. Our study demonstrates the high incidence of many late effects in a large group of survivors: these include local toxicities, such as dysphagia, but also toxicities such as fatigue and thyroid disease that impact multiple organ systems. The majority of these late effects have potential to significantly impact quality of life, and many may be treatable or even preventable. At minimum, PROs should impact patient counseling. The large number of late effects reported by head and neck cancer survivors supports the need for extensive survivorship care planning for this patient population.
Disclosure
The authors report no conflicts of interest in this work.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. | ||
Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110(7):1429–1435. | ||
Young D, Xiao CC, Murphy B, et al. Increase in head and neck cancer in younger patients due to human papillomavirus (HPV). Oral Oncol. 2015;51(8):727–730. | ||
Rathod S, Livergant J, Klein J, Witterick I, Ringash J. A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral Oncol. 2015;51(10):888–900. | ||
cancer.gov [homepage on the Internet]. National Cancer Institute, NCI Dictionary of Cancer Terms. NCI. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=445089, Accessed May 3, 2018. | ||
Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. | ||
Di Maio M, Gallo C, Leighl NB, et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol. 2015;33(8):910–915. | ||
Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol. 2006;7(11):903–909. | ||
Hill-Kayser CE, Vachani C, Hampshire MK, Jacobs LA, Metz JM. An internet tool for creation of cancer survivorship care plans for survivors and health care providers: design, implementation, use and user satisfaction. J Med Internet Res. 2009;11(3):e39. | ||
Moskovitz JM, Moy J, Seiwert TY, Ferris RL. Immunotherapy for head and neck squamous cell carcinoma: a review of current and emerging therapeutic options. Oncologist. 2017;22(6):680–693. | ||
Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Supportive Care Cancer. 2016;24(8):3669–3676. | ||
Petersen MA, Larsen H, Pedersen L, Sonne N, Groenvold M. Assessing health-related quality of life in palliative care: comparing patient and physician assessments. Eur J Cancer. 2006;42(8):1159–1166. | ||
Falchook AD, Green R, Knowles ME, et al. Comparison of patient- and practitioner-reported toxic effects associated with chemoradiotherapy for head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2016;142(6):517–523. | ||
Vainshtein JM, Griffith KA, Feng FY, et al. Patient-reported voice and speech outcomes after whole-neck intensity modulated radiation therapy and chemotherapy for oropharyngeal cancer: prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2014;89(5):973–980. | ||
Chandwani KD, Zhao F, Morrow GR, et al. Lack of patient-clinician concordance in cancer patients: its relation with patient variables. J Pain Symptom Manage. 2017;53(6):988–998. | ||
Basch E, Wood WA, Schrag D, et al. Feasibility and clinical impact of sharing patient-reported symptom toxicities and performance status with clinical investigators during a phase 2 cancer treatment trial. Clin Trials. 2016;13(3):331–337. | ||
Kjaer T, Dalton SO, Andersen E, et al. A controlled study of use of patient-reported outcomes to improve assessment of late effects after treatment for head-and-neck cancer. Radiother Oncol. 2016;119(2):221–228. | ||
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines Head and Neck Cancers, version 1.2015. National Comprehensive Cancer Network; 2015. Available from: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed May 3, 2018. | ||
Duman-Lubberding S, van Uden-Kraan CF, Peek N, et al. An eHealth application in head and neck cancer survivorship care: health care professionals’ perspectives. J Med Internet Res. 2015;17(10):e235. | ||
Simone CB 2nd, Vapiwala N, Hampshire MK, Metz JM. Internet-based survey evaluating use of pain medications and attitudes of radiation oncology patients toward pain intervention. Int J Radiat Oncol Biol Phys. 2008;72(1):127–133. | ||
List MA, Siston A, Haraf D, et al. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: a prospective examination. J Clin Oncol. 1999;17(3):1020–1028. | ||
Franco P, Martini S, Di Muzio J, et al. Prospective assessment of oral mucositis and its impact on quality of life and patient-reported outcomes during radiotherapy for head and neck cancer. Med Oncol. 2017;34(5):81. | ||
Wong AT, Lai SY, Gunn GB, et al. Symptom burden and dysphagia associated with osteoradionecrosis in long-term oropharynx cancer survivors: A cohort analysis. Oral Oncol. 2017;66:75–80. | ||
Goepfert RP, Lewin JS, Barrow MP, et al. Predicting two-year longitudinal MD Anderson Dysphagia Inventory outcomes after intensity modulated radiotherapy for locoregionally advanced oropharyngeal carcinoma. Laryngoscope. 2017;127(4):842–848. | ||
Jansen F, Witte BI, van Uden-Kraan CF, et al. The need for supportive care among head and neck cancer patients: psychometric assessment of the Dutch version of the Supportive Care Needs Survey Short-Form (SCNS-SF34) and the newly developed head and neck cancer module (SCNS-HNC). Support Care Cancer. 2016;24(11):4639–4649. | ||
Rinkel RN, Verdonck-de Leeuw IM, Doornaert P, et al. Prevalence of swallowing and speech problems in daily life after chemoradiation for head and neck cancer based on cut-off scores of the patient-reported outcome measures SWAL-QOL and SHI. Eur Arch Otorhinolaryngol. 2016;273(7):1849–1855. | ||
Karlsson T, Johansson M, Andrell P, Finizia C. Effects of voice rehabilitation on health-related quality of life, communication and voice in laryngeal cancer patients treated with radiotherapy: a randomised controlled trial. Acta Oncol. 2015;54(7):1017–1024. | ||
Macann A, Fua T, Milross CG, et al. Phase 3 trial of domiciliary humidification to mitigate acute mucosal toxicity during radiation therapy for head-and-neck cancer: first report of Trans Tasman Radiation Oncology Group (TROG) 07.03 RadioHUM study. Int J Radiat Oncol Biol Phys. 2014;88(3):572–579. | ||
Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Nigudgi S. Effect of low-level laser therapy on patient reported measures of oral mucositis and quality of life in head and neck cancer patients receiving chemoradiotherapy--a randomized controlled trial. Support Care Cancer. 2013;21(5):1421–1428. | ||
Roh JL, Kim DH, Kim SY, Park CI. Quality of life and voice in patients after laser cordectomy for Tis and T1 glottic carcinomas. Head Neck. 2007;29(11):1010–1016. | ||
Berman AT, DeCesaris CM, Simone CB 2nd, et al. Use of survivorship care plans and analysis of patient-reported outcomes in multinational patients with lung cancer. J Oncol Pract. 2016;12(5):e527–535. | ||
Vainshtein JM, Samuels S, Tao Y, et al. Impact of xerostomia on dysphagia after chemotherapy-intensity-modulated radiotherapy for oropharyngeal cancer: Prospective longitudinal study. Head Neck. 2016;38 Suppl 1:E1605–1612. | ||
Shuman AG, Larkin K, Thomas D, et al. Patient reflections on decision making for laryngeal cancer treatment. Otolaryngol Head Neck Surg. 2017;156(2):299–304. | ||
Jackson LK, Ridner SH, Deng J, et al. Internal lymphedema correlates with subjective and objective measures of dysphagia in head and neck cancer patients. J Palliat Med. 2016;19(9):949–956. | ||
Thor M, Olsson CE, Oh JH, et al. Temporal patterns of patient-reported trismus and associated mouth-opening distances in radiotherapy for head and neck cancer: A prospective cohort study. Clin Otolaryngol. 2018;43(1):22–30. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.