Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Patient-Reported Disease Severity and Quality of Life Among Arabic Psoriatic Patients: A Cross-Sectional Survey
Authors Soliman M
Received 30 June 2020
Accepted for publication 4 August 2020
Published 25 August 2020 Volume 2020:13 Pages 601—609
DOI https://doi.org/10.2147/CCID.S269909
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Moetaza Soliman
Department of Pharmacy Practice, Faculty of Pharmacy, Mansoura University, Mansoura, Egypt
Correspondence: Moetaza Soliman
Department of Pharmacy Practice, Faculty of Pharmacy, Mansoura University, ElGomhoria Street, Mansoura, ElDakahlia, Egypt
Tel +20 1142911306
Email [email protected]
Purpose: Patient-reported measures are of importance in chronic dermatological conditions where psychosocial consequences and quality of life impairment are common. The current study aimed to evaluate patient-reported disease severity and quality of life in Arabic patients with psoriasis.
Patients and Methods: Arabic psoriatic patients were invited to complete an online survey that collected patients’ demographics, self-assessed Simplified Psoriasis Index (saSPI) and the Dermatology Life Quality Index (DLQI). Disease severity and quality of life were compared in relation to patients’ demographics. Correlation between patient-reported measures was calculated using Spearman’s rank correlation test.
Results: A total of 221 patients with psoriasis, from 12 Arabic-speaking countries, participated in the study. The mean (95% CIs) saSPI-severity score was 10.9 (9.6– 12.2). Female patients reported higher saSPI-psychosocial impact scores compared to males (P=0.04) while patients with longer disease duration reported higher saSPI-past history and interventions scores (P=0.0001). The mean (95% CIs) DLQI score was 11.2 (10.2– 12.1). Patients with severe disease reported significantly higher DLQI scores [18.5 (10.5– 2)] (P=0.0001). DLQI was strongly correlated with saSPI-psychosocial impact score (rho= 0.63).
Conclusion: Arabic psoriatic patients participating in this study showed mild to moderate disease severity which had a very large impact on patients’ quality of life. Higher disease severity was associated with more impaired quality of life.
Keywords: self-assessed Simplified Psoriasis Index, psoriasis, quality of life, cross-sectional, patient-reported measures
Introduction
Psoriasis is a chronic condition that requires both professional and personal input to guide treatment and self-management. Professional assessment of psoriasis severity can be done by many instruments as Psoriasis Area and Severity Index (PASI) which is very widely used both in ideal situations during clinical trials and in routine clinical practice during patients’ consultations and monitoring.1 Other common instruments used for professional psoriasis severity assessment include Physician’s Global Assessment (PGA),2 Salford Psoriasis Index and Professional Simplified Psoriasis Index (proSPI).3 All these instruments require patient visits to clinics to attend follow-ups and have their disease severity assessed by a health professional.
Patient-reported assessments are very important in psoriasis to measure physical, social and psychological functioning and to reflect patients’ perspectives on their disease.4 The importance of self-assessed disease severity has increased recently because of social distancing during the Coronavirus disease-19 (COVID-19) pandemic as they can be used during the utilization of telemedicine.5 Using patient-reported disease severity, patients can be monitored remotely, reducing the need for regular travel to attend follow-ups. Patient assessment of disease severity can be done using self-assessed PASI score, Patient’s Global Assessment and self-assessed Simplified Psoriasis Index (saSPI).3,6
The itch, pain and scaling, as main symptoms of psoriasis, negatively impact on patients’ health-related quality of life. The patients often feel embarrassed or ashamed because of their disease which affects their social life by avoiding social activities.7 The state of the skin condition affects other daily activities. Moreover, physical and emotional effects of psoriasis negatively impact patients’ workplaces and work productivity, relationships with family, relatives and friends.8 Sexual difficulties and disease acceptance by partners also affect patients’ quality of life.9 Dealing with treatments, skin self-care, and satisfaction with treatment are also factors of impaired quality of life.10 Many instruments have been developed and validated to measure the quality of life in psoriasis.11 The Dermatology Life Quality Index (DLQI) is the most widely used quality of life measure in psoriasis.12
There are very few studies that addressed patient-reported disease severity and quality of life in Arabic psoriatic patients. A study involving 330 patients from Kuwait reported that physical activities were affected in more than half the patients and sexual activities were affected in one-third of the patients.13 Quality of life was worse in 20 psoriatic patients from Tunisia when psoriasis lesions were located in uncovered areas particularly the face.14 The mean DLQI score was 12.7 in 176 patients with psoriasis in Morocco.15 However, in the Middle East and Africa, low priority is usually given to the assessment of the quality of life; probably due to short consultation times.16
The current study aimed to evaluate the self-assessed disease severity and quality of life in Arabic psoriatic patients.
Patients and Methods
Study Design
A cross-sectional study design was used to collect patient-reported assessments from psoriasis patients. The author created an online survey that contained two sections (Supplementary material 1). The first section included the purpose of the study and information necessary for the patients and collected a required approval of the consent from each patient. Patient characteristics including age, gender, and onset of psoriasis, nationality, and level of education were also collected in this section. The second section included the saSPI and the DLQI. Links to the created survey were posted to different social media sites of Arabic psoriasis patients for participation. Inclusion criteria included (i) diagnosis with psoriasis, (ii) age >18 years, and (iii) Arabic language speaking.
The ethical approval for the study was obtained from The Faculty of Pharmacy – Mansoura University Ethical Committee prior to commencement of patient’s data collection. All participants provided informed consent and the study was in compliance with the Declaration of Helsinki 1975, as revised in 2000.
Patient-Reported Measures
The Self-Assessed Simplified Psoriasis Index
The saSPI is a three-component psoriasis assessment tool; (i) disease severity (saSPI-s), (ii) psychosocial impact (saSPI-p), and (iii) interventions and past history (saSPI-i).3 The three components cannot be simply summed.
The saSPI-s is a composite severity score. The patient is asked to evaluate the extent of psoriasis in 10 unequal areas of the body with a score of 0 (clear or minor), 0.5 (obvious), or 1 (widespread). The response is summed for the 10 areas giving a measure from 0 to 10. The patient is also asked to describe his/her overall state of psoriasis with a score of 0 (clear or just slight redness of staining), 1 (mild redness or scaling with no more than slight thickening), 2 (definite redness, scaling, or thickening), 3 (moderately severe with obvious redness, scaling, or thickening), 4 (very red and inflamed, very scaly, or very thick), or 5 (intensely inflamed skin with or without pustules). This score (0–5) is multiplied by the extent score (0–10) producing the saSPI-s (0–50). The degree of disease severity can be described according to the saSPI-s as mild (<10), moderate (10–20), or severe (>20) psoriasis.17
saSPI-p is a psychosocial impact scale that evaluates the impact of psoriasis on daily life on a scale from 0 to 10 where 0 represents no effect at all and 10 represents the most extreme effect on daily life.
saSPI-i summarizes the past history and interventions used for disease management. It is a score from 0 to 10 in response to 10 questions; four of them address the disease course and six of them address previous interventions (UV treatment or PUVA, methotrexate, acitretin, ciclosporin, biologic therapy, and any other treatments).
The Dermatology Life Quality Index
The DLQI is dermatology specific, patient-reported, quality of life measure that is very widely used to measure the quality of life in psoriasis patients. It consists of ten questions that measure daily activities, leisure, symptoms and feelings, work and school, and personal relationships.12 The patient responds to each question on a scale from 0 to 3 and the DLQI is calculated by adding the scores to each question, generating a score ranging from 0 to 30. The higher the DLQI score, the more impaired the quality of life is, with a score of >10 represents a severely affected life by the skin condition. The score is categorized to no effect at all (0–1), small effect (2–5), moderate effect (6–10), very large effect (11–20), and extremely large effect (21–30) on patients’ life.18
Statistical Analysis
The visibility of the disease was defined by the presence of psoriatic lesions in any of the three most visible areas of the body (i) scalp and hairline, (ii) face, neck, and ears, or (iii) hands, fingers and fingernails.
Descriptive statistics were used to describe quantitative variables while discrete variables were presented as percentages. Nonparametric methods were used with non-normally distributed variables. Mean values were presented along with the 95% Confidence Intervals (95% CIs). Mann–Whitney’s U-test was used to compare two groups and the Kruskal–Wallis test by ranks was used to compare more than two groups.
The correlation between patient-reported measures was calculated using Spearman’s rank correlation test which was interpreted as follows: no correlation (0–0.1), weak correlation (0.11–0.29), moderate correlation (0.3–0.49), strong correlation (0.5–0.69), and very strong correlation (>0.7).19
The p-values <0.05 were considered significant. All statistical analyses were performed using Stata 10.1 software (Stata Corp., College Station, TX, USA).
Results
Patient Characteristics
The study included 221 patients with a median (IQR) psoriasis duration of 12.0 (6.0–20) years. Sixty-eight percent of the patients were men. The median (IQR) age was 35.0 (29.0–41.0) years. Most of patients were university-educated (60.2%). The participating patients were from 12 different Arabic countries (Table 1).
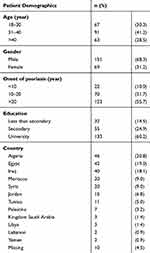 |
Table 1 Patient Demographics |
Disease Severity
The self-assessed Simplified Psoriasis Index-severity
The patients showed mild to moderate disease severity with mean (95% CIs) saSPI-s score of 10.9 (9.6–12.2) ranging from 0 to 50 (Figure 1A). Scalp and hairline area was the highest affected area in this group of patients followed by the area of knees, lower legs, and ankles. The area of feet, toes, and toenails was the least affected area (Figure 2). On a 6-points scale of average plaque severity, 5.5% of the patients reported clear or just slight redness of staining while 3.7% reported intensely inflamed skin with or without pustules (Table 2). Both male and female patients showed similar saSPI-s scores. Patients aged 31–40 years showed the highest disease severity compared to other age groups (P=0.04) (Table 3). Patients with low education levels showed higher disease severity scores than those with higher education levels (P=0.04) (Table 3).
 |
Table 2 Patient-Reported Disease Severity and Quality of Life |
 |
Table 3 Disease Severity and Quality of Life in Relation to Patients’ Demographics |
 |
Figure 2 Response distribution to the extent of psoriasis in the ten body areas according to the self-assessed Simplified Psoriasis Index-severity score. |
The self-assessed Simplified Psoriasis Index-psychosocial Impact
In response to the psychosocial impact score of the saSPI, the mean (95% CIs) saSPI-p score was 6.0 (5.6–6.4) and ranged from 0 to 10. Only 6.0% of the patients reported no impact of psoriasis on their daily life while 19.7% reported having a major impact of psoriasis on daily life (to the highest extent possible) (Figure 1B). Female patients showed higher mean (95% CIs) saSPI-p (6.7 (6.0–7.4)) compared to males (5.7 (5.2–6.2)) (P=0.04).
The self-assessed Simplified Psoriasis Index-interventions and past history
The mean (95% CIs) saSPI-i score was 2.1 (1.9–2.3) and ranged from 0 to 10. In terms of the distribution of the saPSI-i score, 33.0% reported a score of one, 22.2% reported a score of two and 0.5% reported a score of ten (Figure 1C). Fifty-two percent of the patients had psoriasis for more than 10 years while 23.5% of the patients had psoriasis for more than 20 years. Only 10.9% of the patients had previous or current biologic therapy (Table 2). Patients with early onset of psoriasis reported higher saSPI-i scores (P=0.0001).
Quality of Life
The mean (95% CIs) DLQI score was 11.2 (10.2–12.1) and ranged from 0 to 30. Only 5.9% of the patients had no effect of psoriasis on their life while 13.1% of the patients had an extremely large effect of psoriasis on their life (Figure 1D).
The highest percent of patients having an impact of psoriasis on quality of life was seen in response to the first question of the DLQI where 91.6% of the patients reported having problems with itchy, sore painful or stinging skin followed by the second question where 86.9% of the patients reported being embarrassed or self-conscious because of their skin. The lowest impact of psoriasis on quality of life was seen in response to the sixth question where 64.7% of the patients reported having no problems at all, or not relevant, with doing sports followed by the ninth question, where 53.9% of the patients reported having no problems at all or being not relevant to have any sexual difficulties (Figure 3).
 |
Figure 3 Response distribution to the ten questions of the Dermatology Life Quality Index. |
DLQI scores were similar in patients with different age groups, female and male patients, and in patients with different disease durations. Moreover, comparison across different countries revealed similar DLQI scores. Patients with visible psoriasis lesions reported worse quality of life compared to those with non-visible lesions (P=0.007; Table 3). Patients with severe disease reported significantly higher DLQI scores (mean (95% CIs)) of 16.0 (13.3–18.7) than those with moderate (11.6 (9.4–13.5)) and mild (9.3 (8.2–10.4)) disease (P=0.0001).
Correlation Between Different Patient-Reported Measures
DLQI was strongly correlated with the saSPI-p score (rho= 0.63, P=0.0001) while it was moderately correlated with saSPI-s (rho= 0.36, P=0.0001), and saSPI-i (rho= 0.32, P=0.0001). Moderate correlations were also seen between saSPI-s and saSPI-p scores (rho= 0.34, P=0.0001).
Discussion
In this study, patient-reported disease severity and quality of life were evaluated in Arabic psoriatic patients. Mean (95% CIs) self-assessed disease severity was 10.9 (9.6–12.2) with 57.5%, 24.4%, and 18.1% of the patients showed mild, moderate and severe psoriasis, respectively. Similar results were reported for an international sample of 186 psoriasis patients from 15 countries including one Arabic country where mean ± SD sa-SPI-s score was 9.6±8.6 with 59.1%, 30.6%, and 10.2% of the patients reported mild, moderate and severe psoriasis, respectively.20 Literature search revealed no studies that assessed patient-reported disease severity in Arabic countries. However, in comparison to the physician-assessed disease severity (PASI score) in 108 psoriasis patients from the United Arab Emirates, 85.1%, 11.1%, and 3.8% of the patients had mild, moderate and severe psoriasis, respectively.21 These differences in the disease severity may be explained by using different assessment tools and the difference between the patient’s and the physician's perspectives of the disease.
In-depth presentation of aspects of disease severity showed some characteristics that worth attention during medical care. For example, 24.0% of the patients had erythrodermic or generalized pustular psoriasis, 10.0% were diagnosed with psoriasis before turning 10 years of age, 5.4% were hospitalized because of psoriasis, and 23.5% had psoriasis for >20 years.
Patients with severe psoriasis showed the highest impairment in quality of life; this comes in agreement with literature where a strong correlation between self-reported disease severity and DLQI (rho=0.82) was reported.3 Patients with early onset of psoriasis reported higher saSPI-i scores which was an expected result because early-onset patients may have had an enriched history of the disease and may have been treated with many previous interventions. In addition, patients with low education levels showed higher disease severity scores than those with higher education levels (P=0.04). A recent study by the author showed that psoriasis patients with lower levels of education were less likely to accept their disease as measured by Acceptance of Illness Scale;22 which may have contributed to overestimation of their disease severity.
The mean (95% CIs) quality of life of the patients in the current study was 11.2 (10.2–12.1) which represents a very large effect of psoriasis on patients’ life. The literature reported different quality of life scores for different populations.23–25 Cultural background of patients may explain these findings. Within the Arabic countries, a study from Kuwait reported impaired sexual activities in one-third of the patients.13 This was comparable to 23.5% of the patients in the current study taking into account that having sexual difficulties was the least troubling to our patients. Quality of life was worse in a small sample (20 psoriatic patients) from Tunisia when psoriasis lesions were located in uncovered areas particularly the face.14 In the current study, exposed lesions in both the hands and the face areas were associated with worse quality of life. The mean DLQI score was 12.7 in 176 patients with psoriasis in Morocco15 compared to 11.2 in the current study.
The results of the current study identified a strong correlation between the DLQI and saSPI-p (rho= 0.63). This finding comes in agreement with the original developers of the saSPI who reported a very strong correlation between the DLQI and saSPI-p (rho= 0.98).3 The saSPI-p is a simple one question instrument that can provide information that is matched to a ten-question DLQI scale, this may be advantageous.
One of the strengths of the current study is the online collection of patients’ response which allowed including psoriasis patients from 12 different Arabic countries. Besides, the online assessment allowed the participants to respond in their own suitable time without any stress or rush. This study exemplifies how modern social media can facilitate collection of extensive data from a wide range of participants at minimal cost. However, selection bias may be an issue in the current study because illiterate or uneducated individuals may not be able to participate. Besides, elderly patients who are not familiar with participating in online assessment may have lower odds of participation.
The current study focused on the patient self-assessments without interference from health care professionals. It was shown to be feasible to obtain important information about psoriasis extent, distribution and severity remotely from patients themselves by the use of the self-assessment version of the Simplified Psoriasis Index. This has relevance in the current era of social distancing during the COVID-19 pandemic, whereby patients can be monitored remotely, reducing the need for regular travel to attend follow-ups. However, self-assessment of disease severity may be influenced by many factors that could be identified if compared to the professionally-assessed disease severity. For example, patient may tend to overestimate their psoriasis severity to seek more oriented medical care. This might not be the case with all patients; a survey of 120 psoriasis patients revealed a significant correlation between improvements in physician-measured PASI scores and patient’s satisfaction with the treatment (P=0.0003).26
The cross-sectional study design was easy to conduct, cheap, and provided a quick response. The study allowed a true reflection of patients’ perspective towards their disease. However, it was not possible to assess the ease or difficulty of completing the questionnaires by respondents. Presumably, however, any who did have difficulty would not have submitted a response. A follow-up survey of respondents would be useful in this regard.
The quality of life assessment in the current study was limited to the dermatology-specific quality of life as measured by DLQI; however, a future assessment of the general health-related quality of life using generic instruments as the short form-36 (SF-36) and European Quality of life-5 Dimensions score (EQ-5D) will enable researchers to compare the quality of life of psoriasis patients to the general population or to other populations with different conditions.27
Conclusion
Arabic psoriatic patients participating in this study showed mild to moderate disease severity which had a very large impact on patients’ quality of life. Patient-reported disease severity may be implemented for patient monitoring to reduce the number of visits to healthcare professionals in medical institutions or clinics, especially in the current social distancing situations during the COVID-19 pandemic. Patient-reported quality of life assessment should be given the same priority as the disease severity evaluation during patient follow-up.
Acknowledgment
The author would like to thank all the study participants.
Disclosure
The author reports no funding and no conflicts of interest for this work.
References
1. Puzenat E, Bronsard V, Prey S, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(Suppl 2):10–16. doi:10.1111/j.1468-3083.2009.03562.x
2. Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessment. J Am Acad Dermatol. 2004;51(4):563–569. doi:10.1016/j.jaad.2004.04.012
3. Chularojanamontri L, Griffiths CEM, Chalmers RJG. The Simplified Psoriasis Index (SPI): a practical tool for assessing psoriasis. J Invest Dermatol. 2013;133(8):1956–1962. doi:10.1038/jid.2013.138
4. Kitchen H, Cordingley L, Young H, Griffiths CEM, Bundy C. Patient-reported outcome measures in psoriasis: the good, the bad and the missing! Br J Dermatol. 2015;172(5):1210–1221. doi:10.1111/bjd.13691
5. Chat VS, Uppal SK, Kearns DG, Wu JJ. Clinical management of psoriasis patients during the COVID-19 pandemic. J Dermatolog Treat. 2020;1–2. doi:10.1080/09546634.2020.1781045
6. Fleischer ABJ, Feldman SR, Dekle CL. The SAPASI is valid and responsive to psoriasis disease severity changes in a multi-center clinical trial. J Dermatol. 1999;26(4):210–215. doi:10.1111/j.1346-8138.1999.tb03458.x
7. Fortune DG, Richards HL, Griffiths CEM. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23(4):681–694. doi:10.1016/j.det.2005.05.022
8. Pearce DJ, Singh S, Balkrishnan R, Kulkarni A, Fleischer AB, Feldman SR. The negative impact of psoriasis on the workplace. J Dermatolog Treat. 2006;17(1):24–28. doi:10.1080/09546630500482886
9. Molina-Leyva A, Jiménez-Moleón JJ, Naranjo-Sintes R, Ruiz-Carrascosa JC. Sexual dysfunction in psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2015;29(4):649–655. doi:10.1111/jdv.12845
10. Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res. 2018;310(4):271–319. doi:10.1007/s00403-018-1808-x
11. Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4:35. doi:10.1186/1477-7525-4-35
12. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x
13. Al-Mazeedi K, El-Shazly M, Al-Ajmi HS. Impact of psoriasis on quality of life in Kuwait. Int J Dermatol. 2006;45(4):418–424. doi:10.1111/j.1365-4632.2006.02502.x
14. Zghal A, Zeglaoui F, Kallel L, et al. [Quality of life in dermatology: tunisian version of the Skindex-29]. Tunis Med. 2003;81(1):34–37.
15. Khoudri I, Lamchahab FZ, Ismaili N, Senouci K, Hassam B, Abouqal R. Measuring quality of life in patients with psoriasis using the Arabic version for Morocco of the Dermatology Life Quality Index. Int J Dermatol. 2013;52(7):795–802. doi:10.1111/j.1365-4632.2011.05450.x
16. Abdulghani M, Al Sheik A, Alkhawajah M, et al. Management of psoriasis in Africa and the Middle East: a review of current opinion, practice and opportunities for improvement. J Int Med Res. 2011;39(5):1573–1588. doi:10.1177/147323001103900501
17. Chularojanamontri L, Griffiths CEM, Chalmers RJG. Responsiveness to change and interpretability of the simplified psoriasis index. J Invest Dermatol. 2014;134(2):351–358. doi:10.1038/jid.2013.318
18. Basra MKA, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230(1):27–33. doi:10.1159/000365390
19. Schober P, Boer C, Schwarte LA. Correlation Coefficients: appropriate Use and Interpretation. Anesth Analg. 2018;126(5):1763–1768. doi:10.1213/ANE.0000000000002864
20. Henry AL, Kyle SD, Chisholm A, Griffiths CEM, Bundy C. A cross-sectional survey of the nature and correlates of sleep disturbance in people with psoriasis. Br J Dermatol. 2017;177(4):1052–1059. doi:10.1111/bjd.15469
21. Dimitrov D, Ł M, Szepietowski JC. Stigmatization in Arabic psoriatic patients in the United Arab Emirates - a cross sectional study. Postep Dermatologii I Alergol. 2019;36(4):425–430. doi:10.5114/ada.2018.80271
22. Soliman M. Acceptance of illness and need for education to support dermatology self-care in psoriasis patients: a cross-sectional study. Postepy Dermatologii I Alergol Dermatology Allergol. 2020. doi:10.5114/ada.2020.95655
23. Kwan Z, Bong YB, Tan LL, et al. Socioeconomic and sociocultural determinants of psychological distress and quality of life among patients with psoriasis in a selected multi-ethnic Malaysian population. Psychol Health Med. 2017;22(2):184–195. doi:10.1080/13548506.2016.1220603
24. Jung S, Lee S-M, Suh D, Shin HT, Suh D-C. The association of socioeconomic and clinical characteristics with health-related quality of life in patients with psoriasis: a cross-sectional study. Health Qual Life Outcomes. 2018;16(1):180. doi:10.1186/s12955-018-1007-7
25. Fortune DG, Main CJ, O’Sullivan TM, Griffiths CE. Quality of life in patients with psoriasis: the contribution of clinical variables and psoriasis-specific stress. Br J Dermatol. 1997;137(5):755–760. doi:10.1111/j.1365-2133.1997.tb01113.x
26. Patruno C, Ayala F, Megna M, Napolitano M, Balato N. Patient-physician relationship in patients with psoriasis. Indian J Dermatol Venereol Leprol. 2012;78(2):228. doi:10.4103/0378-6323.93657
27. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi:10.3109/07853890109002087
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

