Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Patient Reported Central Nervous System Adverse Events of Efavirenz-Based Antiretroviral Therapy in People Living with HIV in Northwest Ethiopia
Authors Muche EA , Kiflu M, Ayalew MB
Received 12 August 2020
Accepted for publication 16 September 2020
Published 20 October 2020 Volume 2020:12 Pages 601—609
DOI https://doi.org/10.2147/HIV.S276111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Esileman Abdela Muche,1 Mekdes Kiflu,2 Mohammed Biset Ayalew1
1Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Pharmacy, College of Medicine and Health Sciences, Debremarkose University, Debremarkos, Ethiopia
Correspondence: Mohammed Biset Ayalew
Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Tel +251943056330
Email [email protected]
Background: Central nervous system (CNS) toxicities from regimens containing efavirenz are the main reasons for non-adherence, switch and discontinuation of antiretroviral therapy (ART). We aimed to assess prevalence of CNS adverse events and associated factors among HIV patients taking efavirenz-based regimens at the University of Gondar Comprehensive and Specialized Hospital (UoGCSH), Northwest Ethiopia.
Methods: A hospital-based cross-sectional study was conducted from March 15 to May 15, 2018 on 345 patients living with HIV who were taking efavirenz-based regimens. Information on sociodemographic and clinical characteristics was taken from medical records and patient interview. Binary logistic regression analysis was done to determine association. Statistical significance was declared at P value of ≤ 0.05.
Results: About 52.8% of participants experienced CNS adverse events. Vivid dreams, confusion, insomnia and somnolence were the most frequently reported adverse events. Most of the CNS adverse events occurred in the first year of treatment initiation and resolved within 1– 4 weeks. Age, economic status, CD4 count, disease stage, presence of comorbidities and concurrent use of other medication had a significant association with the occurrence of CNS adverse events.
Conclusion: More than half of HIV patients taking efavirenz-based regimens at UoGCSH experienced CNS adverse events. Health-care providers should give attention to patients on efavirenz therapy to monitor for CNS adverse events, especially for patients who have low CD4 count, advanced disease, comorbidities, low income and are older in age.
Keywords: CNS, adverse effect, efavirenz, antiretroviral therapy, low- and middle-income country
Introduction
Antiretroviral therapy (ART) regimens have the capacity to reduce patients’ human immunodeficiency virus (HIV) load to undetectable levels and decreasing HIV-related opportunistic infections by increasing their CD4 T-lymphocyte counts. In spite of the benefits, antiretroviral drugs might cause adverse reactions which may be the main reason for drug discontinuation, switching and non-adherence. As a result, intended benefits from antiretroviral drug therapy may not be achieved or treatment failure may occur.1–3 Antiretroviral therapy comprising of two nucleoside reverse transcriptase inhibitors (NRTIs) as a backbone and one non-nucleoside reverse transcriptase inhibitor (NNRTI) is maintained as the preferred first-line treatment regimen in adults because of its high viral suppression capacity.4 First-line antiretroviral regimens recommended by the Ethiopian HIV treatment guidelines for treatment-naive individuals are: tenofovir + lamivudine + efavirenz/nevirapine (TDF+3TC+EFV/NVP) or zidovudine + lamivudine + nevirapine/efavirenz (AZT+3TC+NVP/EFV).5
Efavirenz (EFV), one of the NNRTIs, has high antiretroviral efficacy and prolonged half-life allowing once-daily dosing.6 However, it crosses the blood–brain barrier (BBB) and can cause CNS adverse effects.7 As compared with structurally similar agents such as nevirapine, neuropsychiatric effects from EFV occur more frequently and with greater severity.8 A systematic review and meta-analysis study conducted by Shubber et al. reported that patients receiving EFV were more likely to experience severe central nervous system events than people receiving nevirapine-based regimens.9 A study conducted on Chinese HIV patients receiving tenofovir + lamivudine + efavirenz (TDF+3TC+EFV) as their first-line ART regimen reported that the most commonly reported adverse events (95.6%) were related to the CNS.10
CNS adverse effects reduce the patient’s quality of life which leads to loss of confidence in the health care and the drug, which in turn makes the patient non-adherent to the medication. A prospective observational study showed that adverse effects of highly active antiretroviral therapy (HAART) was a reason for discontinuation of medications for about a quarter of HIV patients.11 Scourfield et al. reported that of 89 patients who switched from an EFV-based ART regimen, 71% was due to CNS toxicity.12 According to Fumaz et al. the risk of discontinuation of treatment as a result of experiencing symptoms was higher in EFV-treated patients compared with non-EFV-based regimens.13
Sub-Saharan Africa is a region of high HIV burden, increased ART need and limited treatment options. As a result, the impact of ART-related adverse effects in the region is serious. In addition, the risk of developing CNS adverse events from EFV for people in sub-Saharan Africa is high due to single nucleotide polymorphismin many patients living in this region, which exposes them to increased EFV plasma concentration. Nyakutira et al. estimated that about one-fifth of the population in sub-Saharan Africa are genetically slow metabolizers which may increase the risk of adverse events from EFV.14 In such resource-limited settings information regarding the magnitude of adverse drug effects and associated factors is very important for monitoring risks. In addition, investigating treatment adverse events is crucial because of their direct impact on treatment adherence and patients’ quality of life.
To the authors’ knowledge, no detailed data exist in Ethiopia on the prevalence of EFV-related CNS adverse effects. The aim of this study is to determine the prevalence of CNS adverse events and to identify the possible contributing factors in adult patients taking EFV-based regimens at the University of Gondar Comprehensive and Specialized Hospital (UoGCSH), Northwest Ethiopia.
Methods
Study Area and Period
This study was conducted at UoGCSH, Gondar, Northwest Ethiopia. Gondar has a latitude and longitude of 12°36′N 37°28′E. It is located 737 kilometers away from Addis Ababa, the capital city of Ethiopia. The hospital is one of the biggest tertiary level referral and teaching hospitals in the region. It provides a service for an estimated 7 million people. The hospital has an ART clinic and a special pharmacy dedicated to serving people living with HIV. The study was conducted from March 15 to May 15, 2018.
Study Design
A hospital-based cross-sectional study design was employed.
Inclusion and Exclusion Criteria
Inclusion Criteria
HIV patients who were taking an EFV-based regimen.
Exclusion Criteria
Patients under the age of 15, pregnant women, patients with documented CNS illness prior to starting EFV and those who did not give consent to participate.
Sample Size and Sampling Methods
About 1900 patients were taking an EFV-based regimen at the clinic. The sample size was calculated using the following single population proportion formula. N= ((Z α/2)2 (p) (1-p))/d2 Z α/2 (95% confidence interval) =1.96; d (margin of error) =5%, p (proportion of people developing an EFV-related adverse drug event (ADE)) =50% (no previous study at the study area). Since the total population is <10,000 the sample was recalculated using the following corrective formula. Nf=n/(1+(n/N)); n = sample size calculated by single population proportion formula, Nf = actual sample size, N = total number of patients taking an EFV-based regimen in the study area (1900). Therefore, the sample size was: Nf = 384/(1 + (384/1900)) = 319. By adding 8% contingency, a total of 345 patients were sampled. Participants were selected using a simple random sampling technique from eligible patients available at the hospital on each day of the study period.
Data Collection Procedure
Data were collected by interviewing the patient using a well-structured questionnaire and reviewing paper-based medical records. The data collection tool was pretested on 17 patients (5% of calculated sample size), after which some language-related modifications were made to clarify some of the questions. Data were collected by two graduating pharmacy students. The collected data were revised and double-checked during data entry by two of the authors.
Data Processing and Analysis
The collected data were cleaned, checked for completeness, categorized, coded, and entered in to SPSS version 20. Descriptive statistics was used to summarize demographic and clinical characteristics. Both univariate and multivariate binary logistic regression analysis were done to identify factors associated with the occurrence of CNS adverse events. Statistical significance was declared at p-value ≤ 0.05.
Ethics Approval
Letter of ethical clearance was obtained from an ethical review committee of School of Pharmacy of the University of Gondar (reference number SoPs 254/2018). Letter of cooperation was obtained from the medical director of UoGCSH. The study was conducted in accordance with the Declaration of Helsinki. Participants and parents/legal guardians of participants under the age of 18 years were informed about the purpose of the study. Written consent was taken from participants aged 18 years or above. For participants under the age of 18 years, in addition to their assent, parental/legal guardian informed consent was obtained. Privacy and confidentiality were ensured during patient interview and review of patients’ medical records. Thus, the name and address of participants were not recorded in the data collection tool.
Results
Sociodemographic Characteristics
From the total of 345 patients included in the current study more than half (59.7%) were males, nearly a quarter (23.5%) had no formal education and the majority (58.0%) were 25–44 years old. The age range of participants was 15–70 years with a mean of 37.7±10.7 years. Most (71.7%) of the participants had a low economic status. A large proportion (36.5%) of the study participants were single and 35.1% were unemployed. Forty percent of participants had an alcohol use history while only 8.1% were smokers. Table 1 shows the detail of sociodemographic characteristics of study participants.
 |
Table 1 Sociodemographic Characteristics of Participants |
Clinical Characteristics
Regarding clinical characteristics of participants, about half (50.7%) had a CD4 count of <200cells/mm3 and 42.0% had stage 3 disease. Most (78.8%) of the participants were on a tenofovir + lamivudine + efavirenz (TDF+3TC+EFV) regimen and nearly three-quarters (73.0%) were taking the medication at night. Some of the participants (12.5%) had other concomitant chronic illnesses and more than a quarter (26.1%) were taking other medications in addition to their ART regimen. Table 2 illustrates the clinical characteristics of participants.
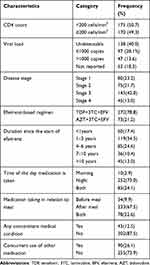 |
Table 2 Clinical Characteristics of Participants |
CNS Adverse Events
As indicated in Figure 1, more than half (52.8%) of the study participants reported at least one type of CNS adverse event. Vivid dreams (29.9%), confusion (27.5%), insomnia (24.1%) and somnolence (20.9%) were the most frequently reported adverse events. The majority (89.0%) of participants who reported an ADE experienced the event within a year of starting the EFV-based regimen and in 68.7% of participants ADEs were resolved within 1–4 weeks.
 |
Figure 1 CNS adverse events. |
Sociodemographic Factors Associated with Reported CNS Adverse Events
As indicated in Table 3, the result of multivariate binary logistic regression analysis showed that age and economic status had a significant association with the occurrence of CNS adverse events. Accordingly, patients who are 45 years old or above are three times more likely to have an ADE than patients who are less than 25 years old (AOR =3.183 (1.302–7.782), P=0.011). Patients with good (monthly income of >5000 Ethiopian birr) and moderate (monthly income of 2000–5000 Ethiopian birr) economic status are less likely to have CNS adverse events as compared with those with low economic status (monthly income of less than 2000 Ethiopian birr).
 |
Table 3 Sociodemographic Factors Associated with Reported CNS Adverse Effects of Efavirenz |
Clinical Factors Associated with Reported CNS Adverse Events
CD4 count, World Health Organization (WHO) disease stage, concomitant medical condition and concurrent use of other medication were found to have a significant association with occurrence of EFV-related CNS adverse events. Patients with a CD4 count of 200 or above are 88% less likely to have CNS adverse events than those with a CD4 count of below 200 (AOR=0.120 (0.038–0.383), P<0.0001). As indicated by the larger odds ratio in Table 4, patients who had advanced WHO stage disease (stages 3 or 4) were very much more likely to develop CNS adverse events than patients with WHO stage 1 disease. The absence of a concomitant medical condition is associated with a lower rate of CNS adverse events (AOR=0.107 (0.016–0.714), P=0.021). However, patients who were not taking other medications concurrently were five times more likely to report having CNS adverse events (AOR=5.466 (1.545–19.336), p=0.008).
 |
Table 4 Clinical Factors Associated with Reported CNS Adverse Effects of Efavirenz |
Discussion
The prevalence of CNS adverse events in the current study was 52.8%. Similarly, a study conducted in Kenya reported a prevalence of 48.6%,15 a study in Spain reported 54%,13 a study in the USA and one systematic review showed a prevalence of 50%.16,17 However, the prevalence rate reported in this study is higher than the report from Ambo referral hospital, Ethiopia, which is 35.7%.8 This difference might be because the study in Ambo referral hospital included patients who took ART for more than 6 months, obtained data from patient charts only and included patients on non-EFV-based ART regimens.
The most commonly reported CNS adverse events in our study were vivid dreams (29.9%), confusion (27.5%), insomnia (24.1%) and somnolence/drowsiness (20.9%). A study conducted in Ghana reported that insomnia, headaches, abnormal dreams and drowsiness were significantly more common among patients on EFV-based ART.18 A study conducted by Shikuma et al. on HIV-positive subjects taking EFV-based therapy found that the rate of sleep disordered breathing (SDB) was substantially higher in study subjects compared with published age-matched norms.19 The majority (89.0%) of participants who reported CNS adverse events experienced the event within a year of starting an EFV-based regimen and in 68.7% of participants the adverse events were resolved within 1–4 weeks. The Ghanaian study indicated that most neuropsychiatric toxicities were reported in the first year of therapy after which the occurrence of events declined.18 A review article by Apostolova et al. reported that patients treated with EFV revealed neuropsychological symptoms during the first 4 weeks of treatment and that these resolved later on.7
Age and economic status had a significant association with the occurrence of CNS adverse events. Patients who are 45 years old or above are three times more likely to develop the adverse events than patients who are less than 25 years old. Likewise, many other African studies reported that occurrence of ART-related adverse events were significantly higher in older patients compared with younger ones.1,20–22 Patients with good and moderate economic status are less likely to have CNS adverse events as compared with those with low economic status. This could be because people with better economic status could have improved living standards and better health-care access.
Patients who had severe disease (WHO stages 3 or 4 disease or low CD4 count) have higher risk of CNS adverse events from EFV-based ART regimens. In the current study patients with a CD4 count of below 200 are more likely to have CNS adverse events than those with a CD4 count of 200 or above. Patients who had advanced stage disease (WHO stages 3 or 4) were very much more likely to develop CNS adverse events than patients with stage 1 disease. This may be explained by the fact that patients with WHO stages 3 or 4 disease or a CD4 count of below 200 have deteriorated medically and may not cope with the drug effect, or may have multiple comorbidities and opportunistic infections which they may consider and report as ADEs.
The presence of a concomitant medical condition is one of the clinical factors having a significant association with the occurrence of CNS adverse events. In support of this finding, the study conducted at Mbagathi District Hospital’s comprehensive care centre in Kenya reported that the presence of concomitant medical conditions was significantly associated with the occurrence of CNS adverse events.15 However, concurrent use of other medication is negatively associated with CNS adverse events. This may be because concurrently used medications may sometimes mask symptoms of some of the adverse events or may induce the metabolism of EFV which in turn reduces its plasma level and lead to lesser risk of an adverse event.
The result of multivariate logistic regression analysis indicated the absence of a significant association between CNS adverse events and the type of ART regimen. In line with this, Wambura reported that none of the EFV-based ART regimens had a significant association with the occurrence of CNS adverse events.15 Even though a large proportion of alcohol users in our study reported CNS adverse events the association between alcohol use and occurrence of CNS adverse events was not significant (p=0.055). In line with this, a retrospective study by Salome et al. reported that alcohol users were at no more risk of showing CNS adverse events than those who denied a history of alcohol use.23 Smoking status was not found to be significantly associated with the occurrence of CNS adverse events. This finding may not represent the true picture of association because of the smaller number of respondents (8.1% only) reporting as having a smoking history, which would probably be due to not being willing to disclose this information for personal reasons.
The results of the current study should be interpreted cautiously due to some limitations. Firstly, recall bias may affect the findings as patients may not be able to recall what happened since the start of their ART regimen. Secondly, being a cross-sectional study, all the limitations associated with this type of design may be manifested in our study. Thirdly, generalization of the findings may be difficult as it is a single center study.
Conclusion
More than half of HIV patients taking efavirenz-based regimens at the University of Gondar Comprehensive and Specialized Hospital (UoGCSH) experienced CNS adverse events. Vivid dreams, confusion, insomnia and somnolence were the most frequently reported adverse events. Most of the CNS adverse events experienced by the respondents occurred in the first year of treatment and resolved within a month. Health-care providers should give attention to patients on efavirenz therapy to monitor for CNS adverse events, especially those patients who have low CD4 count, advanced stage disease, comorbidities, low income and are older in age.
Data Sharing Statement
The datasets during and/or analyzed during the current study is available from the corresponding author on reasonable request.
Consent to Publish
Participants consent was taken to publish this work.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from the ethical review committee of the School of Pharmacy, University of Gondar. The respondents were informed about the purpose of the study and their consent to participate was obtained.
Acknowledgments
We are very grateful to the staff of ART pharmacy at UoGCSH for their cooperation during the data collection process. We also extend our special thanks to Rahawa Kiyar for her support in the data collection.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Masenyetse LJ, Manda SO, Mwambi HG. An assessment of adverse drug reactions among HIV positive patients receiving antiretroviral treatment in South Africa. AIDS Res Ther. 2015;12(1):6. doi:10.1186/s12981-015-0044-0
2. Agu K, Ochei U, Oparah A, Onoh O. Treatment outcomes in patients receiving combination antiretroviral therapy in Central Hospital, Benin City, Nigeria. Trop J Pharm Res. 2010;9(1). doi:10.4314/tjpr.v9i1.52028
3. Agu KA, King RC, Isah MA, and Iyaji PG. Medication adherence and risk factors for non-adherence among patients taking highly active antiretroviral therapy. West Afr J Pharm. 2011;22:1.
4. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach - Second edition Geneva, Switzerland. 2016 [updated June 2016; cited July 23, 2020]. Available from: https://www.who.int/hiv/pub/arv/arv-2016/en/.
5. Federal Ministry of Health. National Guidelines for Comprehensive HIV Prevention, Care and Treatment. Addis Ababa, Ethiopia; 2017:236.
6. Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15(1):71–75.
7. Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, Esplugues JV. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother. 2015;70(10):2693–2708. doi:10.1093/jac/dkv183
8. Chelkeba L, Abdissa G. Assessment of ART adverse reactions and determinants at primary hospital in Ethiopia. Int J Basic Clin Pharmacol. 2013;2(2):208–215. doi:10.5455/2319-2003.ijbcp20130317
9. Shubber Z, Calmy A, Andrieux-Meyer I, et al. Adverse events associated with nevirapine and efavirenz-based first-line antiretroviral therapy: a systematic review and meta-analysis. AIDS. 2013;27(9):1403–1412. doi:10.1097/QAD.0b013e32835f1db0
10. Dai L, Su B, Liu A, et al. Adverse events in Chinese human immunodeficiency virus (HIV) patients receiving first line antiretroviral therapy. BMC Infect Dis. 2020;20(1):1–9. doi:10.1186/s12879-020-4878-2
11. Meeyannoor K, Bhuvana K. A prospective observational study of adverse drug reactions to antiretroviral therapy: type and risk factors in a tertiary care teaching hospital. Int J Basic Clin Pharmacol. 2014;3(2):380.
12. Scourfield A, Zheng J, Chinthapalli S, et al. Discontinuation of atripla as first-line therapy in HIV-1 infected individuals. AIDS. 2012;26(11):1399–1401. doi:10.1097/QAD.0b013e328353b047
13. Fumaz CR, Munoz-Moreno JA, Moltó J, et al. Long-term neuropsychiatric disorders on efavirenz-based approaches: quality of life, psychologic issues, and adherence. J Acquir Immune Defic Syndr. 2005;38(5):560–565. doi:10.1097/01.qai.0000147523.41993.47
14. Nyakutira C, Röshammar D, Chigutsa E, et al. High prevalence of the CYP2B6 516G→ T (* 6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur J Clin Pharmacol. 2008;64(4):357–365. doi:10.1007/s00228-007-0412-3
15. Wambura CA. Assessment of the Prevalence of Central Nervous System Adverse Reactions in Adult Patients Taking Efavirenz Based Regimens at Mbagathi District Hospital’s Comprehensive Care Centre. JKUAT; 2015.
16. Treisman GJ, Kaplin AI. Neurologic and psychiatric complications of antiretroviral agents. AIDS. 2002;16(9):1201–1215. doi:10.1097/00002030-200206140-00002
17. Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of efavirenz. AIDS Behav. 2011;15(8):1803–1818. doi:10.1007/s10461-011-9939-5
18. Sarfo FS, Sarfo MA, Chadwick D. Incidence and risk factors for neuropsychiatric events among Ghanaian HIV patients on long-term non-nucleoside reverse transcriptase inhibitor-based therapy. Eneurologicalsci. 2016;3:21–25. doi:10.1016/j.ensci.2015.12.002
19. Shikuma CM, Kohorn L, Paul R, et al. Sleep and neuropsychological performance in HIV+ subjects on efavirenz-based therapy and response to switch in therapy. HIV Clin Trials. 2018;19(4):139–147.
20. Eluwa GI, Badru T, Akpoigbe KJ. Adverse drug reactions to antiretroviral therapy (ARVs): incidence, type and risk factors in Nigeria. BMC Clin Pharmacol. 2012;12(1):7. doi:10.1186/1472-6904-12-7
21. Abah IO Adverse drug reactions to Antiretroviral drugs: effect on virologic faliure in a Nigerian cohort of HIV-infected adults on first-line Antiretroviral therapy. Masters thesis. 2017.
22. Oumar AA, Dakouo M, Tchibozo A, et al. Antiretroviral-induced adverse drug reactions in HIV-infected patients in Mali: a resource-limited setting experience. Int J Basic Clin Pharmacol. 2019;8(5):831–836. doi:10.18203/2319-2003.ijbcp20191565
23. Salomé NJ, Warren S, Judith AA. Tolerance of efavirenz-induced central nervous system side effects in HIV-infected individuals with a history of substance abuse. HIV Clin Trials. 2003;4(3):145–149. doi:10.1310/P7MJ-K2WD-T1FY-AU8L
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
