Back to Journals » Patient Preference and Adherence » Volume 13
Patient Preferences For Chemotherapy In The Treatment Of Non-Small Cell Lung Cancer: A Multicenter Discrete Choice Experiment (DCE) Study In China
Authors Sun H, Wang H, Xu N, Li J, Shi J, Zhou N, Ni M, Hu X, Chen Y
Received 24 July 2019
Accepted for publication 20 September 2019
Published 8 October 2019 Volume 2019:13 Pages 1701—1709
DOI https://doi.org/10.2147/PPA.S224529
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Naifeng Liu
Hui Sun,1,2 Huishan Wang,3 Ningze Xu,1 Junling Li,4 Jufang Shi,4 Naitong Zhou,5 Ming Ni,6 Xianzhi Hu,7 Yingyao Chen1
1Key Lab of Health Technology Assessment, National Health Commission, School of Public Health, Fudan University, Shanghai, People’s Republic of China; 2Department of Health Technology Assessment Research, Shanghai Health Development Research Center, Shanghai Medical Information Center, Shanghai, People’s Republic of China; 3The Second Clinical Medical School of Nanjing Medical University, Nanjing, People’s Republic of China; 4Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 5West China School of Pharmacy, Sichuan University, Chengdu, People’s Republic of China; 6Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China; 7Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China
Correspondence: Yingyao Chen
School of Public Health, Fudan University, Shanghai 200032, People’s Republic of China
Tel +86-21-33565183
Fax +86-21-64169552
Email [email protected]
Objective: The study aims to quantify patients’ risk-benefit preferences for chemotherapy in the treatment of non-small cell lung cancer (NSCLC), and to elicit their willingness to pay (WTP) for treatment outcomes.
Methods: A face-to-face discrete choice experiment (DCE) was conducted on NSCLC patients in four tertiary hospitals each from Beijing, Shanghai, Guangzhou and Chengdu in China. Patients were invited to complete choice questions that constructed by seven attributes: progression-free survival (PFS), disease control rate (DCR), rash, nausea and vomiting, tiredness, mode of administration and out-of-pocket costs. A mixed logit model was used to evaluate the choice model. Estimates of relative preferences and marginal willingness to pay for each attribute were then explored.
Results: A total of 361 patients completed the survey. Improvements in PFS (10, 95% CI: 8.4–11.6) were the most important attribute for patients, followed by increase in DCR (4.6, 95% CI: 3.4–5.8). Tiredness (3.9, 95% CI: 2.9–5.1) was judged to be the most important risk. While remaining attributes were ranked in decreasing order of importance: nausea and vomiting (1.9, 95% CI: 0.9–3.0), mode of administration (0.8, 95% CI: 0.2–1.4) and rash (0.5, 95% CI: −0.6–1.5). There was little variation in preferences among patients with different sociodemographic characteristics. Patients were monthly willing to pay $2304 (95% CI, $1916–$2754) that guaranteed 11 months of PFS, followed by $1465 (95% CI, $1163-$1767) per month to improve their disease control rate by 90%.
Conclusion: The results suggested that efficacy was the most important attribute for patients. Side effects, mode of administration and treatment cost significantly influenced patient preferences. Patient engagement in prioritizing their treatment preferences should be emphasized during the clinical decision-making process and regimen implementation.
Keywords: non-small cell lung cancer, chemotherapy, patient preferences, discrete choice experiment
Introduction
Lung cancer is the most commonly diagnosed cancer worldwide and the most common cause of cancer-related mortality, its health and economic burden on society are significant.1 According to the annual Chinese cancer registry report in 2011, the mortality of lung cancer in China was 39.27/100,000.2 In 2014, the mortality of lung cancer increased further to 45.80/100,000.3 The total treatment cost (including chemotherapy, biologic agents, targeted and radiation therapy) for lung cancer in China reached 24.31 billion yuan in 2015.4 Non-small cell lung cancer (NSCLC) accounted for approximately 85% of all primary lung cancers, including squamous cell carcinoma (25–30%), adenocarcinoma (40%) and large cell carcinoma (10–15%).5
Implementation of NSCLC therapy aims to prolong the survival time, control tumor-related symptoms as well as improve patients’ quality of life.5,6 Currently, surgery is the standard of care for resectable, early-stage and functionally operable NSCLC.7 Chemotherapy will be recommended for patients with stage IV NSCLC and negative or unknown test results for ALK or ROS1 rearrangements, sensitizing EGFR mutations, or PD-L1 expression.5 Recommended agents include platinum agents, taxanes, vinorelbine, etoposide, pemetrexed, and gemcitabine.5,6,8 Patients with advanced metastasis may benefit from palliative chemotherapy.6 Different chemotherapy regimens offer different clinical outcomes in terms of efficacy, potential risks, dosing option and administration mode, with different expenditures for patients as well.
Patient engagement in prioritizing their treatment preferences can have an impact on the treatment effects.9–12 Understanding patient preferences is important to inform the regimens selection, as well as promote patient-centered health care.13–15 However, in China, studies of NSCLC were mainly concerned about the efficacy, safety, cost of different therapies,16–18 there are very few studies that investigating patient preferences in the treatment of NSCLC. The objective of this study is to quantify patients’ risk-benefit preferences for chemotherapy in the treatment of NSCLC, and to elicit the WTP they make. For this study, the discrete choice experiment (DCE) was applied with data collected from multicenter settings in China.
Methods
Sample And Study Design
Multicenter face-to-face survey was conducted in four tertiary hospitals each from Beijing, Shanghai, Guangzhou and Chengdu between September 30, 2017 and December 31, 2017. The inclusion criteria were: 1) participants were required to be at least 18 years old with a physician diagnosis of NSCLC. 2) participants had received chemotherapy practice. We aimed to recruit 400 respondents (100 in each region) in the survey, the final sample included 361 respondents in our study. Earlier studies have shown that this number of respondents is sufficiently large for reliable statistical analyses.19,20
For this study, after detailed explanations of the questionnaire by interviewers, participants were invited to participate in a 10 mins structured interview (based on a questionnaire) with one trained interviewers. Copies of their written informed consent were provided to participants upon recruitment. All eligible participants were informed of the purpose of the study and their right to refuse to participate. The study protocol and questionnaires were approved by the Fudan University School of Public Health Institutional Review Board (IRB#2017-09-0638, September 8, 2017–December 31, 2018). All of the procedures were performed in accordance with the Declaration of Helsinki and relevant policies in China.
The Discrete Choice Experiment Questionnaire
The discrete choice experiment (DCE) is a quantitative survey-based method, which has been extensively used to assess patient preferences, and marginal rates of substitution (e.g. marginal willingness to pay) in health care.21–24 In a DCE, respondents are presented with a sequence of hypothetical scenarios (choice sets) composed by defined attributes (efficacy, safety, mode of administration, costs, etc.) that are assigned with different levels. For each choice set, respondents are asked to choose their preferred scenario. Thus, relative preferences of given attributes can be determined and the trade-offs that respondents make can be quantified. There are several checklists available during the design of DCE study.23,25–28
Selection Of Attributes And Their Levels
Three criteria were considered when we selected attributes: relevance to patient concern about the NSCLC chemotherapy, ease of quantifying the attribute within a DCE framework, overlap or correlation with other attributes.
Based on a critical literature review,29–34 consultation with clinical experts and patients focus group, key attributes were identified with different levels to describe the NSCLC treatment alternatives, with each of these attributes assigned two or three levels (Table 1). The key attributes in the survey are progression-free survival (PFS), disease control rate (DCR), side effect of skin, nausea and vomiting, tiredness and mode of administration. We used out-of-pocket costs per month as a value attribute to explore patients’ marginal willingness to pay for each attribute level. For this study, the levels of progression-free survival, levels of disease control rate, and levels of side effects were based on evidence from clinical trials or real-world data.35–41 The levels of out-of-pocket costs and administration mode were identified by published literature and calibrated by physicians and patients.42–44
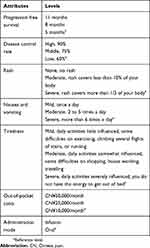 |
Table 1 Attributes And Their Levels |
Construction Of The DCE Questionnaire
The combination of these attributes and levels (six attributes with three levels, one attribute with two levels) resulted in 1458 hypothetical scenarios (36×21), which obviously could not be used in a questionnaire. Therefore, we applied fractional factorial design (SAS OPTEX procedure) to generate optimal scenarios. We firstly applied macro %Mktruns to calculate reasonable design sizes, then macro %Mktex was used to create the combinations.45–47 The resulting experimental design consisted of 18 choice pairs. The survey instrument included an introduction to choice sets with a description of the attributes and their levels. Each respondent answered 18 trade-off questions under the interviewers’ assistance (Figure 1 for a DCE survey example).
 |
Figure 1 Sample of DCE survey question. |
In addition to DCE questions, the survey instrument included questions on demographic characteristics (e.g. gender, age, education level, and household income) and patients’ treatment experience (e.g. time since diagnosis, cancer type, cancer stage, and past treatment). We also conducted a pre-test with 10 patients at one hospital to test the understandability of the survey instrument.
Data Analysis
Mixed logit model was used to estimate the relative preferences of the attributes and patient WTP for each attribute level. The mixed logit model controlled for clustering and unobserved preference heterogeneity among patients by estimating a distribution for each preference parameter.48 The coefficients from the mixed logit model represented estimates of the probability of choosing a chemotherapy for NSCLC. Then, WTP was calculated to resemble the real-world situation, where patients’ valuation for obtaining improvement in certain treatment outcome. Effects coding was applied to represent a categorical variable in the mixed logit regression model to assure all attribute levels can be estimated including the inference level.49
In the current study, we firstly estimated the mixed logit model, then the results from the model were used to calculate the marginal WTP for each attribute level. All analyses were performed in Stata statistical software (version 14 SE, Stata Corp).
Results
Study Participants
Three hundred and sixty-one patients participated in and completed the survey, Beijing (95), Shanghai (87), Guangzhou (90), Chengdu (89). The socio-demographic characteristics of participating patients are summarized in Table 2. Of the 361 patients, the majority were male (63%) and had received senior high school education (51%). The mean age of the patients was 58 years, spanning a range of 31 to 82 years. Most patients were diagnosed as having adenocarcinoma NSCLC histology (62%), with the time since diagnosis of less than 1 year (74%).
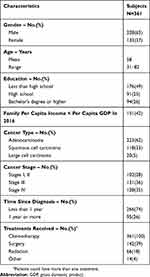 |
Table 2 Demographic Characteristics Of The Study Sample |
Patient Preferences For Treatment Of NSCLC
The main effects mixed logit model results are displayed in Table 3. The coefficients were significant for nearly all attributes regarding PFS, DCR, nausea and vomiting, tiredness, mode of administration and treatment costs, meaning that these attributes played significant roles when patients engaging in the treatment decisions. The coefficient of rash, however, had no significant impact on patients’ decision.
 |
Table 3 Main Effects Mixed Parameter Logit Model Results |
For this study, 11 months of PFS (coefficient, 0.59 [standard error (SE), 0.05]; P < 0.001) was the most preferred for patient, followed by 90% disease control rate (coefficient, 0.37 [SE, 0.04]; P < 0.001). Severe tiredness (coefficient, −0.29 [SE, 0.04]; P < 0.001) was judged to be the most important risk, followed by severe nausea and vomiting (coefficient, −0.13 [SE, 0.04]; P < 0.001). Oral administration (coefficient, 0.05 [SE, 0.02]; P < 0.05) was preferred to infusion. As expected, patients had higher positive preferences for better clinical outcomes. For instance, patient preferences for 11 months of PFS were far greater than 8 months. Meanwhile, patients had negative preferences for side effects.
Patient Preferences Intensity
The relative preferences intensity results are illustrated in Figure 2, with 10 representing the most preferred attributes and 0 representing the least preferred. The vertical bars around each level mean estimate denoted the 95% confidence interval about the point estimate. In relation to the level of other attributes, patients’ strongest positive preference was to prolong progression-free survival by 11 months. Patients also had strong positive preferences for improving with a 90% disease control rate, mild tiredness and oral administration.
 |
Figure 2 Patient preferences intensity. |
Figure 2 also illustrates the mean relative preferences intensity score with a 95% confidence interval. The mean relative preferences intensity score for each attribute was estimated as an improvement from the worst level to the best level (over the ranges presented in this study). Taking tiredness as an example, its mean relative preferences intensity was the improvement from severe to mild. In the current study, having an improvement from 5 months of PFS to 11 months of PFS was the most important (10.0; 95% [confidence interval (CI)]: 8.4–11.6), followed by improvement with 30% DCR (4.6; 95% CI: 3.4–5.8). Next were tiredness (3.9; 95% CI: 2.9–5.1), nausea and vomiting (1.9; 95% CI: 0.9–3.0), oral administration (0.8; 95% CI: 0.1–1.4) and rash (0.5; 95% CI: −0.6–1.5).
Variation In Patient Preferences For NSCLC Treatment
We estimated interaction terms between patients’ sociodemographic characteristics (e.g. age, cancer type, tumor stage) and preference for different levels of chemotherapy attributes (Table 4). We found that compared with patients aged over 40, patients aged 30–40 had stronger preference for longer PFS, and they had negative preferences for infusion mode. Squamous cell carcinoma patients tend to favor no rash than patient with other types. Adenocarcinoma patients have positive preference for mild tiredness. As the tumor stage evolved, patients have more demand for longer PFS. Despite being statistically significant, the magnitude of differences in preferences across groups was small.
 |
Table 4 Variation In Patient Preferences For NSCLC Treatment |
Patients’ Willingness To Pay For Treatment Of NSCLC
Some earlier studies reported patient expenditures for NSCLC treatments in China, and the treatment costs per cycle for NSCLC ranged from $730 to $2924.42–44 Exchange rate as of September 2018: US$1= ¥6.84. In the current study, patients’ marginal WTP was elicited by stated preference discrete choice analysis. The results revealed patients were monthly willing to pay $2340 (95% CI, $1927-$2754) for obtaining 11 months of PFS (Table 5).
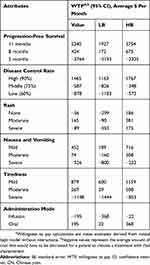 |
Table 5 Patients’ Marginal WTP For Each Attribute Level |
In addition, patients were willing to pay $195 (95% CI, $22–$368) per month for oral administration, $1,465 (95% CI, $1163–$ 1767) per month to improve their disease control rate by 90%, $145 (95% CI, $90–$381) per month for rash less than 10% on the body, $571 (95% CI, $189–$716) per month to reduce nausea and vomiting to one time a day and $879 (95% CI, $600–$1159) per month to get mild tiredness. The wide ranges of CIs showed that there were considerable differences in WTP, which proved the existence of preference heterogeneity.
Discussion
In this study with a multicenter sample of patients, we apply a DCE framework to investigate patient preferences for chemotherapy in the treatment of NSCLC. We found that prolonging progression-free survival, increasing disease control rate and reducing side effects were the primary consideration for NSCLC patients (over the ranges of attributes and levels presented in the survey). Mode of administration and out-of-pocket cost have statistically influence on patient preference. We also estimated interaction terms between patients’ sociodemographic characteristics, we found the magnitude of differences in preferences across groups was small. Furthermore, we elicited the extent to which patients were willing to pay for achieving an improvement of efficacy or reduction of potential side effects from the treatment. The result showed that the highest willingness to pay was obtained for 11 months of PFS.
The findings of the current study were consistent with some earlier studies in most countries. Bridges et al31 reported that PFS benefit was the most important attribute for patients when considering treatment decisions in the US. In the study of Muhlbacher et al32 in Germany, they found patients had clear preferences for an increase in “progression-free survival,” and “oral administration” preferred to “infusion.” Studies conducted in the US,30 Canada50,51 and Japan29,52 revealed that most cancer patients (>50%) judged moderate survival benefits sufficient to make chemotherapy worthwhile. In contrast to the study in the Germany, which ranks “tumor-associated symptoms” in the first place of all included side effects, 29 the patients in the present study assessed the “tiredness” as the most important side effect in their treatment decision. A possible explanation might be that patient are severely affected by “tiredness” in their daily life and possibly with a reduction in their quality of life. However, through literature reviews in selected Chinese and English databases, no studies have been found in relation to patients’ risk-benefit preferences for NSCLC chemotherapy in China, this study could add informative and applied data to this field.
More analysis could be needed to further understand patient’ WTP for the treatment outcomes. First, the cost input in this study was determined by literature and calibrated by patients focus group, more sensitivity analysis was needed to validate the WTP value. Second, the estimated WTP did not consider the absence or presence of side effects and risk of drugs abuse that may have impacts on WTP estimation, further research is needed to investigate these potential factors that may have impacts on WTP.
Some limitations should be noted in this study. Firstly, potential selection bias might exist among patients, because the participants were healthy enough to complete the survey. Secondly, although we identified seven key attributes with different levels patient concerned most, the limited number of attributes and levels presented may not reflect the patient treatment decision in real world. However, the attributes used in this study were similar to those used in other studies on patient preferences towards NSCLC chemotherapy,29,31,32 incorporating more attributes and levels could increase respondents burden, which could not benefit in DCEs study. Finally, the DCEs survey was conducted at regional large-scale tertiary hospitals in China. Thus, the patients’ relative preferences for NSCLC chemotherapy and their WTP for treatment outcome may not be generalizable to other countries.
Conclusion
The current study is the first attempt to examine patient preferences for NSCLC chemotherapy in China. The findings from this study have provided some useful insight into understanding patients’ relative preferences for NSCLC chemotherapy and the willingness to pay they make for achieving a single treatment outcome, which can further help to inform the clinical decision-making and promote patient-centered care.
Acknowledgments
The study was funded by China Medical Board Health Technology Assessment Collaborating Program (Grant No.16-251). We would like to thank all the staff involved in the field survey for their excellent research assistance.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21262
2. Chen WQ, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
3. Chen WQ, Sun KX, Zheng RS, et al. Report of cancer incidence and mortality in different areas of China, 2014. China Cancer. 2018;27:1-14.
4. Cai Y, Yan BH, Zhou GW. Analysis of direct economic burden and average hospitalization cost of lung cancer in China in 2011–2015. Chin J Health Stat. 2018;35:334-337.
5. Ettinger DS, Wood DE, Akerley W, et al. NCCN guidelines insights: non-small cell lung cancer, version 4.2016. J Natl Compr Canc Netw. 2016;14:255–264. doi:10.6004/jnccn.2016.0031
6. Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):i27–i39. doi:10.1093/annonc/mdu199
7. Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:v1–v21. doi:10.1093/annonc/mdx222
8. Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. ONCOLOGIST. 2008;13(Suppl 1):5–13. doi:10.1634/theoncologist.13-S1-5
9. Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psychooncology. 2006;15:9–19. doi:10.1002/pon.907
10. Hopmans W, Damman OC, Senan S, et al. A patient perspective on shared decision making in stage I non-small cell lung cancer: a mixed methods study. BMC Cancer. 2015;15:959. doi:10.1186/s12885-015-1584-3
11. Kehl KL, Landrum MB, Arora NK, et al. Association of actual and preferred decision roles with patient-reported quality of care: shared decision making in cancer care. Jama Oncol. 2015;1:50–58. doi:10.1001/jamaoncol.2014.112
12. Mokhles S, Nuyttens J, de Mol M, et al. Treatment selection of early stage non-small cell lung cancer: the role of the patient in clinical decision making. BMC Cancer. 2018;18:79. doi:10.1186/s12885-018-4242-8
13. Arora NK, McHorney CA. Patient preferences for medical decision making: who really wants to participate? Med Care. 2000;38:335–341. doi:10.1097/00005650-200003000-00010
14. Spatz ES, Spertus JA. Shared decision making: a path toward improved patient-centered outcomes. Circ Cardiovasc Qual Outcomes. 2012;5:e75–e77. doi:10.1161/CIRCOUTCOMES.112.969717
15. Sekimoto M, Asai A, Ohnishi M, et al. Patients’ preferences for involvement in treatment decision making in Japan. BMC Fam Pract. 2004;5:1. doi:10.1186/1471-2296-5-1
16. Li Z, Jiang S, He R, et al. Trajectories of hospitalization cost among patients of end-stage lung cancer: a retrospective study in China. Int J Environ Res Public Health. 2018;15(12):2877. doi:10.3390/ijerph15061188
17. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28:2443–2450. doi:10.1093/annonc/mdx359
18. Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14:953–961. doi:10.1016/S1470-2045(13)70355-3
19. Marshall D, Bridges JF, Hauber B, et al. Conjoint analysis applications in health – how are studies being designed and reported: an update on current practice in the published literature between 2005 and 2008. PATIENT. 2010;3:249–256. doi:10.2165/11539650-000000000-00000
20. Orme B. Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research. Madison, WI: Research Publishers LLC; 2006.
21. Ryan M, Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy. 2003;2:55–64.
22. de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21:145–172. doi:10.1002/hec.1697
23. Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19:300–315. doi:10.1016/j.jval.2016.04.004
24. Ryan MGKAM. Using Discrete Choice Experiments to Value Health and Health Care. Dordrecht, The Netherlands: Springer; 2008.
25. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26:661–677. doi:10.2165/00019053-200826080-00004
26. Lancsar E, Fiebig DG, Hole AR. Discrete choice experiments: a guide to model specification, estimation and software. Pharmacoeconomics. 2017;35:697–716. doi:10.1007/s40273-017-0506-4
27. Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health – a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14:403–413. doi:10.1016/j.jval.2010.11.013
28. Hensher DARJGW. Applied Choice Analysis: A Primer. Cambridge: Cambridge University Press; 2005.
29. Hirose T, Horichi N, Ohmori T, et al. Patients preferences in chemotherapy for advanced non-small-cell lung cancer. Intern Med. 2005;44:107–113. doi:10.2169/internalmedicine.44.107
30. Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews. BMJ. 1998;317:771–775. doi:10.1136/bmj.317.7161.771
31. Bridges JF, Mohamed AF, Finnern HW, et al. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77:224–231. doi:10.1016/j.lungcan.2012.01.016
32. Muhlbacher AC, Bethge S. Patients’ preferences: a discrete-choice experiment for treatment of non-small-cell lung cancer. Eur J Health Econ. 2015;16:657–670. doi:10.1007/s10198-014-0622-4
33. Bridges JF, Jones C. Patient-based health technology assessment: a vision of the future. Int J Technol Assess Health Care. 2007;23:30–35. doi:10.1017/S0266462307051549
34. Blinman P, Alam M, Duric V, et al. Patients’ preferences for chemotherapy in non-small-cell lung cancer: a systematic review. Lung Cancer. 2010;69:141–147. doi:10.1016/j.lungcan.2010.05.001
35. Li B, Ren S, Wang Y, et al. Efficacy of third-generation chemotherapeutic agents combined with cisplatin or carboplatin in 3100 Chinese patients with advanced non-small-cell lung cancer. Thorac Cancer. 2013;4:117–122. doi:10.1111/j.1759-7714.2012.00173.x
36. Luo L, Hu Q, Jiang JX, et al. Comparing single-agent with doublet chemotherapy in first-line treatment of advanced non-small cell lung cancer with performance status 2: a meta-analysis. Asia Pac J Clin Oncol. 2015;11:253–261. doi:10.1111/ajco.12359
37. de Castria TB, Da SE, Gois AF, et al. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2013;8:CD009256.
38. Schuette WH, Groschel A, Sebastian M, et al. A randomized phase II study of pemetrexed in combination with cisplatin or carboplatin as first-line therapy for patients with locally advanced or metastatic non-small-cell lung cancer. Clin Lung Cancer. 2013;14:215–223. doi:10.1016/j.cllc.2012.10.001
39. Kim ES, Moon J, Herbst RS, et al. Phase II trial of carboplatin, paclitaxel, cetuximab, and bevacizumab followed by cetuximab and bevacizumab in advanced nonsquamous non-small-cell lung cancer: SWOG S0536. J Thorac Oncol. 2013;8:1519–1528. doi:10.1097/JTO.0000000000000009
40. Hattori Y, Satouchi M, Katakami N, et al. A phase II study of pemetrexed in patients with previously heavily treated non-squamous non-small cell lung cancer (HANSHIN Oncology Group 001). Cancer Chemother Pharmacol. 2014;73:17–23. doi:10.1007/s00280-013-2290-y
41. Zhou C, Wu YL, Chen G, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33:2197–2204. doi:10.1200/JCO.2014.59.4424
42. Yang CC, Hu Q, Liu YJ. Progress in research on disease burden of non-small cell lung cancer. China Licensed Pharmacist. 2016;13:40–45.
43. XL W. Clinical effect and pharmacoeconomics of gefitinib and erlotinib in advanced non-small-cell lung cancer. China Medicine and Pharmacy. 2015;05:100–102.
44. Zhang MM, Pang TY, Gao WH. Pharmacoeconomics evaluations of four different chemotherapy regimens against advanced non-small cell lung cancer. Chin Hosp Pharm J. 2016;36:2001–2004.
45. Reed JF, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16:3–13. doi:10.1016/j.jval.2012.08.2223
46. Burgess L, Street DJ. Optimal designs for choice experiments with asymmetric attributes. J Stat Plan Infer. 2005;134:288–301. doi:10.1016/j.jspi.2004.03.021
47. Kuhfeld’s WF. Marketing Research Methods in SAS: Experimental Design, Choice, Conjoint and Graphical Techniques. Cary, NC: SAS Institute Inc; 2010.
48. Train KE. Discrete Choice Methods with Simulation. Cambridge: Cambridge University Press; 2003.
49. Bech M, Gyrd-Hansen D. Effects coding in discrete choice experiments. Health Econ. 2005;14:1079–1083. doi:10.1002/hec.984
50. Brundage MD, Davidson JR, Mackillop WJ. Trading treatment toxicity for survival in locally advanced non-small cell lung cancer. J Clin Oncol. 1997;15:330–340. doi:10.1200/JCO.1997.15.1.330
51. Brundage MD, Feldman-Stewart D, Cosby R, et al. Cancer patients’ attitudes toward treatment options for advanced non-small cell lung cancer: implications for patient education and decision support. Patient Educ Couns. 2001;45:149–157. doi:10.1016/s0738-3991(01)00155-0
52. Hirose T, Yamaoka T, Ohnishi T, et al. Patient willingness to undergo chemotherapy and thoracic radiotherapy for locally advanced non-small cell lung cancer. Psychooncology. 2009;18:483–489. doi:10.1002/pon.1450
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
