Back to Journals » Patient Preference and Adherence » Volume 14
Patient Preferences for Biologic and Biosimilar Osteoporosis Treatments in Colombia
Authors Graham-Clarke PL, Hauber B , Boeri M, Leonardi F, Burge RT , Fernandez M, Tockhorn-Heidenreich A , Florez S
Received 21 February 2020
Accepted for publication 1 June 2020
Published 23 June 2020 Volume 2020:14 Pages 1049—1064
DOI https://doi.org/10.2147/PPA.S250745
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Peita L Graham-Clarke,1 Brett Hauber,2 Marco Boeri,3 Felice Leonardi,4 Russel T Burge,5 Maria Fernandez,2 Antje Tockhorn-Heidenreich,6 Sandra Florez4,7
1Global Patient Outcomes and Real World Evidence, Eli Lilly Australia, West Ryde, NSW 2114, Australia; 2Health Preference Assessment Group, RTI Health Solutions, Research Triangle Park, NC 27709, USA; 3Health Preference Assessment Group, RTI Health Solutions, Belfast BT2 8LA, UK; 4Eli Lilly Interamerica Inc, Bogotá, Colombia; 5Global Patient Outcomes and Real World Evidence, Eli Lilly and Co, Lilly Corporate Center, Indianapolis, IN 46285, USA; 6Global Patient Outcomes and Real World Evidence, Eli Lilly and Co, Erl Wood Manor, Surrey GU20 6PH, UK; 7Pain and Palliative Care Unit, Universidad De La Sabana, Bogotá, Colombia
Correspondence: Marco Boeri
RTI Health Solutions, Forsyth House, Cromac Square Belfast, Belfast BT2 8LA, UK
Tel +44 (0)161 447 6016
Fax +1.919.541.7222
Email [email protected]
Purpose: Teriparatide is used to treat patients with established osteoporosis but is often reserved for patients who have inadequate response to antiresorptive therapy. Biosimilar teriparatide, which is believed to have efficacy and safety similar to the originator product, is now available in Colombia. However, little is known about patients’ preferences for originator biologic and biosimilar treatments. Our objective was to quantify the relative importance that patients in Colombia place on features of injectable osteoporosis treatments including whether the treatment is an originator biologic or a biosimilar.
Patients and Methods: We used a discrete choice experiment (DCE) to elicit preferences of patients with osteoporosis treatment devices in Colombia. The survey was completed by 200 respondents at high risk of fracture, with or without teriparatide experience. Each treatment alternative within the DCE was characterized by five attributes: type of medicine (originator biologic, biosimilar), needle length, angle of injection, how to measure the medicine dose, and how long the medicine can be left unrefrigerated. A random parameters logit regression was used to estimate preferences and conditional relative attribute importance, while controlling for preference heterogeneity.
Results: A total of 200 patients (mean age = 58.3 years) completed the survey. Most were female (84.5%) and married (54.5%); 50.5% had secondary education or less, 21% had current teriparatide exposure. The attribute with the highest conditional relative importance estimate (standard error) was biologic versus biosimilar (10 [1.11]), followed by needle length (8.06 [1.11]), dose measurement (6.38 [0.87]), refrigeration (3.81 [1.18]), and angle of injection (1.30 [0.66]). Unobserved preference heterogeneity was present and controlled for in the analyses.
Conclusion: Despite the availability of biosimilar teriparatide in Colombia, patients expressed a strong preference for an originator biologic osteoporosis medicine over a biosimilar osteoporosis medicine, when the efficacy, safety, and cost of the two options were assumed to be the same.
Keywords: discrete choice experiment, injection, devices, teriparatide, fractures
Introduction
Osteoporosis is a progressive disease characterized by low bone mineral density (BMD), deterioration of bone architecture, and compromised bone strength resulting in fractures.1 It causes significant morbidity and burden in aging populations and a growing impact on health system budgets. It was estimated in 2010, that 27.5 million people (5.5 million men and 22 million women) in Europe had osteoporosis,2 resulting in approximately 3.5 million new fractures, at an estimated cost of 37 billion Euros. Similarly, Wright et al3 estimated that in 2010, 10.2 million US adults aged 50 years and older had osteoporosis. In Latin America, it was estimated in 2012 that more than 1.4 million women in Colombia aged 50 years or older were living with osteoporosis, with this number projected to increase to more than 1.5 million by 2020, and to over 2 million by 2050.4 A report by the National Institute of Health of Colombia showed a higher overall prevalence of osteoporosis in Colombia compared with other locations for all age groups and an abrupt increase in the number of Colombian women with osteoporosis during the fifth and sixth decade of life.5
Teriparatide (recombinant human parathyroid hormone) is a bone-forming medication delivered by subcutaneous injection that preferentially stimulates osteoblasts to produce new bone tissue, thereby increasing bone mass and strength. Once-daily administration of teriparatide over the course of up to 24 months of treatment in postmenopausal women and men with osteoporosis reduces vertebral and non-vertebral fractures6–9 but is often restricted to more advanced disease. It may be cost-effective as a second-line therapy in patients who have more severe osteoporosis and who are not responding to conventional antiresorptive therapies as demonstrated by continued fracture.10
Recently, biosimilar teriparatide has been registered for use in Colombia. Biosimilar medicines are biologic products that are highly similar to originator reference products. Biosimilar products are not exact replicas of the originator biologic medicine; however, despite minor differences in clinically inactive components, biosimilars are assumed to have no clinically meaningful differences in terms of safety profile, purity, and potency compared with the originator biologic.11 The introduction of biosimilar agents in the health-care system has the potential for significant cost-savings, mitigating rising drug costs, or expanding patient access to biologic therapies.12 However, cost is not the only factor influencing biosimilar product uptake. Studies have demonstrated that despite a reduction in cost of 20–30% compared with the originator reference product, uptake of the biosimilar product does not always eclipse the utilization of the originator product.13 Available biosimilar products may vary in other ways that are important to prescribers and patients. Uptake greatly depends on health-care provider willingness to promote, prescribe, and use biosimilars in clinical practice and on patient acceptance of the alternative options. Health-care providers have been shown to approach biosimilar medicines with caution, citing limited biosimilar knowledge, low prescribing comfort, and safety and efficacy concerns as deterrents to biosimilar use.14
Previous patient preference studies of osteoporosis treatments have examined the relative importance of attributes that varied among available treatment options including efficacy and safety, frequency of dosing (once daily, weekly, monthly, yearly), cost, and route of administration (oral, injectable), and have demonstrated that these factors influence patients’ preferences, adherence, and satisfaction with osteoporosis treatments.15–19 However, none of the studies elicited patients’ preferences for biosimilar alternatives to existing biologic osteoporosis treatments among patients with advanced disease. Therefore, the aim of this study was to elicit preferences for osteoporosis treatment attributes from patients at high risk of fracture who are candidates for injectable treatment. A discrete choice experiment (DCE) survey was used to elicit preference for treatment attributes that patients identified as being important when choosing among injectable osteoporosis treatments when the efficacy, safety, and cost of the alternatives are assumed to be the same. In addition, we explored the importance to patients of educational and/or support programs by using an object-case best-worst scaling (BWS) exercise.20
Patients and Methods
Study Design
The discrete choice experiment (DCE) is one among a number of methods used to elicit patient preferences and has been used increasingly in recent years to quantify preferences for multiple attributes, the relative importance of those attributes to patients, and the tradeoffs patients are willing to make among those attributes.21–25 In a DCE, respondents are presented with a series of questions, each presenting a choice between two or more profiles defined by varying levels of a common set of attributes.26 The pattern of responses across the series of choices can be used to infer the implicit weights that respondents place on changes in the level of one attribute relative to changes in the levels of other attributes. The attributes used to define injectable medicine alternatives in this study were whether the medicine was an originator biologic or biosimilar, needle length, angle of injection, dose measurement, and refrigeration requirements. These attributes and the levels used to define each profile are presented in Table 1.26
 |
Table 1 Attributes and Levels of the Discrete Choice Experiment |
The attributes were identified by participants attending two qualitative focus groups conducted in Bogotá, Colombia, on July 13, 2017. Each focus group included five participants who were eligible to participate if they were 18 years of age or older, had a self-reported physician diagnosis of osteoporosis, and could provide informed consent. During the focus group, participants were first asked about what they liked most and what they liked least about their current or most recent osteoporosis treatment. After the initial discussion during which the explanation of a biosimilar product was provided (see Appendix), nurses demonstrated the use of two teriparatide drug-delivery devices: FORTEO (Eli Lilly and Company, Indianapolis, Indiana USA) and OSTEOTIDE (Virchow Biotech Private Limited). The devices used in the focus group were educational devices for demonstration purposes only, containing no medication, needle, or branding; neither device was identified as an originator biologic device or a biosimilar device. The nurses briefly explained how each device worked and summarized the features of each device (eg, needle length, cartridge design). Participants were asked to write one aspect they liked most and one they liked least about each device on an index card and then discuss these aspects with the group.
A draft English-language survey instrument was developed based on the findings from the qualitative focus groups, and pretesting of the survey was conducted via face to face interviews in December 2017. Pretests were conducted with a convenience sample of 10 patients in the US. Participants in the pre-tests had a self-reported physician diagnosis of severe osteoporosis and who either took daily injections to treat severe osteoporosis or were told by a health-care provider that they were at risk for fracture due to osteoporosis. The goal of the pretest interviews was to identify potential improvements in the content, formatting, language, and graphics used in the survey instrument. Patients were asked a series of debriefing questions to determine whether they understood the definitions and instructions in the survey instrument, accepted the hypothetical context of the survey, and successfully completed the choice questions in the survey instrument as instructed. The responses to the comprehension questions in the survey instrument and the patients’ verbal reasoning were considered in qualitatively assessing the performance of the survey instrument. The pretest interviews resulted in no significant changes to the survey instrument.
The English-language survey instrument was then translated into Colombian Spanish and reviewed by a Spanish speaker native to Colombia who was not involved in the translation process. To test the translation of the survey instrument and ensure that the concepts and questions presented in the survey were understandable to Spanish speakers, the translated survey instrument was further pretested with native Spanish speakers. The Spanish pretest interviews were conducted using individual telephone interviews with a convenience sample of 10 patients in the US who met the criteria for the English-language pretest interviews and spoke Spanish commonly used in Colombia, Mexico, or another South American country. The Spanish-language pretests were conducted by a native Spanish speaker in March 2018.
Survey Instrument
The final DCE presented each respondent with a series of questions, each of which asked them to select between two unlabeled hypothetical treatments for osteoporosis, characterized by the attributes and levels presented in Table 1. Multiple fractional factorial experimental designs were generated with no prior expectations on the coefficients in Sawtooth and compared for D-efficiency and correlation across attributes.27–30 The final experimental design comprised three blocks of 12 choice questions each. Respondents were randomly assigned to one block, and the questions within the block were presented in random order to avoid reduce effects. A sample choice question is shown in Figure 1.
 |
Figure 1 Example discrete choice experiment question (English-language version). |
The survey also included questions to elicit patients’ experiences with osteoporosis treatment and socioeconomic and demographic characteristics. Throughout the administration of the DCE, a series of comprehension questions were administered. The results indicated a high awareness of the difference between biologic and biosimilar. Finally, the survey included an exploratory object-case BWS to assess patients’ preferences for different types of educational and/or support programs that could be provided with biologic or biosimilar teriparatide, namely, in-person nurse training, starter kit with user guide, web-based video training, ongoing nurse support by phone, ongoing technical support by phone, and online website. The evaluation of education and/or support programs was exploratory and therefore excluded from the main analysis.
Data Collection
To be eligible to complete the survey, respondents were required to meet the following criteria:
- Be a resident of Colombia
- Be aged 18 years or older
- Have self-reported having taken teriparatide or having been told by a physician that they are at risk of serious fracture due to osteoporosis (overlap between the two was acceptable).
- Be able to read or understand Spanish and provide informed consent
To conduct the DCE with five attributes and a maximum of three levels for each attribute, a target of 200 respondents was planned.26 Respondents were recruited either through an online panel via email or intercepted in person in Colombia. In-person recruitment was implemented either through physician or personal contact referrals or were intercepted at key locations in different cities in Colombia. Respondents recruited in person were then invited to complete the survey on site using a tablet linked to the same online survey presented to respondents recruited through the online panel.
The survey instrument was programmed and hosted online by Survey Sampling International (SSI). On November 16, 2017, this study was reviewed by RTI International’s Institutional Review Board (IRB) and was deemed eligible for IRB exemption (RTI IRB No. 14139). The final survey administered in Colombia was reviewed and approved by the Ethics Committee for Research in Health Sciences Division of Universidad del Norte on May 31, 2018 (evaluation No. 174).
Data Analysis
The questions in a DCE generate cross-section/time-series data that require analysis using advanced statistical techniques.31 The DCE data were analyzed using a random parameters logit (RPL) model. The RPL model relates respondents’ treatment choices to the attribute levels of each treatment profile in the choice questions. The RPL model mitigates potential estimation bias in the mean preference weight estimates due to unobserved preference heterogeneity among respondents by estimating a distribution around each mean preference parameter and accounts for the fact that each respondent made multiple treatment choices over a series (panel) of choice questions.32–34 Given the two methods of recruiting, we tested whether data from respondents recruited online and respondents recruited in person could be pooled in a single data set using the test proposed by Swait and Louviere and found that it was possible to pool the data.35 In the second stage of the test, which looks for scale heterogeneity if preferences can be pooled, we found that statistically significant scale heterogeneity was not present in this data set.
For all analyses, a main-effects specification of the utility function as described in Equation 1 was used in estimations, and parameters estimated for each attribute level were assumed to be normally distributed across respondents to capture heterogeneity.
Eq (1) V = βBIOL × BIOL + βBIOS × BIOS
+ βMM4 × MM4 + βMM6 × MM6 + βMM12 × MM12
+ βDEG90 × DEG90 + βDEG45 × DEG45
+ βAUTO × AUTO + βMANU × MANU
+ βNEVR × NEVR + βH24 × H24 + βH36 × H36
where V is the value function for a particular treatment profile (specified as a function of the attributes as in Eq. 1), and β is a parameter estimate for each attribute level. The explanatory variables included in the final utility model are defined in Table 1.
In the final model specification, all independent variables were effects coded so that the mean effect of each attribute was normalized at zero. To have an identifiable model, one level of each attribute was omitted during estimation and recovered after estimation (as the negative sum of the included-category parameters) using the delta method to obtain standard errors.36 Effects-coded independent variables for each attribute level (eg, for a 3-level attribute: 0 1, 1 0, –1 –1, such that the parameter for the omitted category is the negative sum of the included categories) will be used instead of dummy-coded variables (eg, 0 1, 1 0, 0 0) so that the mean effect for each attribute is normalized at zero, rather than having a zero value corresponding to the one level omitted.
Once a functional form has been determined for the distribution of preferences, it is also possible to specify the variance-covariance matrix of the RPL model in a manner consistent with either density independence (by identifying only its diagonal values) or density correlation (by allowing for nonzero off-diagonal values). The latter can be used to account for both preference and variance (often referred to as scale) heterogeneity.37 However, a correlated model requires a greater number of degrees of freedom than a model with independent densities. We estimated uncorrelated and correlated RPL models and, we found that the two model specifications led to the same conclusions and that the correlated RPL model, which required many more degrees of freedom, resulted in a worse Bayesian information criterion. Therefore, the results of the uncorrelated RPL model are presented here. To determine the relative importance of an attribute from the model estimates, the difference between the attribute level with the highest preference weight and the level with the lowest preference weight was calculated. This difference represents the maximum change in utility achievable with any attribute, given the levels chosen for the attributes in the study.
Although the RPL model controls for unobserved preference heterogeneity among respondents in the sample, it does not identify observable characteristics that may be systematically associated with such differences in preferences. To test for systematic differences in preferences between these groups, treatment choices were analyzed using an RPL model with the same specification as the full-sample model. We created a dummy-coded variable that was equal to 1 if the respondent was recruited through the panel and interacted the dummy variable with each of the explanatory variables in Equation 1. The parameter for each of these interaction terms can be interpreted as the difference between the respondents recruited in person and the respondents recruited through the online panel for the preference weights for the corresponding attribute level. Systematic differences in preferences were evaluated using a test of the joint significance of all interaction terms.
All analyses were performed with Stata 15 (Stata Corp LLC, College Station, Texas).
Results
Respondents
Two hundred total respondents with osteoporosis who met the inclusion criteria participated in this study and were included in the final sample: 65 (32.5%) were recruited through the SSI online panel via email and 135 (67.5%) were intercepted in person in Colombia and invited to participate in the interview on a tablet. Table 2 presents respondent characteristics for both groups and for the full sample, including the result from the Fisher’s test to compare the two groups. The mean age was 58 years, with a median of 57. The high prevalence of women in the sample (84.5%, with 92.6% in the subsample recruited in person) is not surprising, as osteoporosis is more prevalent in females than in males. Over 50% of the respondents were married or living as married/civil partnership and had either basic elementary education or secondary education. Thirty-two percent of respondents were homemakers, 16% were employed full time, and 18% were retired. All characteristics listed in Table 2, except current and prior injectable treatment, were statistically significantly different between the groups recruited through the online panel and those intercepted in person.
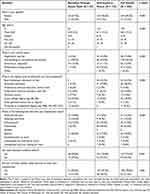 |
Table 2 Respondent Characteristics and Comparison Between Respondents Intercepted in Person and Respondents Recruited Through the Online Panel (Fisher Exact Test) |
Preference Weight Estimates
Table 3 presents the mean parameter estimates from the RPL model, including the parameter estimate for the omitted level of each attribute calculated using post-estimation. These estimates include the estimated parameters for each attribute level included in the model (mean preference estimates for the sample, which we refer to as preference weights), P values for the means, and 95% confidence intervals for the mean preference estimates. Because all attribute levels included in the DCE were modeled using effects-coded variables, the P value for each preference weight indicates the significance of each attribute level relative to the mean effect of that attribute (normalized to be zero). In addition to the mean preference weights and their significance levels, the RPL model output includes the standard deviations on all random parameters (presented in the last column of Table 3). All standard deviations were statistically significantly different from zero and relatively large, suggesting preferences heterogeneity for all attribute levels across respondents in the sample.
 |
Table 3 Mean Coefficients from the Random Parameters Logit Model Estimates for the Full Sample (N = 200) |
The results indicate that respondents prefer an original biologic medicine to a biosimilar medicine, shorter needle lengths, a 45-degree injection angle to a 90-degree angle, automatic dosing to manual dosing, and a medicine that can be left out of the refrigerator for longer periods of time. The mean preference weights for all levels of each attribute are statistically significantly different from one another at the 5% level of significance.
Conditional Relative Attribute Importance
The difference between the most- and least-preferred levels of each attribute is a measure of the conditional relative importance of that attribute, ie, the importance of an attribute relative to the other attributes in the study given the range of levels of that attribute. Computing these differences emphasizes that, among the full sample, type of osteoporosis medicine was the most important attribute followed by needle length, how to measure the medicine dose for each injection, how long the medicine can be left out of the refrigerator when traveling, and the angle of injection. Type of osteoporosis medicine was more than 7 times as important as the angle of injection and more than 2.5 times as important as the amount of time the medicine can be left out of the refrigerator, while needle length was approximately 6 times as important as the angle of injection and approximately twice as important as the amount of time the medicine can be left out of the refrigerator. Finally, type of osteoporosis medicine was statistically significantly more important than the amount of time the medicine can be left out of the refrigerator when traveling and the angle of injection. The injection angle was also statistically significantly less important than needle length and medication dosing. These results are also presented graphically in Figure 2 for a simpler overview; note that in the graphic, the results are scaled to show the conditional relative importance estimate for each attribute, such that the attribute with the highest conditional relative importance—type of osteoporosis medicine—is set to 10 and the conditional importance of each of the other attributes is scaled relative to the conditional importance of the attribute with the highest conditional relative importance.
Subgroup Analysis
We first explored differences in preferences between respondents who were recruited through the online panel (n = 65) vs those who were recruited in person (n = 135). The joint test of significance was statistically significant (P = 0.005), indicating that, overall, respondents who were recruited through the online panel had statistically significant, systematically different preferences compared with respondents who were recruited in person. The conditional relative importance estimates for respondents stratified by recruiting method are presented in Figure 3. Both groups preferred original biologic medicine to biosimilar medicine, shorter needle lengths, automatic dosing to manual dosing, and medicine that can be left out of the refrigerator for longer periods of time. However, only the respondents recruited through the online panel had a statistically significant preference for a 45-degree injection angle over a 90-degree injection angle. Additionally, both groups preferred being able to leave the medicine out of the refrigerator for 36 hours to never being able to leave the medicine unrefrigerated. However, respondents recruited in person had different preferences among the three levels of this attribute compared with respondents recruited through the online panel. Among respondents recruited in person, being able to leave the medicine out of the refrigerator for 36 hours was statistically significantly preferred to 24 hours, while the preference weights for these two attribute levels were not statistically significantly different among respondents recruited through the online panel.
We explored preferences across different subgroup pairs. The subgroup pairs, the sample size of each subgroup, and the P value of each joint test of significance are presented in Table 4.
 |
Table 4 Descriptions of the Subgroups Analyzed (N = 200) |
When a test of joint significance with seven degrees of freedom (the number of different parameters in the model with subgroups) was performed, preferences across subgroups based on age, marital status, fracture experience, and injection experience were not significantly systematically different, while preferences between subgroups based on education and employment were systematically different at more than a 5% level (0.2% and 2.3%, respectively; see Table 4). The results for these two subgroup pairs are summarized below, and Figures 4 and 5 present the conditional relative attribute importance estimates for each subgroup.
The results of the employment subgroup analysis indicate that respondents not employed outside the home had a stronger preference for original biologic medicine over biosimilar medicine than employed or self-employed respondents. The results also show, at least qualitatively, that employed and self-employed respondents placed a higher conditional relative importance on needle length and how to measure the medicine dose.
Among the education subgroups, respondents with a college education or higher placed greater importance on how the medicine dose is measured (preferring the automatic dosing) and considered the type of medicine to be as important as needle length and how long the medicine can be kept outside the refrigerator while traveling. In contrast, respondents with lower education considered the type of medicine and needle length to be the most important attributes. Additionally, the subgroup analysis based on education yielded results that were very similar to the results of the comparison between preferences of respondents recruited through the online panel and respondents recruited in person. Therefore, it is likely that differences in education explain the differences in preferences observed between respondents recruited using the two different recruitment methods.
Discussion
This study elicited preferences of patients with osteoporosis in Colombia for features of injectable originator biologic and biosimilar devices for osteoporosis following good research practices for the conduct of discrete choice experiments.26,38 Because efficacy and safety are assumed to be the same between originator biologics and biosimilars, and the out of pocket costs would not differ from the patient’s perspective, this study focused on eliciting patients’ preferences for treatment attributes other than efficacy, safety, and cost, that patients indicated were important when choosing among alternative injectable treatments for osteoporosis. On average, Colombian patients with osteoporosis indicated that having an originator biologic product rather than a biosimilar product was the most important driver of treatment choice even after they were informed that a biosimilar treatment works about as well as the originator biologic and has side effects that are very similar to those of the originator biologic. The remaining treatment attributes were, on average, less important to these patients. Specifically, needle length was the second most important attribute followed by (in decreasing order of importance) how the dose is measured, how long the medicine can be left unrefrigerated, and the angle of injection.
The attributes included in this study were based directly on input from patients. In this regard, our study is not entirely unique. Previous preferences studies in osteoporosis also used qualitative research with patients to identify treatment attributes. For example, Silverman et al conducted qualitative interviews with osteoporosis patients.39 Hiligsmann et al conducted group discussions with patients to prioritize a list of 12 medication attributes identified from the literature and discussion with clinicians.40 The attributes included in the current study were identified by patients as being important through focus groups conducted in Colombia and were confirmed as being relevant to patients in qualitative pretest interviews in both the US and Colombia. In the focus groups, patients were asked to indicate which features of injection devices used to deliver biologic and biosimilar osteoporosis medicines were important to patients. Attribute levels were defined based on publicly available consumer information for injectable products. Unlike in other studies, route of administration was effectively excluded from this study because the focus of the study was on biologic and biosimilar treatments which can only be delivered by injection.
Previous research has demonstrated that patients understand differences between biologic and biosimilar medicines, however, this study is the first to use a DCE to elicit patients’ preferences for both biologic and biosimilar treatments for osteoporosis.41 Baiji and colleagues used a DCE to elicit gastroenterologists’ preferences for using biologic and biosimilar drugs in Crohn’s disease and ulcerative colitis.42,43 These studies found that treatment type (biologic versus biosimilar) was important to gastroenterologists, particularly when considering treatment for a patient who was already on an originator biologic. Azevedo and colleagues asked Portuguese patients direct questions to elicit their opinions about biosimilar treatments for psoriasis and found that the majority opposed the use of biosimilars because of the lack of clinical studies of these products in European populations or in patients with psoriasis.44 Two additional studies used surveys to elicit attitudes toward biosimilars among patients in Germany and found that patients were reluctant to accept biosimilar products in part because they believed not enough was known about the treatment.45,46 Finally, a number of studies used simulated-use or interview methods to elicit patients’ impressions of autoinjectors for biosimilar formulations of etanercept and adalimumab; however, none of these studies used a DCE to elicit preferences.47–50
To our knowledge, this is one of the first DCEs to be administered to patients in Colombia. Most of the previous studies of patients’ preferences for osteoporosis treatments were conducted in the US and Western Europe and none of the existing studies were conducted in Latin America.40 As a result, the vast majority of research designed to understand preferences for osteoporosis treatments involved eliciting preferences among middle-class Caucasian women. A multi-country European study demonstrated that statistically significant differences in preferences between countries for some, but not all, osteoporosis treatment attributes indicate that cultural and socioeconomic differences among countries may influence treatment preferences.51 Silverman found no differences in preferences among postmenopausal women from different racial and ethnic groups; however, education and income were found to be predictors of patient preferences.39 Similar to Silverman, we found that preferences differed systematically by education and employment, indicating that preferences within what is likely a culturally homogeneous population vary based on socioeconomic status.39
This study focused on eliciting preferences of osteoporosis patients who had either injectable osteoporosis treatment experience or who had been told they were at high risk of fracture. Some previous studies of patient preferences for osteoporosis treatment included osteoporosis patients at lower risk of fracture who were eligible for pharmacologic treatment or for whom medication or lifestyle changes had been proposed.16,51 Other studies included patients with severe osteoporosis or high fracture risk in the sample.15,52 Among previous stated-preference studies in osteoporosis, only Silverman and colleagues sampled only patients with prior fracture or who were at high risk of fracture.39 Among those studies that included high-risk patients in the sample, one study found that patients at high risk for fracture were more concerned about reducing the risk of fracture than those at lower risk.15 In this study, sampling patients at higher risk of fracture were chosen because biologic treatments for osteoporosis are accessible in Colombia only to patients as a second-line option and because higher fracture risk has been shown to affect patient preferences for medications.15
Patient preference studies provide important information about the relative importance that patients place on different aspects of treatment or healthcare delivery. Stated-preference studies similar to the one reported here have been used to inform approval decisions by regulators and to support shared decision-making between patients and their health-care providers.53,54 Patient preference studies often demonstrate that patients’ values may differ from those of their doctors.55 In addition, such studies can provide insights into why patients may choose not to adhere to treatment despite the risk of poor health outcomes.56 Therefore, patient preference information can inform health-care decisions throughout the healthcare system.
This study was patient-centered and used rigorous methods to elicit patient preferences following good research practices; however, our study also has some limitations. First, stated preferences may differ from preferences implied by actual treatment choices. An inherent limitation of all stated-preference methods is that respondents are evaluating hypothetical medications, and what respondents declare they will do may potentially be different from what they would actually do if faced with the choice in real life. However, the purpose of the study was not to predict future behavior but to elicit preferences and quantify the relative importance of attributes that differentiate biologic and biosimilar osteoporosis treatments. Our study focused on five attributes of a hypothetical injectable treatment, we did not incorporate attributes related to other types of osteoporosis medications that are available to patients at high risk of osteoporosis. We do not know whether our findings would be different if we included additional attributes in our study. Noteworthy is that when examining the list of attributes, no teriparatide treatment, biologic or biosimilar, is positive on all five attributes and originator teriparatide does not necessarily differentiate from biosimilar teriparatide on the four attributes. Originator teriparatide, for example, cannot be left unrefrigerated for any length of time and while angle of injection was included as a device attribute, it is not included in product labels. Therefore, we cannot use these results to accurately predict the likelihood that any individual patient or group of patients would choose biologic or biosimilar teriparatide.
Another potential limitation is that the survey was administered in 2018, shortly after the introduction of biosimilar teriparatide; therefore, patients may not have been familiar or comfortable with biosimilar treatments beyond what was explained during the conduct of the study. Treatment patterns and patient awareness of or comfort with biosimilar alternatives could have changed since the data were collected.
The sample included in the study may not be representative of patients who would be offered teriparatide – originator or biosimilar. The average age of respondents was 58 years, and the majority of respondents had not experienced a fracture, suggesting that their baseline risk of subsequent fracture may be lower than for patients currently treated with teriparatide in Colombia. Furthermore, the sample size for the study may be insufficient to detect some differences in preferences between subgroup pairs. For example, our study was unable to examine the impact of secondary factors including income, chronological age, and prior teriparatide treatment on patient weighting of attributes for injectable osteoporosis medications.
Finally, the results from patients recruited through the online panel differed from those recruited in person indicating that the results may be sensitive to the method of recruitment or that the method of recruitment resulted in some selection bias. However, differences appear to be driven by differences in education rather than differences in recruitment method per se which may provide an explanation for the differences in results between the two groups.
Conclusion
Clinical and policy decisions, as well as product development could benefit from knowledge about what patients’ value and prefer regarding their treatment. In this study, we demonstrated that despite the availability of biosimilar teriparatide in Colombia, when given the choice between hypothetical osteoporosis treatments, when the efficacy, safety, and cost of the two options were assumed to be the same, patients in Colombia expressed a strong preference for an original biologic osteoporosis medicine over a biosimilar osteoporosis medicine. In addition, patients of higher socioeconomic status (defined by higher employment and education) likely place greater emphasis on convenience (dose measurement and refrigeration requirements) than do patients who are unemployed or have lower educational attainment. Colombian physicians and policymakers should therefore not only take patient preferences but also individual patient characteristics, into account when determine the appropriate approaches to manage osteoporosis among those at high risk of fracture.
Abbreviations
BA, Bachelor of Arts; BIOL, biologic; BIOS, biosimilar; BMD, bone mineral density; BS, Bachelor of Science; BWS, best-worst scaling; DCE, discrete choice experiment; IRB, Institutional Review Board; LL, loglikelihood; Max, maximum (age); MBA, Master of Business Administration; MD, Medical Doctor; Min, minimum (age); mm, millimeter; MS, Master of Science; PhD, Doctor of Philosophy; Q1, 25th percentile; Q3, 75th percentile; RPL, random parameters logit; SD, standard deviation.
Data Sharing Statement
The data used in the analyses are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The RTI International Institutional Review Board (IRB) determined that this study meets the criteria for IRB exemption. In the qualitative phase, each interview participant signed and dated an informed consent form before the interview. The online survey administered in Colombia was reviewed and approved by the Ethics Committee for Research in Health Sciences Division of Universidad del Norte on May 31, 2018 (evaluation No. 174). All respondents to the discrete choice experiment survey provided electronic informed consent and received compensation for time spent participating. The study complied with the Declaration of Helsinki.
Acknowledgments
The authors gratefully acknowledge Kimberly Moon of RTI Health Solutions for project management support and Kathleen Gallaher for assistance with data analysis. The abstract of this paper and details contained within the manuscript were presented at the ISPOR Latin America 2019 Conference in Bogotá, Colombia, 12–14 September 2019. The poster’s abstract was published in Value in Health Regional Issues as Leonardi Reyes F. et al PMS17 PATIENT PREFERENCES FOR BIOLOGIC AND BIOSIMILAR OSTEOPOROSIS TREATMENTS IN COLOMBIA: A DISCRETE-CHOICE EXPERIMENT Value in Health Regional Issues, Volume 19, S54.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The research was funded by Eli Lilly and Company.
Disclosure
Dr Peita Graham-Clarke reports being an Employee and Shareholder of Eli Lilly; Dr Brett Hauber is an employee of RTI Health Solutions during the conduct of the study; Dr Marco Boeri report Marco Boeri is an employee of RTI Health Solutions; Dr Russel Burge is a Salaried employee and stockholder of Eli Lilly and Company during the conduct of the study; Dr Maria Fernandez is an employee of RTI-HS which received funding from Eli Lilly & Co. to conduct the study which is the subject of this manuscript; Ms Antje Tockhorn-Heidenreich is an Employee and minor stockholder of Eli Lilly and Company during the conduct of the study; Dr Sandra Florez report personal fees from Eli Lilly, during the conduct of the study and is an employee of Eli Lilly; Felice Leonardi is an employee of Eli Lilly and Company. The analysis reported in this manuscript was funded by Eli Lilly and Company through a research contract with RTI Health Solutions. The authors report no other conflicts of interest in this work.
References
1. Kanis JA, Melton LJ
2. Hernlund E, Svedbom A, Ivergrd M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos. 2013;8(1–2):136. doi:10.1007/s11657-013-0136-1
3. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. doi:10.1002/jbmr.2269
4. Latin America Regional Audit. Epidemiology, costs and burden of osteoporosis in 2012. International osteoporosis foundation; 2012. Available at: https://www.iofbonehealth.org/sites/default/files/media/PDFs/Regional%20Audits/2012-Latin_America_Audit-ES_0_0.pdf.
5. Ardila E. Epidemiology of osteoporosis in Colombia. Bone. 2001;29(3):297. doi:10.1016/S8756-3282(01)00518-X
6. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. doi:10.1056/NEJM200105103441904
7. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16(5):510–516. doi:10.1007/s00198-004-1713-3
8. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a randomized, double-blind, double-dummy, randomized controlled trial. Lancet. 2018;391(10117):230–240. doi:10.1016/S0140-6736(17)32137-2
9. Geusens P, Marin F, Kendler DL, et al. Effects of teriparatide compared with risedronate on the risk of fractures in subgroups of postmenopausal women with severe osteoporosis: the VERO trial. J Bone Miner Res. 2018;33(5):783–794. doi:10.1002/jbmr.3384
10. Murphy DR, Smolen LJ, Klein TM, Klein RW. The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet Disord. 2012;13(1):213. doi:10.1186/1471-2474-13-213
11. United States Food and Drug Administration. Biosimilar and interchangeable products. Available at: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm580419.htm#biosimilar.
12. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the united states: initial experience and future potential. Rand Health Q. 2018;7:3.
13. Marciano I, Ingrasciotta Y, Giorgianni F, et al. Pattern of use of biosimilar and originator somatropin in Italy: a population-based multiple databases study during the years 2009–2014. Front Endocrinol (Lausanne). 2018;9:95. doi:10.3389/fendo.2018.00095
14. Leonard E, Wascovich M, Oskouei S, Gurz P, Carpenter D. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019;25(1):102–112. doi:10.18553/jmcp.2019.25.1.102
15. de Bekker-grob EW, Essink-Bot ML, Meerding WJ, Pols HAP, Koes BW, Steyerberg EW. Patients’ preferences for osteoporosis drug treatment: a discrete choice experiment. Osteoporos Int. 2008;19(7):1029–1037. doi:10.1007/s00198-007-0535-5
16. Fraenkel L, Gulanski B, Wittink D. Patient treatment preferences for osteoporosis. Arthritis Rheum. 2006;55(5):729–735. doi:10.1002/art.22229
17. Greenfield S, Kaplan S, Ware JE
18. Daltry LH. Doctor-patient communication in rheumatological disorders. Ballieres Clin Rheumatol. 1993;7(2):221–239. doi:10.1016/S0950-3579(05)80087-1
19. Lee S, Glendenning P, Inderjeeth C. Efficacy, side effects and route of administration are more important than frequency of dosing of anti-osteoporosis treatments in determining patient adherence: a critical review of published articles from 1970 to 2009. Osteoporos Int. 2011;22(3):741–753. doi:10.1007/s00198-010-1335-x
20. Flynn TN, Louviere JJ, Peters TJ, Coast J. Best-worst scaling: what it can do for health care research and how to do it. J Health Econ. 2007;26(1):171–189. doi:10.1016/j.jhealeco.2006.04.002
21. Soekhai V, Whichello C, Levitan B, et al. Methods for exploring and eliciting patient preferences in the medical product lifecycle: a literature review. Drug Discov Today. 2019;24(7):1324–1331. doi:10.1016/j.drudis.2019.05.001
22. Medical Device Innovation Consortium. Medical Device Innovation Consortium (MDIC) patient centered benefit-risk project report: a framework for incorporating information on patient preferences regarding benefit and risk in regulatory assessments of new medical technology; 2015. Available at: https://mdic.org/wp-content/uploads/2018/05/MDIC_PCBR_Framework_Web.pdf.
23. Soekhai V, de Bekker-grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–226. doi:10.1007/s40273-018-0734-2
24. Clark MD, Determann D, Petrou S, Moro D, de Bekker-grob EW. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics. 2014;32(9):883–902. doi:10.1007/s40273-014-0170-x
25. de Bekker-grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21(2):145–172. doi:10.1002/hec.1697
26. Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
27. Kuhfeld W, Tobias F, Garratt M. Efficient experimental design with marketing research applications. J Marketing Res. 1994;31(4):545–557. doi:10.1177/002224379403100408
28. Kuhfeld W Efficient experimental designs using computerized searches.
29. Chrzan K, Orme B. An Overview and Comparison of Design Strategies for Choice-Based Conjoint Analysis. Technical Paper Series. Sawtooth Software Inc; 2000.
30. Kuhfeld W. Marketing Research Methods in SAS: Experimental Design, Choice, Conjoint, and Graphical Techniques. Cary, NC: SAS Institute Inc; 2010.
31. Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–315. doi:10.1016/j.jval.2016.04.004
32. McFadden D, Train K. Mixed MNL models for discrete response. J Appl Econ. 2000;15(5):447–470. doi:10.1002/1099-1255(200009/10)15:5<447::AID-JAE570>3.0.CO;2-1
33. Train K. Discrete Choice Methods with Simulation.
34. Train K, Sonnier G. Mixed logit with bounded distributions of correlated partworths. In: Scarpa R, Alberini A, editors. Applications of Simulation Methods in Environmental and Resource Economics. Dordrecht, Netherlands: Springer; 2005.
35. Swait J, Louviere J. The role of the scale parameter in the estimation and comparison of multinomial logit models. J Marketing Res. 1993;30(3):305–314. doi:10.1177/002224379303000303
36. Hensher DA, Rose JM, Greene WH. Applied Choice Analysis. Cambridge (UK): Cambridge University Press; 2005.
37. Hess S, Rose JM. Can scale and coefficient heterogeneity be separated in random coefficients models? Transportation. 2012;39(6):1225–1239. doi:10.1007/s11116-012-9394-9
38. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making. Pharmacoeconomics. 2008;268(8):661–677. doi:10.2165/00019053-200826080-00004
39. Silverman S, Calderon A, Kaw K, et al. Patient weighting of osteoporosis medication attributes across racial and ethnic groups: a study of osteoporosis medication preferences using conjoint analysis. Osteoporos Int. 2013;24(7):2067–2077. doi:10.1007/s00198-012-2241-1
40. Hiligsmann M, Bours SP, Boonen A. A review of patient preferences for osteoporosis drug treatment. Curr Rheumatol Rep. 2015;17(9):61. doi:10.1007/s11926-015-0533-0
41. Van Overbeeke E, de Beleyr B, de Hoon J, Westhovens R, Huys I. Perception of originator biologics and biosimilars: a survey among Belgian rheumatoid arthritis patients and rheumatologists. BioDrugs. 2017;31(5):447–549. doi:10.1007/s40259-017-0244-3
42. Baji P, Gulácsi L, Lovász BD, et al. Treatment preferences of originator versus biosimilar drugs in Crohn’s disease; discrete choice experiment among gastroenterologists. Scand J Gastroenterol. 2016;51(1):22–27. doi:10.3109/00365521.2015.1054422
43. Baji P, Gulácsi L, Golovics PA, et al. Perceived risks contra benefits of using biosimilar drugs in ulcerative colitis: discrete choice experiment among gastroenterologists. Value Health Reg Issues. 2016;10:85–90. doi:10.1016/j.vhri.2016.07.004
44. Azevedo A, Bettencourt A, Selores M, Torres T. Biosimilar agents for psoriasis treatment: the Perspective of Portuguese patients. Acta Med Port. 2018;31(9):496–500. doi:10.20344/amp.10127
45. Waller J, Sullivan E, Piercy J, Black CM, Kachroo S. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence. 2017;11:519–530. doi:10.2147/PPA.S129333
46. Sullivan E, Piercy J, Waller J, Black CM, Kachroo S. Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PLoS One. 2017;12:e0175826. doi:10.1371/journal.pone.0175826
47. Tischer B, Mehl A. Patients’ and nurses’ preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Prefer Adherence. 2018;12:1413–1424. doi:10.2147/PPA.S169339
48. Egeth M, Soosaar J, Nash P, et al. Patient and healthcare professionals preference for Brenzys vs. Enbrel autoinjector for rheumatoid arthritis: a randomized crossover simulated-use study. Adv Ther. 2017;34(5):1157–1172. doi:10.1007/s12325-017-0523-x
49. Thakur K, Biberger A, Handrich A, Rezk MF. Perceptions and preferences of two etanercept autoinjectors for rheumatoid arthritis: a new European Union-approved etanercept biosimilar (Benepali®) versus etanercept (Enbrel®) - findings from a nurse survey in Europe. Rheumatol Ther. 2016;3(1):77–89. doi:10.1007/s40744-016-0035-1
50. Fenwick S, Thakur K, Munro D. Nurse and patient perceptions and preferences for subcutaneous autoinjectors for inflammatory joint or bowel disease: findings from a European survey. Rheumatol Ther. 2019;6(2):195–206. doi:10.1007/s40744-019-0144-8
51. Hiligsmann M, Dellaert BG, Dirksen CD, et al. Patients’ preferences for anti-osteoporosis drug treatment: a cross-European discrete choice experiment. Rheumatology. 2017;56(7):1167–1176. doi:10.1093/rheumatology/kex071
52. Darbà J, Restovic G, Kaskens L, et al. Patient preferences for osteoporosis in Spain: a discrete choice experiment. Osteoporos Int. 2011;22(6):1947–1954. doi:10.1007/s00198-010-1382-3
53. Ho MP, Gonzalez JM, Lerner HP, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29(10):2984–2993. doi:10.1007/s00464-014-4044-2
54. Weernink MGM, van Til JA, Witteman HO, Fraenkel L, IJzerman MJ. Individual value clarification methods based on conjoint analysis: a systematic review of common practice in task design, statistical analysis, and presentation of results. Med Decis Making. 2018;38(6):746–755. doi:10.1177/0272989X18765185
55. Johnson FR, Hauber AB, Özdemir S, Siegel CA, Hass S, Sands BE. Are gastroenterologists less tolerant of treatment risks than patients? Benefit-risk preferences in Crohn’s disease management. J Manag Care Pharm. 2010;16(8):616–628. doi:10.18553/jmcp.2010.16.8.616
56. Laba TL, Essue B, Kimman M, et al. Understanding patient preferences in medication nonadherence: a review of stated preference data. Patient. 2015;8(5):385–395. doi:10.1007/s40271-014-0099-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




