Back to Journals » Clinical Ophthalmology » Volume 14
Patient Preferences Associated with Anti-Vascular Endothelial Growth Factor Therapies for Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema
Authors Bhagat D , Kirby B, Bhatt H, Jager R, George M , Sheth V
Received 24 July 2020
Accepted for publication 14 September 2020
Published 1 October 2020 Volume 2020:14 Pages 2975—2982
DOI https://doi.org/10.2147/OPTH.S273564
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Davis Bhagat,1 Breanne Kirby,1 Harit Bhatt,1,2 Rama Jager,1,2 Meena George,1,2 Veeral Sheth1,2
1University Retina and Macula Associates, Oak Forest, IL, USA; 2University of Illinois, Chicago, IL, USA
Correspondence: Veeral Sheth
University Retina and Macula Associates, 15947 W 127th St Suite E, Lemont, IL 60439, USA
Tel/Fax +1 630 410 8357
Email [email protected]
Purpose: To evaluate treatment-related preferences among patients receiving intravitreal anti-vascular endothelial growth factor (VEGF) therapy for neovascular age-related macular degeneration (nAMD) or diabetic macular edema (DME).
Patients and Methods: We conducted a prospective survey of patients with nAMD or DME treated at one of three US-based retina clinics. Prior to survey development, small focus groups with anti-VEGF-treated patients identified five treatment-related “attributes” considered important to those with nAMD or DME: vision outcomes, cost to the insurance provider, cost to the patient, frequency of treatment, and drug label status. Attributes were described using two to three “levels”, and hypothetical treatment profiles were generated by assigning one level to each attribute. Surveyed patients were asked to indicate their preference between two given treatment profiles for a total of eight pairwise comparisons. Discrete choice conjoint analysis was performed to estimate the relative importance of each attribute for the overall patient cohort, and for subgroups stratified by age and highest education level.
Results: Among 300 respondents, 54% were female, 78% were aged ≥ 65 years, and 67% indicated that high school was their highest level of education. Achieving good vision was the most important factor associated with anti-VEGF therapy for nAMD or DME (relative importance, 40.4%), followed by low cost to the patient, on-label drug status, less frequent treatment intervals, and low cost to the insurance provider (23.1%, 21.3%, 12.2%, and 3.0%, respectively). When patients were stratified by age group or highest education level, preference trends across subgroups were generally comparable with the overall cohort.
Conclusion: Our data suggest that treatment decisions regarding anti-VEGF therapies for nAMD or DME are most likely driven by their efficacy, and that patients may be willing to accept less desirable treatment attributes, such as increased cost and/or injection frequency, in order to achieve superior vision outcomes.
Keywords: anti-VEGF therapy, conjoint analysis, diabetic macular edema, neovascular age-related macular degeneration, patient preferences
Introduction
Diabetic macular edema (DME) and neovascular age-related macular degeneration (nAMD) are leading causes of blindness among working-age and elderly populations, respectively. Intravitreal anti-vascular endothelial growth factor (VEGF) therapy is considered the standard of care for nAMD and DME,1,2 with four agents commonly used in current clinical practice: US Food and Drug Administration (FDA)–approved ranibizumab and aflibercept for nAMD and DME; FDA-approved brolucizumab for nAMD; and off-label bevacizumab for both conditions.3–6 Landmark trials suggest that these agents have comparable safety and efficacy in their respective indications;7–12 therefore, treatment preferences among physicians and patients may instead be driven by other factors, such as differences in cost and labeled injection frequency. For example, monthly injections of ranibizumab 0.5 mg and 0.3 mg are FDA-approved for the treatment of nAMD and DME, respectively,3 whereas aflibercept 2.0 mg is indicated every 8 weeks after three to five monthly loading doses,4 and brolucizumab 6.0 mg is recommended every 8–12 weeks after three monthly loading doses in patients with nAMD.5 Moreover, wholesale acquisition costs for these on-label therapies currently range between $1170–$1950 per dose, while the cost of repackaged bevacizumab 1.25 mg has been estimated at $50–$60.13–16
As treatment decisions become more complex and the delivery of patient-centered care becomes increasingly important, physicians are often encouraged to involve patients in decisions regarding their health. This paradigm shift towards shared decision-making has been driven by evidence that treatment adherence, patient satisfaction, and clinical outcomes are improved when patient preferences are taken into consideration.17–19 Patient preferences in health care are often evaluated using conjoint analysis, a survey-based technique used to quantify the value placed on characteristics (or “attributes”) related to a health product or service. Through a series of trade-off exercises, patient preferences are used to generate utility values that demonstrate the relative importance of each attribute in treatment decisions.20,21 Conjoint analyses have been used to elicit patient preferences for the management of several ophthalmic conditions, including glaucoma,22,23 cataracts,24 diabetic retinopathy,25 and nAMD.26
In light of the similarities and differences between anti-VEGF therapies for nAMD and DME, we sought to better understand the treatment-related attributes that patients consider most important in current clinical practice. This prospective survey and conjoint analysis aimed to elicit and evaluate patient preferences among those receiving intravitreal anti-VEGF therapy for the management of nAMD or DME.
Materials and Methods
Study Design and Patient Population
This was a prospective study of patients with nAMD or DME surveyed at one of three University Retina clinics (Bedford Park, IL; Lemont, IL; Oak Forest, IL) between August and December 2018. Patients aged ≥18 years and receiving intravitreal anti-VEGF therapy for nAMD or DME were eligible to participate; non-English-speaking patients and those with an impaired ability to understand and provide verbal consent were excluded. Details of the study were explained to those deemed eligible by the investigators, and patients who provided verbal consent completed the electronic survey during their scheduled clinic visit. Patients completed the survey anonymously and no identifiable data were collected; therefore, this study was exempted from requiring ethical approval by the Quorum Review institutional review board.
Survey Design
Prior to survey development, small focus groups with patients who had received three or more anti-VEGF injections (three groups of five patients) were conducted to identify treatment-related attributes that may be considered important to those with nAMD or DME. Five attributes were identified and subsequently evaluated in the survey: vision outcomes with treatment, cost of treatment to the insurance provider, cost of treatment to the patient, frequency of treatment, and drug label status.
The survey was developed and administered using SurveyAnalytics software (QuestionPro Inc, Austin, TX).27 The five treatment attributes to be assessed were each assigned two to three “levels” to reflect differing features of anti-VEGF therapy; attributes, levels, and definitions provided to patients are described in Table 1. During the survey, patients were asked to indicate their preference between two hypothetical anti-VEGF treatment profiles, each comprising a set of attributes described by a set of levels (Figure 1). Based on the number of treatment attributes and levels assessed in this study, 48 hypothetical treatment profiles are available for comparison; however, it is not feasible for patients to meaningfully consider every possible combination in one survey. As such, patients completed eight pairwise comparisons in this study, which were dynamically assigned by the software using a D-optimal design algorithm.
 |
Table 1 Anti-Vascular Endothelial Growth Factor Treatment Attributes, Levels, and Definitions Provided to Patients |
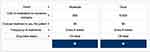 |
Figure 1 Illustrative treatment profile comparison presented to patients. |
Statistical Analysis
Demographic data collected in the survey (age, sex, and highest level of education) are presented using descriptive statistics. Discrete choice conjoint analysis was performed with SurveyAnalytics software, which used survey responses to generate utility values for each attribute level using multinomial logistic regression and Nelder–Mead simplex methodology.28 The relative importance of each attribute was expressed as a percentage by dividing the utility range for a given attribute (ie, highest minus lowest utility level) by the sum of utility ranges for all attributes and multiplying by 100. The relative importance of the five attributes was calculated for the overall patient cohort, and for subgroups stratified by age and highest education level.
Results
Study Population
In total, 300 patients receiving intravitreal anti-VEGF therapy for nAMD or DME completed the survey. Patient demographics at the time of the survey are summarized in Table 2. Fifty-four percent of patients were female, the majority (78%) were aged ≥65 years, and high school was the highest level of education achieved for 67% of the cohort.
 |
Table 2 Baseline Patient Demographics |
Overall Preferences
Conjoint analysis found that achieving good vision was the most important attribute for patients receiving intravitreal anti-VEGF therapy for nAMD or DME (Table 3). The relative importance of good vision was 40.4% for the overall cohort, followed by cost to the patient (preference for low cost; 23.1% relative importance), and drug label status (preference for on-label status; 21.3% relative importance). The least important attributes were treatment frequency (preference for less frequent treatment; 12.2% relative importance) and cost to the insurance provider (preference for low cost; 3.0% relative importance).
 |
Table 3 Utility Values and Relative Importance of Anti-Vascular Endothelial Growth Factor Treatment Attributes |
Preferences by Age
When patients were stratified by age, good vision remained the most desirable feature of intravitreal anti-VEGF therapy across age groups (Figure 2). Compared with other age groups, patients aged 18–39 years placed greater importance on vision (63% vs 39–43% relative importance) and placed less importance on treatment frequency (0% vs 9–16% relative importance); however, these results are based on a small sample size (n=4) and should be interpreted with caution. The relative importance of drug label status was greater for those aged 65–79 years than other age groups (26% vs 14–19% relative importance); otherwise, preference trends across age groups were generally consistent with the overall cohort.
Preferences by Education Level
The relative importance of treatment attributes for patients stratified by highest education level is presented in Figure 3. Vision remained the most important factor for patients who had completed high school or an undergraduate degree (39% and 50% relative importance, respectively). The importance of cost to the patient was comparable to vision outcomes among those who did not complete high school (33% vs 32% relative importance), while patients with a graduate/professional degree placed slightly greater importance on drug label status than vision outcomes (31% vs 27% relative importance). Patients who completed a graduate/professional degree also considered treatment frequency to be more important than any other subgroup (21% vs 1–13% relative importance), while those who did not complete high school placed greater importance on cost to the insurance provider than treatment frequency (12% vs 3% relative importance).
Discussion
Our conjoint analysis of 300 patients in current clinical practice revealed that vision was the most important factor when considering intravitreal anti-VEGF therapies for nAMD or DME. These data suggest that treatment decisions regarding anti-VEGF agents are most likely driven by their efficacy, and that patients may be willing to accept less desirable treatment attributes, such increased cost and/or injection frequency, in order to achieve superior vision outcomes.
Our results coincide with previous analyses that have demonstrated the importance of vision outcomes among patients receiving treatment for nAMD or DME.26,29,30 In particular, Baxter et al used conjoint analysis to evaluate preferences regarding nAMD management in UK clinical practice and found that patients ranked good vision above a one-stop clinic setup (whereby injections are administered during the same scheduled visit), less frequent visits, clinic waiting time, drug label status, cost to the National Health Service, and the qualification of the injector (doctor vs nurse practitioner).26 Similarly, a discrete choice experiment by Mueller et al found that “change of visual acuity” was the most important factor among patients receiving anti-VEGF therapy for nAMD in Germany, followed by “waiting, treatment, and travel time”, and “treatment scheme” (comparing injections every 4 weeks, every 8 weeks, and as-needed with monthly visits).29 Due to the manner in which health care is funded in the UK and Germany, costs incurred to patients were not considered in these studies; however, the results of our analysis suggest that when out-of-pocket costs are factored into treatment decisions, patients are nevertheless willing to trade-off increased costs for improved vision gains. Our findings are also consistent with other conjoint analyses that identified vision-related outcomes as the most important factors among patients with glaucoma,22 cataracts,24 and diabetic retinopathy;25 collectively demonstrating the high value that patients with ophthalmic conditions place on preserving vision to maintain their quality of life.
Cost of treatment to the patient and drug label status were the second- and third-most important attributes in our analysis, respectively. Assuming that vision outcomes are generally similar between off-label bevacizumab and its on-label comparators for nAMD or DME,7–10 our findings suggest that the significantly lower cost of off-label bevacizumab may favor its use in clinical practice. However, the influence of treatment costs on patient preferences may be substantially reduced when costs are covered by insurance providers, which was found to be the least important factor in our analysis. For patients with DME, willingness to accept an off-label treatment might also be dependent on visual acuity, based on evidence that off-label bevacizumab may be less efficacious than on-label anti-VEGF therapies in those with lower baseline vision.7,8
Treatment frequency was found to be the fourth-most important factor in our conjoint analysis, suggesting that many patients are prepared to tolerate an intensive treatment regimen in exchange for good vision. Previous studies have similarly demonstrated the high treatment burden that patients will accept to preserve their vision, including Mueller et al who estimated that patients with nAMD were willing to spend an additional 12.7 hours per clinic visit to achieve stable rather than worsening vision, and 21.2 hours to improve their vision outcomes.29 In a survey that compared patient preferences associated with focal grid laser and intravitreal anti-VEGF therapy for DME, Mason et al found that 83% of patients were prepared to receive 15–16 injections in order to gain two lines of Snellen visual acuity, 86% would sacrifice zero lines of vision to receive 4 lasers versus 15–16 injections, and 76% would be willing to receive treatment 12 times per year to maintain their vision.30 Given that endophthalmitis is a potentially vision-threatening complication of intravitreal injection therapy,31 further studies are needed to examine whether an increasing risk of endophthalmitis may impact a patient’s willingness to accept frequent injections for improved vision outcomes.
We stratified patients by age to examine whether advancing age, and possibly Medicare eligibility, may influence anti-VEGF treatment preferences; however, the relative importance of each attribute was similar between age subgroups. Moreover, preference trends for patients grouped according to highest education level were generally comparable with that of the overall cohort. These findings indicate that decisions regarding anti-VEGF therapy for nAMD and DME are driven by similar factors across all patients, irrespective of demographic profile.25 One notable exception was the subgroup of patients with graduate/professional degrees, who ranked drug label status as the most important attribute of anti-VEGF therapy for nAMD and DME and placed greater relative importance on treatment frequency than any other education level subgroup. These observations are based on a small sample size (4.7% of the overall cohort) and require validation in larger studies; nevertheless, they may suggest that patients with higher degrees have a greater awareness of the research and regulatory processes that ensure the safety and effectiveness of approved medications. Moreover, these patients may be less able to attend frequent treatment visits due to employment or other lifestyle reasons and are thus more willing to absorb increased costs in exchange for fewer injections.
Treatment preferences reported herein are based on a small, English-speaking patient population surveyed across three US-based retina clinics; therefore, further studies are needed to validate our survey and assess the generalizability of our results in other patient groups, countries, and/or health care systems. Patient demographics collected in this study were limited to age, sex, and education level; as such, we were unable to compare preferences between nAMD and DME cohorts, nor examine the influence of other patient-related factors on anti-VEGF treatment decisions (eg, visual acuity, ethnicity, treatment experience, and insurance type). As conjoint analyses are inherently limited by the number of attributes that patients can meaningfully rank in one survey, further studies are needed to assess the importance of other treatment-related factors, such as the safety profiles and adverse events associated with different anti-VEGF therapies. Finally, although patients preferred vision to treatment frequency when monthly and bimonthly regimens were compared, our survey was conducted prior to the FDA approval of brolucizumab for nAMD (indicated every 8–12 weeks5) and did not consider other extended dosing schedules, including as-needed and treat-and-extend protocols. Given that these regimens were developed to individualize therapy and/or reduce treatment burden in clinical practice,32 future studies may seek to determine their impact on anti-VEGF treatment choices; however, our findings suggest that a patient’s preference for fewer injections will be largely dependent on the vision outcomes achievable with these strategies.
In conclusion, patients with nAMD or DME placed greater importance on vision outcomes than treatment costs, drug label status, and injection frequency when receiving intravitreal anti-VEGF therapy for the management of these conditions. These preferences may be used to guide treatment decisions between patients and physicians, promote patient-centered care in ophthalmology, and ultimately improve health outcomes among patients with nAMD or DME.
Abbreviations
DME, diabetic macular edema; FDA, US Food and Drug Administration; nAMD, neovascular age-related macular degeneration; VEGF, vascular endothelial growth factor.
Acknowledgments
The authors thank Karina D Hamilton-Peel, PhD, CMPP, for medical writing assistance during the preparation of this manuscript.
Disclosure
Dr Meena George has served on the advisory board of Allergan, outside the submitted work. Dr Veeral S Sheth has served as a consultant for Alimera, Eyepoint, Genentech, Inc., Notal Vision, and Novartis; and reports personal fees from Genentech, Novartis, Alimera, Notal Vision, and Eyepoint, and grant support from Apellis Pharmaceuticals, Chengdu Kanghong Biotechnology Co., Ltd., Graybug Vision, Inc., Ionis Pharmaceuticals, Outlook Therapeutics, and Regeneron, outside the submitted work. The authors report no other conflicts of interest with respect to this work.
References
1. Flaxel CJ, Adelman RA, Bailey ST, et al. Age-related macular degeneration preferred practice pattern®. Ophthalmology. 2020;127(1):P1–P65. doi:10.1016/j.ophtha.2019.09.024
2. Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy preferred practice pattern®. Ophthalmology. 2020;127(1):P66–P145. doi:10.1016/j.ophtha.2019.09.025
3. Genentech, Inc. Lucentis® (ranibizumab injection) for intravitreal injection. Available from: https://www.gene.com/download/pdf/lucentis_prescribing.pdf.
4. Regeneron Pharmaceuticals, Inc. Eylea® (aflibercept) injection, for intravitreal use. Available from: https://www.regeneron.com/sites/default/files/EYLEA_FPI.pdf.
5. Novartis Pharmaceuticals Corporation. Beovu® (brolucizumab-dbll) injection, for intravitreal use. Available from: https://www.novartis.us/sites/www.novartis.us/files/beovu.pdf.
6. Genentech, Inc. Avastin® (bevacizumab) injection, for intravenous use. Available from: https://www.gene.com/download/pdf/avastin_prescribing.pdf.
7. Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. doi:10.1056/NEJMoa1414264
8. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351–1359. doi:10.1016/j.ophtha.2016.02.022
9. Schmid MK, Bachmann LM, Fäs L, Kessels AG, Job OM, Thiel MA. Efficacy and adverse events of aflibercept, ranibizumab and bevacizumab in age-related macular degeneration: a trade-off analysis. Br J Ophthalmol. 2015;99(2):141–146. doi:10.1136/bjophthalmol-2014-305149
10. Solomon SD, Lindsley KB, Krzystolik MG, Vedula SS, Hawkins BS. Intravitreal bevacizumab versus ranibizumab for treatment of neovascular age-related macular degeneration: findings from a cochrane systematic review. Ophthalmology. 2016;123(1):70–77.e1. doi:10.1016/j.ophtha.2015.09.002
11. Sarwar S, Clearfield E, Soliman MK, et al. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016;2016(2):CD011346. doi:10.1002/14651858.CD011346.pub2
12. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi:10.1016/j.ophtha.2019.04.017
13. Hurley SF, Matthews JP, Guymer RH. Cost-effectiveness of ranibizumab for neovascular age-related macular degeneration. Cost Eff Resour Alloc. 2008;6:12. doi:10.1186/1478-7547-6-12
14. Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR. Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmol. 2016;134(8):888–896. doi:10.1001/jamaophthalmol.2016.1669
15. Holekamp N, Duff SB, Rajput Y, Garmo V. Cost-effectiveness of ranibizumab and aflibercept to treat diabetic macular edema from a US perspective: analysis of 2-year protocol T data. J Med Econ. 2019;23(3):287–296. doi:10.1080/13696998.2019.1666855
16. Kiss S, Malangone-Monaco E, Wilson K, et al. Real-world injection frequency and cost of ranibizumab and aflibercept for the treatment of neovascular age-related macular degeneration and diabetic macular edema. J Manag Care Spec Pharm. 2020;26(3):253–266. doi:10.18553/jmcp.2020.19245
17. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410–417. doi:10.1370/afm.1161
18. Lindhiem O, Bennett CB, Trentacosta CJ, McLear C. Client preferences affect treatment satisfaction, completion, and clinical outcome: a meta-analysis. Clin Psychol Rev. 2014;34(6):506–517. doi:10.1016/j.cpr.2014.06.002
19. Shingler SL, Bennett BM, Cramer JA, Towse A, Twelves C, Lloyd AJ. Treatment preference, adherence and outcomes in patients with cancer: literature review and development of a theoretical model. Curr Med Res Opin. 2014;30(11):2329–2341. doi:10.1185/03007995.2014.952715
20. Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320(7248):1530–1533. doi:10.1136/bmj.320.7248.1530
21. Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
22. Bhargava JS, Patel B, Foss AJE, Avery AJ, King AJ. Views of glaucoma patients on aspects of their treatment: an assessment of patient preference by conjoint analysis. Invest Ophthalmol Vis Sci. 2006;47(7):2885–2888. doi:10.1167/iovs.05-1244
23. Bhargava JS, Bhan-Bhargava A, Foss AJE, King AJ. Views of glaucoma patients on provision of follow-up care; an assessment of patient preferences by conjoint analysis. Br J Ophthalmol. 2008;92(12):1601–1605. doi:10.1136/bjo.2008.140483
24. Ross MA, Avery AJ, Foss AJE. Views of older people on cataract surgery options: an assessment of preferences by conjoint analysis. Qual Saf Health Care. 2003;12(1):13–17. doi:10.1136/qhc.12.1.13
25. Wirostko B, Beusterien K, Grinspan J, et al. Patient preferences in the treatment of diabetic retinopathy. Patient Prefer Adherence. 2011;5:229–237. doi:10.2147/PPA.S11972
26. Baxter JM, Fotheringham AJ, Foss AJE. Determining patient preferences in the management of neovascular age-related macular degeneration: a conjoint analysis. Eye. 2016;30(5):698–704. doi:10.1038/eye.2016.18
27. QuestionPro, Inc. Conjoint analysis for market research. Available from: https://www.surveyanalytics.com/conjoint/index.html.
28. QuestionPro, Inc. Conjoint analysis: definition, example, types, algorithm and model. Available from: https://www.questionpro.com/blog/what-is-conjoint-analysis/.
29. Mueller S, Agostini H, Ehlken C, Bauer-Steinhusen U, Hasanbasic Z, Wilke T. Patient preferences in the treatment of neovascular age-related macular degeneration: a discrete choice experiment. Ophthalmology. 2016;123(4):876–883. doi:10.1016/j.ophtha.2015.12.001
30. Mason L, Crosson JN, Mason JO, McGwin G. Patient preferences with regard to laser versus intravitreal injections in the treatment of diabetic macular edema. J Ophthalmol. 2017;2017:7398470. doi:10.1155/2017/7398470
31. Jager RD, Aiello LP, Patel SC, Cunningham ET. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24(5):676–698. doi:10.1097/00006982-200410000-00002
32. Freund KB, Korobelnik J-F, Devenyi R, et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases. Retina. 2015;35(8):1489–1506. doi:10.1097/IAE.0000000000000627
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


