Back to Journals » Patient Preference and Adherence » Volume 9
Patient experience and practice trends in multiple sclerosis – clinical utility of fingolimod
Received 29 January 2015
Accepted for publication 4 April 2015
Published 21 May 2015 Volume 2015:9 Pages 685—693
DOI https://doi.org/10.2147/PPA.S57354
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Johnny Chen
Jong-Mi Lee,1 May H Han1,2
1Stanford Healthcare, Multiple Sclerosis Center, 2Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford University, Stanford, CA, USA
Abstract: Targeting sphingosine-1-phosphate pathway with orally available immune-modulatory fingolimod (Gilenya™) therapy ameliorates relapsing–remitting multiple sclerosis (RRMS) by decreasing relapse rate as shown in FREEDOMS and TRANSFORMS. Fingolimod has also been shown to be superior to interferon-beta therapy as evidenced by TRANSFORMS. Albeit multiple benefits in treatment of multiple sclerosis including high efficacy and ease of administration, potential untoward effects such as cardiotoxicity, risk of infection, and cancer exist, thus mandating careful screening and frequent monitoring of patients undergoing treatment with fingolimod. This review outlines mechanism of action, observations, side effects, and practice guidelines on use of fingolimod in treatment of RRMS.
Keywords: sphingosine-1-phosphate, RRMS, FREEDOMS, TRANSFORMS, side effects, IFNβ
Introduction
Fingolimod (Gilenya™) is the first US Food and Drug Administration (FDA)-approved oral therapy for treatment of multiple sclerosis (MS) based on two Phase III pivotal trials, FREEDOMS and TRANSFORMS.1–4 Fingolimod targets the sphingosine-1-phosphate (S1P) pathway by regulation of lymphocyte trafficking from secondary lymphoid organs into the systemic circulation (Table 1).5–8 Interaction of the sphingolipid ligand, S1P, in the blood or lymph with the G protein-coupled receptor S1P receptor 1 (S1PR1) on lymphocytes is necessary for lymphocyte egress from lymph nodes into blood and lymph.9–11 The critical role played by S1P–S1PR1 interaction in immune trafficking is perturbed by fingolimod, a functional antagonist of S1PR.12,13 Fingolimod sequesters lymphocytes in the spleen and lymph nodes by inducing receptor internalization and degradation, causing lymphopenia and sparing the central nervous system from immune attack by myelin-reactive lymphocytes.11 Fingolimod has been shown to effectively decrease relapse rate up to 50% and is superior to interferon-beta (IFNβ) therapy.14–17 However, since fingolimod signals via most of the S1PRs (S1PR1 and 3–5), untoward effects in systems expressing these receptors, including cardiovascular and visual systems (such as cardiac rhythm abnormalities and macular edema), have been observed in patients treated with fingolimod.18–21 Furthermore, due to fingolimod’s action on lymphopenia, side effects related to serious infections and cancer risk, possibly by interfering immune surveillance function of lymphocytes, are also observed.18 In the post-market experience, rebound disease activity (most likely due to reversing fingolimod’s effect on lymphocyte egress) is observed upon discontinuation of the therapy.22–25 Thus, careful patient selection with rigorous and frequent monitoring and pre-consideration of optimal treatment sequencing are required for patients undergoing fingolimod therapy.26–29 This review article presents a comprehensive review of screening, monitoring, side effects, and efficacy in the clinical practice utilizing fingolimod for the treatment of relapsing–remitting MS (RRMS).
  | Table 1 S1PR in the immune system |
Screening before initiating therapy
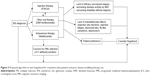
Baseline screening and ongoing monitoring are required for fingolimod treatment to avoid potential serious side effects (Figure 1).29,30 The recommended baseline screening includes electrocardiogram (ECG) for cardiac rhythm abnormalities, ophthalmologic examination to evaluate for macular edema, serological test for immunity to varicella zoster virus (VZV) infection, complete blood count, liver function tests, blood pressure, and urine pregnancy test for females during reproductive age.30 Additional recommendations include pulmonary function test, dermatological examination, and serological test for viral hepatitis and tuberculosis, when clinically indicated. A detailed evaluation such as referral to cardiology (for abnormal ECG) and dermatology proceeds if baseline screening is abnormal. Active immunization against VZV is recommended for nonimmune cases.30,31 Review of past medical history and medication list for potential drug interactions is also recommended as part of prescreening. Special attention is paid to patients with diabetes and uveitis due to the increased risk of developing macular edema with fingolimod therapy.32,33 Cardiac rhythm abnormalities such as torsades de pointes, bradycardia, or conduction block could occur, especially in patients who are concurrently on medications that can cause prolonged QT interval (eg, citalopram, chlorpromazine, haloperidol, methadone, erythromycin), or interfere with cardiac conduction (eg, beta blockers, diltiazem, verapamil, digoxin).34,35 Interval monitoring of follow-up ophthalmologic examination (at 3–4 months following treatment initiation), complete blood counts, and hepatic function tests are recommended.1,2 Continuous monitoring of infection is recommended until 2 months after discontinuation of fingolimod. Women of childbearing potential should use effective contraception during and for 2 months after stopping therapy, since fingolimod therapy may cause fetal abnormalities.1,2 Regular monitoring for hypertension is also recommended throughout the duration of therapy.1,2,30
Patients undergoing fingolimod therapy receive more rigorous screening compared to those undergoing other disease-modifying therapies (DMTs). This may delay the initiation of therapy; however, patients in our practice have expressed satisfaction with thorough screening prior to starting therapy.
First-dose observation
The first-dose observation (FDO) is a 6-hour monitoring session assessing for cardiac rhythm abnormalities, especially bradycardia, after initiation of fingolimod therapy. FDO includes monitoring for heart rate, cardiac rhythm, and blood pressure every hour, and ECG at 0 hour and 6 hours after taking first dose of fingolimod.36 The cardiovascular side effects during FDO from FREEDOMS and TRANSFORMS studies are bradycardia as being the most common cardiovascular event followed by first-degree atrioventricular (AV) block, second-degree AV block Mobitz type 1, sinus arrhythmia, and ventricular premature beats.36 The new FDA-revised FDO monitoring includes a repeat ECG prior to discharge based on potential cardiac rhythm abnormalities following first dose of fingolimod.37,38 FDO is carried out as an outpatient procedure in most facilities where patients are observed on a cardiac monitor for 6 hours with access to a rapid response team. Extended monitoring is recommended for those patients who demonstrate the following: 1) heart rate <45 beats per minute, 2) a continued downward trend, 3) new-onset second-degree or more severe conduction block, 4) symptomatic bradycardia, and 5) prolonged QTc interval (QTc >470 ms in females and >450 ms in males) following 6-hour FDO monitoring.37 Overnight continuous cardiac monitoring in a medical facility for FDO is recommended for patients with a preexisting cardiac condition or on concurrent medication that can interfere with cardiac conduction; patients with a prior cardiac history have not shown increased incidence of adverse events with FDO.39–41 Common complaints during FDO in our practice include headache, mild nausea, and symptomatic bradycardia in order of frequency. The guidelines for patients requiring repeat FDO are discontinuation within first 2 weeks of therapy, interruption of 1 or more days during weeks 3–4 of therapy, interruption of 7 or more days after 4 weeks of therapy, and interruption of 14 or more days anytime during treatment.1,37
Untoward effects associated with fingolimod use
Side effects associated with fingolimod reported in FREEDOMS and TRANSFORMS are abnormal laboratory finding, infections, cardiovascular side effects, macular edema, malignancies, pulmonary side effects, and rare cases of posterior reversible encephalopathy syndrome. Less serious side effects include headache and hypertension.1,2
Laboratory test abnormalities
Lymphopenia was the most common abnormal laboratory test leading to drug discontinuation in the FREEDOMS studies.42,43 Peripheral blood lymphocyte counts decreased up to 20%–30% from baseline within the first month of initiation of fingolimod therapy.20 Lymphopenia is often reversible and normalized approximately 45–135 days following discontinuation of fingolimod.44 Elevated alanine aminotransferase (ALT) (up to three or more times the upper limit of normal) was observed as the second most common abnormal laboratory finding.1,2 ALT levels also normalized spontaneously after discontinuation of fingolimod without permanent hepatic dysfunction.1,2
Risk of infection
The overall incidence of infections in FREEDOMS and TRANSFORMS studies was similar in the treatment and placebo groups; however, a slightly higher incidence of bronchitis, influenza, and herpes viral infections (including herpes zoster infection) was observed in the fingolimod treatment groups.30 Other common infections included upper respiratory tract infections, nasopharyngitis, urinary tract infections, and sinusitis.30 Two fatal cases of reactivation of latent herpes virus (in the fingolimod 1.25 mg treatment group), a case of fatal disseminated VZV infection, and a case of fatal herpes simplex virus type 1 encephalitis in TRANSFORMS study were also observed.1–3,30 Other infections such as reactivation of human papilloma virus, John Cunningham virus (in a post-natalizumab case), tuberculosis, and cytomegalovirus were also reported; however, post-marketing data did not reveal an increased rate of occult infections.45 About 20% of our patient cohort on fingolimod (n=50) have reported symptoms of vaginitis, recurrent upper respiratory, and sinus infections; however, the etiology is unclear.
Cardiovascular side effects
Fingolimod binds to S1PR1 in the heart, which can result in heart rhythm abnormalities such as bradycardia, therefore necessitating FDO.36–41 Maximal decrease in heart rate occurs at 4–6 hours, which is the basis of rationale for the 6-hour monitoring period.20 Symptomatic (eg, dizziness, chest discomfort, palpitations, and/or fatigue) bradycardia was observed in less than 1% of subjects.43,46 In FREEDOMS and TRANSFORMS studies, symptomatic bradycardia during FDO resolved within 24 hours without pharmacological interventions.1,2 Fingolimod is also known to induce cardiac conduction abnormalities, including first- and second-degree AV block, on the 6-hour post-dose ECG; patients who continued on treatment did not have persistent cardiac conduction abnormalities.34,35 We have had infrequent cases of symptomatic bradycardia leading to discontinuation of fingolimod as seen with patients in our practice.
Macular edema
Another potential side effect associated with the upregulation of S1PR in the vascular endothelial cells of the macula is fingolimod-associated macular edema (FAME).28–32 Most of the cases with FAME in FREEDOMS and TRANSFORMS studies were asymptomatic; the overall incidence of FAME was 0.5% in the 0.5 mg group, with complete resolution following discontinuation of therapy.1,2 FAME generally occurred within 3–4 months of fingolimod initiation, although there has been a case report of early bilateral macular edema following fingolimod therapy within the first 3 months.32,33 Therefore, a follow-up ophthalmologic examination at 3–4 months post-fingolimod initiation is recommended. Few cases of unresolved macular edema were identified in the higher dose (1.25 mg) fingolimod group.20 In our patient cohort, we have seen two cases of symptomatic FAME which appeared 3–4 months after fingolimod initiation. Both cases had complete resolution of visual disturbance within 2–3 months after discontinuing fingolimod.
Risk of malignancy
An increased risk of malignancies was found in association with fingolimod therapy in the TRANSFORMS and FREEDOMS studies. Most common malignancies found in association with fingolimod use are dermatological malignancies (Bowen’s disease, n=1; basal cell carcinoma, n=10; and malignant melanoma, n=4).20,21 Other malignancies reported in the studies are breast cancer (n=5) with a fatal case of metastatic breast cancer in a patient who died 10 months after discontinuing fingolimod.47 Although there were at least three case reports of lymphoma in the fingolimod treatment group during drug development, a general consensus has not been reached on the risk of lymphoma with fingolimod.48
Pulmonary side effects
Respiratory effects including mild reductions in 1-second forced expiratory volume and diffusion capacity for carbon monoxide were observed in FREEDOMS and TRANSFORMS.1,2 Spirometry and diffusion lung capacity tests are recommended if clinically indicated.1 We have a standard protocol assessing for pulmonary function status through spirometry testing prior to FDO.
Pregnancy
Although fingolimod is classified as pregnancy category C, there have been cases of teratogenicity in live births during fingolimod clinical development.49 Exposure to fingolimod in the first trimester resulted in five cases of abnormal fetal development in 66 pregnancies.49,50 The available pregnancy registry data continue to provide important information regarding use of fingolimod in women of childbearing potential with the known risk of possible fetal malformation and teratogenic effects.51,52 The current recommendation for women of childbearing potential is to use effective contraception during fingolimod therapy and for at least 2 months after discontinuation of fingolimod.1
Tumefactive MS and rebound relapses
To date, 16 case reports in the literature describe worsening MS disease activity or even development of tumefactive demyelinating lesions (TDL) following fingolimod therapy.53–55 TDL are extremely rare and were observed in patients with established diagnosis of MS leading to the suspicion of a causal relationship between the use of fingolimod and development of TDL.55,56 The diagnosis of progressive multifocal leukoencephalopathy (PML) was entertained in this particular patient with TDL, since the patient was on natalizumab therapy before starting fingolimod; however, the patient was found to have “rebound” disease activity along with the development of TDL.57–60 It would seem prudent to monitor clinical progression and magnetic resonance imaging (MRI) activity for worsening disease activity in fingolimod-treated patients regardless of disease duration and prior DMT history.
Post-market experience
Sudden death in a hypertensive patient on calcium-channel blockers and beta blockers within 24 hours following first-dose fingolimod prompted the FDA and the European Medicines Agency (EMA) to recommend a modification in the FDO to include hourly heart rate and blood pressure monitor and an ECG (either continuous, according to the EMA, or pre-dose and 6 hours post-dose, according to the FDA) as well as excluding patients on medications that can cause cardiac rhythm abnormalities.37,38 Despite the recommendations, two open-label studies on fingolimod treatment initiation resulted in overall satisfactory safety and tolerability in patients with concomitant diseases, and no cardiac adverse events were observed in association with fingolimod use.34,39 On the contrary, a case report in 2013 identified that three (out of 59) patients without known cardiovascular disease were found to have cardiac rhythm abnormalities (eg, sinus bradycardia with idioventricular escape rhythm that lasted 45 seconds and second-degree AV block Mobitz type 1).34 Additional post-marketing reports have raised concern over the risk for PML in patients who were originally treated with natalizumab.60 A confirmed case of PML was reported in 2012 in a patient who was treated with natalizumab for 42 months prior to fingolimod therapy.61 The second case of PML was observed in a patient treated with fingolimod, who did not have prior exposure to natalizumab.62 The third reported case of PML was observed in a patient 3.5 months after fingolimod initiation and 4.5 months after natalizumab discontinuation.61,62
Efficacy
Fingolimod met the primary end point of annualized relapse rate reduction across both Phase III trials. With the exception of time to disability progression in TRANSFORMS, secondary end point measures including MRI data were statistically significant in FREEDOMS and TRANSFORMS.63,64 More importantly, TRANSFORMS showed greater efficacy in relapse rate reduction over IFNβ in a 12-month study (Table 2).1,2 Brain volume loss has been specifically studied in FREEDOMS, which found that fingolimod 0.5 mg dose significantly reduced brain volume loss up to 24 months vs placebo irrespective of the presence or absence of gadolinium-enhancing lesions, T2 lesion load, previous treatment status, or level of disability.65 Long-term data to support ongoing reduction in disability progression and brain volume loss are not available at this time, but studies to assess fingolimod safety and tolerability continue (Table 3).66,67
  | Table 2 Summary of pivotal trials |
Suggested treatment algorithm
Clinicians and patients across MS centers continue to struggle with selecting the most effective MS therapy for a particular patient with RRMS, and to assess whether drug benefits outweigh risks of treatment (Figure 2).68–70 As the first of three first-line oral therapies for the treatment of RRMS, fingolimod presents a suitable option for patients with recent diagnosis of MS or those with suboptimal response and/or compliance to injectable first-line immune therapies.71 Clinicians tend to switch patients to fingolimod (from natalizumab) based on the patients’ risk for development of PML based on serological status for John Cunningham virus, and those with neutralizing antibodies against natalizumab.72 A washout period of approximately 3 months has been recommended when switching from natalizumab to fingolimod, but duration of washout period depends on the disease activity and other comorbidities such as the immune status of the patient.73
Patient adherence
It is well recognized that medication adherence is not always 100% with either oral or injectable DMTs for multiple reasons.74,75 Fingolimod provides an attractive alternative to injectable DMTs due to the ease of administration. However, a retrospective study on medication compliance showed that approximately 27% of fingolimod users discontinued within 1 year of treatment initiation.75 Socioeconomic factors play a role in patient adherence to drug treatment with increasing out-of-pocket and copayments and lack of insurance coverage.76,77 The Consortium of MS Centers recently outlined that the main reasons for patients switching DMTs in MS included efficacy, safety, prescriber- or payer-related reasons, and patient-related reasons which included difficulty with adherence, desire to try different administration methods, and perceived lack of efficacy.78 Most of the patients in our practice have discontinued fingolimod primarily due to side effects of the medication and perceived lack of efficacy. Typically, patients who have switched from injection therapy are pleased with the ease of oral administration of fingolimod, despite the lack of comprehensive long-term safety data.
Conclusion
Fingolimod is the first FDA-approved oral therapy for the treatment of RRMS as shown by two large Phase III studies, FREEDOMS and TRANSFORMS.1 Clinical efficacy of fingolimod was observed to be superior to placebo and IFNβ-1a in reducing relapse rate and MRI activity. However, thorough screening, FDO, and long-term follow-up are recommended in order to avoid potential side effects associated with fingolimod therapy. Fingolimod presents as a treatment option as a first-line therapy for patients with new-onset RRMS or those switching therapies due to intolerability and/or lack of efficacy of prior DMTs. However, studies on long-term efficacy, safety, and mechanism of action of fingolimod remain to be further pursued for therapeutic optimization and to avoid undesirable side effects.
Disclosure
Jong-Mi Lee has received consulting agreements or service as speaker (Biogen Idec, Teva Pharmaceuticals, and Genzyme). The other author reports no conflicts of interest in this work.
References
Novartis Pharmaceuticals. Fingolimod Prescribing Information; 2014. Available from: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf. Accessed December 8, 2014. | ||
European Medicines Agency. European Public Assessment Report (EPAR) for Gilenya; 2014. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002202/human_med_001433.jsp&mid=WC0b01ac058001d125. Accessed December 8, 2014. [Gilenya: EPAR: Summary report for the public]. | ||
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. [Pivotal Trial FREEDOMS]. | ||
Cohen JA, Barkhof F, Comi G, TRANSFORMS Study Group, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. [Pivotal Trial FREEDOMS]. | ||
Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): physiology and the effects of S1P receptor modulation. Neurology. 2011;76:S3–S8. | ||
Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158:1173–1182. | ||
Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002; 277(24):21453–21457. | ||
Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69(5):759–777. | ||
Mehling M, Johnson TA, Antel J, Kappos L, Bar-Or A. Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurology. 2011;76(8 suppl 3):S20–S27. | ||
Choi JW, Gardell SE, Herr DR, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor (S1P1) modulation. Proc Natl Acad Sci U S A. 2011;108(2):751–756. | ||
Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects on sphingosine 1-phosphate (S1) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci. 2013;328(1–2):9–18. | ||
Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5(6): 428–434. | ||
Van Doorn R, Van Horssen J, Verzijl D, et al. Sphingosine 1-phosphate receptors 1 and 3 are upregulated in multiple sclerosis lesions. Glia. 2010;58(12):1465–1476. | ||
Scott LJ. Fingolimod: a review of its use in the management of relapsing-remitting multiple sclerosis. CNS Drugs. 2011;8:673–698. | ||
Devonshire V, Havrdova E, Radue EW, et al; FREEDOMS study group. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomized, placebo-controlled FREEDOMS study. Lancet Neurol. 2012;11:420–428. | ||
Gasperini C, Ruggieri S. Development of oral agent in the treatment of multiple sclerosis: how the first available oral therapy, fingolimod will change therapeutic paradigm approach. Drug Des Devel Ther. 2012;6:175–186. | ||
Gasperini C, Ruggieri S, Mancinelli CR, Pozzilli C. Advances in the treatment of relapsing-remitting multiple sclerosis – critical appraisal of fingolimod. Ther Clin Risk Manag. 2013;9:73–85. | ||
Mehling M, Kappos L, Derfuss T. Fingolimod for multiple sclerosis: mechanism of action, clinical outcomes, and future directions. Curr Neurol Neurosci Rep. 2011;11:492–497. | ||
Rommer PS, Zettl UK, Kieseier B, et al. Requirement for safety monitoring for approved multiple sclerosis therapies: an overview. Clin Exp Immunol. 2013;175(3):397–407. | ||
Ward M, Jones DE, Goldman MD. Overview and safety of fingolimod hydrochloride use in patients with multiple sclerosis. Expert Opin Drug Saf. 2014;13(7):989–998. | ||
Kappos L, Cohen J, Collins W, et al. Fingolimod in relapsing multiple sclerosis: an integrated analysis of safety findings. Multiple Sclerosis and Related Disorders. 2014;3(4):494–504. | ||
Beran RG, Hegazi Y, Schwartz RS, Cordato DJ. Rebound exacerbation multiple sclerosis following cessation of oral treatment. Mult Scler Relat Disord. 2013;2(3):252–255. | ||
Alroughani R, Alroughani R, Almulla A, Lamdhade S, Thussu A. Severe reactivation of multiple sclerosis after discontinuation of fingolimod: an IRIS-associated phenomenon. Mult Scler Relat Disord. 2014;3(6):748. | ||
Havla JB, Pellkofer HL, Meinl I, Gerdes LA, Hohfeld R, Kumpfel T. Rebound of disease activity after withdrawal of fingolimod (FTY720) treatment. Arch Neurol. 2012;69(2):262–264. | ||
Ghezzi A, Rocca MA, Baroncini D, et al. Disease reactivation after fingolimod discontinuation in two multiple sclerosis patients. J Neurol. 2013;260(1):327–329. | ||
Rommer PS, Zettl UK, Kieseier B, et al. Requirement for safety monitoring for approved multiple sclerosis therapies: an overview. Clin Exp Immunol. 2014;175(3):397–407. | ||
Singer BA. Initiating oral fingolimod treatment in patients with multiple sclerosis. Ther Adv Neurol Disord. 2013;6(4):269–275. | ||
Ontaneda D, Hara-Cleaver C, Rudick RA, Cohen JA, Bermel RA. Early tolerability and safety of fingolimod in clinical practice. J Neurol Sci. 2012;323(1–2):167–172. | ||
Thomas K, Ziemssen T. Management of fingolimod in clinical practice. Clin Neurol Neurosurg. 2013;115(suppl 1):S60–S64. | ||
Thöne J, Ellrichmann G. Oral available agents in the treatment of relapsing remitting multiple sclerosis: an overview of merits and culprits. Drug Healthc Patient Saf. 2013;5:37–47. | ||
Berger JR. Varicella vaccination after fingolimod: a case report. Mult Scler Relat Disord. 2013;2(4):391–394. | ||
Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence, detection, and management. Neurology. 2012;78(9):672–680. | ||
Coppes O, Gutierrez I, Reder AT, Ksiazek S, Bernard J. Severe early bilateral macular edema following fingolimod therapy. Mult Scler Relat Disord. 2013;2(3):256–258. | ||
Gold R, Comi G, Palace J, FIRST Study Investigators, et al. Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: a phase 3b, open-label study. J Neurol. 2014;261(2):267–276. | ||
Camm J, Hla T, Bakshi R, Brinkmann V. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am Heart J. 2014;168(5):632–644. | ||
Horga A, Castrillo J, Montalban X. Fingolimod for relapsing multiple sclerosis; an update. Expert Opin Pharmacother. 2010;11:1183–1196. | ||
FDA. Revised Drug Monitoring: US Food and Drug Administration; 2014. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm303192.htm. Accessed December 8, 2014. | ||
FDA. First Reported Death on Gilenya: 24hrs Post FDO: US Food and Drug Administration; 2014. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm284240.htm. Accessed December 8, 2014. | ||
Thomas K, Schrötter H, Halank M, Ziemssen T. Fingolimod in a patient with heart failure on the background of pulmonary arterial hypertension and coronary artery disease. BMC Neurol. 2014;14:126. | ||
Laroni A, Brogi D, Morra VB, et al; EAP Investigators. Safety of the first dose of fingolimod for multiple sclerosis: results of an open-label clinical trial. BMC Neurol. 2014;14:65. | ||
Faber H, Fischer H-J, Weber F. Prolonged and symptomatic bradycardia following a single dose of fingolimod. Mult Scler. 2013;19(1):126–128. | ||
Singer B, Ross AP, Tobias K. Oral fingolimod for the treatment of patients with relapsing forms of multiple sclerosis. Int J Clin Pract. 2011;65(8):887–895. | ||
Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomized, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–556. | ||
Johnson TA, Shames I, Keezer M, et al. Reconstitution of circulating lymphocyte counts in FTY720-treated MS patients. Clin Immunol. 2010;137(1):15–20. | ||
Rosenstiel PV, Gottschalk R, Cappiello L, Zhang Y, Said M, Kappos L. Long-term safety of fingolimod: interim evaluation of data from the longterms trial. Mult Scler Relat Disord. 2014;3(6):752. | ||
Hughes B, Cascione M, Freedman MS, et al. First-dose effects of fingolimod after switching from injectable therapies in the randomized, open-label, multicenter, evaluate patient outcomes (EPOC) study in relapsing multiple sclerosis. Mult Scler Relat Disord. 2014;3(5):620–628. | ||
Conzett KB, Kolm I, Jelcic I, et al. Melanoma occurring during treatment with fingolimod for multiple sclerosis: a case report. Arch Dermatol. 2011;147(8):991–992. | ||
Lorvik KB, Bogen B, Corthay A. Fingolimod blocks immunosurveillance of myeloma and B-cell lymphoma resulting in cancer development in mice. Blood. 2012;119(9):2176–2177. | ||
Karlsson G, Francis G, Koren G, et al. Pregnancy outcomes in the clinical development program of fingolimod in multiple sclerosis. Neurology. 2014;82(8):674–680. | ||
Jones B. Multiple sclerosis: study reinforces need for contraception in women taking fingolimod. Nat Rev Neurol. 2014;10(3):125. | ||
Alwan S, Chambers CD, Armenti VT, Dessa-Sadovnick A. The need for disease-specific prospective pregnancy registry for multiple sclerosis (MS). Mult Scler Relat Disord. 2015;4(1):6–17. | ||
Houtchens MK, Kolb CM. Multiple sclerosis and pregnancy: therapeutic considerations. J Neurol. 2013;260(5):1202–1214. | ||
Hellmann MA, Lev N, Lotan I, et al. Tumefactive demyelination and a malignant course in an MS patient during and following fingolimod therapy. J Neurol Sci. 2014;344(1–2):193–197. | ||
Pilz G, Harrer A, Wipfler P, et al. Tumefactive MS lesions under fingolimod: a case report and literature review. Neurology. 2013;81(19): 1654–1658. | ||
Castrop F, Kowarik MC, Albrecht H, et al. Severe multiple sclerosis relapse under fingolimod therapy: incident or coincidence? Neurology. 2012;78(12):928–930. | ||
Jander S, Turowski B, Kieseier BC, Hartung H-P. Emerging tumefactive multiple sclerosis after switching therapy from natalizumab to fingolimod. Mult Scler. 2012;18:1650–1652. | ||
Daelman L, Maitrot A, Maarouf A, Chaunu MP, Papeix C, Tourbah A. Severe multiple sclerosis reactivation under fingolimod 3 months after natalizumab withdrawal. Mult Scler. 2012;11:1647–1649. | ||
Hakiki B, Portaccio E, Giannini M, Razzolini L, Pasto L, Amato MP. Withdrawal of fingolimod treatment for relapsing-remitting multiple sclerosis: report of six cases. Mult Scler. 2012;18(11):1636–1639. | ||
Centonze D, Rossi S, Rinaldi F, Gallo P. Severe relapses under fingolimod treatment prescribed after natalizumab. Neurology. 2012; 79(19):2004–2005. | ||
Alroughani R, Ahmed S, Al-Hashel J. The risk of short-term relapse in patients switching from natalizumab to fingolimod. Mult Scler Relat Disord. 2014;3(6):748–749. | ||
FDA. Brain Infection Case. US Food and Drug Administration; 2014. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm366529.htm. Accessed December 8, 2014. | ||
Calic Z, Cappelen-Smith C, Hodgkinson SJ, McDougall A, Cuganesan R, Brew BJ. Treatment of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome with intravenous immunoglobulin in a patient with multiple sclerosis treated with fingolimod after discontinuation of natalizumab. J Clin Neurosci. 2014;22(3):598–600. | ||
Khatri BO, Pelletier J, Kappos L, et al. Effect of prior treatment status and reasons for discontinuation on the efficacy and safety of fingolimod vs interferon B-1a intramuscular: subgroup analyses of the trial assessing injectable interferon vs. fingolimod oral in relapsing-remitting multiple sclerosis (TRANSFORMS). Mult Scler Relat Disord. 2013;3(3):355–363. | ||
Khatri B, Barkhof F, Comi G, et al; TRANSFORMS Study Group. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomized extension of the TRANSFORMS study. Lancet Neurol. 2011;10(6):520–529. | ||
Radue EW, O’Connor P, Polman CH, et al; FTY720 Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) Study Group. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurol. 2012;69(10):1259–1269. | ||
Yamout B, Khoury S, Zeineddine M, Hourany R. Efficacy and safety of fingolimod treatment in multiple sclerosis: the clinical experience of the AUBMC Multiple Sclerosis Center in Lebanon. Mult Scler Relat Disord. 2014;3(6):749. | ||
Ziemmsen T, Diaz-Lorente M, Abdelkader M, Cornelissen C. PANGAEA: post-authorization Noninterventional German safety Study of Gilenya in relapsing-remitting multiple sclerosis (RRMS) patients: a 24-month interim analysis of a German five-year fingolimod registry study. Mult Scler Relat Disord. 2014;3(6):751. | ||
Fazekas F, Bajenaru O, Berger T, et al. How does fingolimod (gilenya(®)) fit in the treatment algorithm for highly active relapsing-remitting multiple sclerosis? Front Neurol. 2013;4:10. | ||
Hanson KA, Agashivala N, Wyrwich KW, Raimundo K, Kim E, Brandes DW. Treatment selection and experience in multiple sclerosis: survey of neurologists. Patient Prefer Adherence. 2014;8:415–422. | ||
Ontaneda D, Cohn S, Fox RJ. Risk stratification and mitigation in multiple sclerosis. Mult Scler Relat Disord. 2014;3(5):639–649. | ||
Fox E, Edwards K, Burch G, et al. Outcomes of switching directly to oral fingolimod from injectable therapies: results of the randomized, open-label, multicenter, evaluate patient outcomes (EPOC) study in relapsing multiple sclerosis. Mult Scler Relat Disord. 2014;3(5): 607–619. | ||
Havla J, Tackenberg B, Hellwig K, et al. Fingolimod reduces recurrence of disease activity after natalizumab withdrawal in multiple sclerosis. J Neurol. 2013;260(5):1382–1387. | ||
de Seze J, Ongagna JC, Collongues N, et al. Reduction of the washout time between natalizumab and fingolimod. Mult Scler. 2013;19(9):1248. | ||
Halpern R, Agarwal S, Borton L, Oneacre K, Lopez-Bresnahan MV. Adherence and persistence among multiple sclerosis patients after one immunomodulatory therapy failure: retrospective claims analysis. Adv Ther. 2011;28(9):761–775. | ||
Agashivala N, Wu N, Abouzaid S, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol. 2013;13:138. | ||
Tan H, Cai Q, Agarwal S, Stephenson JJ, Kamat S. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther. 2011;28(1):51–61. | ||
Menzin J, Caon C, Nichols C, White LA, Friedman M, Pill MW. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm. 2013;19(1 suppl A):S24–S40. | ||
Therapeutic decision making in multiple sclerosis: best practice algorithms for the MS care clinician. Int J MS Care. 2014;16(suppl 6):1–36. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.