Back to Journals » Vascular Health and Risk Management » Volume 19
Patient Characteristics and Predictors of Pulmonary Embolism in Patients Infected with COVID – 19 in Upper Egypt
Authors Hussein A , M Khalaf A, Alsharawy LA, Abdelrazek G, Shafiq Awad M
Received 2 January 2023
Accepted for publication 28 March 2023
Published 6 April 2023 Volume 2023:19 Pages 201—210
DOI https://doi.org/10.2147/VHRM.S403391
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Harry Struijker-Boudier
Ahmed Hussein,1 Ahmed M Khalaf,2 Laila A Alsharawy,3 Gomaa Abdelrazek,4 Mohammad Shafiq Awad5
1Cardiology Unit, Department of Internal Medicine, Faculty of Medicine, Sohag University, Sohag, Egypt; 2Department of Internal Medicine, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt; 3Department of Chest Diseases, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt; 4Cardiology Department, Faculty of Medicine, Fayoum University, Fayoum, Egypt; 5Cardiology Department, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt
Correspondence: Ahmed Hussein, Sohag University, Faculty of Medicine, Nasser City, Sohag, 82524, Egypt, Tel +2 01011145537, Fax +2 0934600349, Email [email protected]
Background: A little is known about the risk factors and predictors of pulmonary embolism (PE) in Coronavirus disease 2019 (Covid-19) infected patients. Therefore, we directed this study to investigate the predictors of PE in patients infected with Covid – 19 in Upper Egypt.
Methods: We conducted a retrospective cohort study on 297 patients infected with COVID-19, aged ≥ 18 years old. Suspicion of COVID-19 infection was based on the World Health Organization (WHO) criteria and confirmed by nasal and pharyngeal swab for real-time reverse transcriptase-polymerase chain reaction (RT-PCR) analysis. The patient was also determined to have COVID-19 when CT results that were thought to be typical for COVID-19 and clinical data that were compatible were present.
Results: PE was diagnosed in 18.2% of patients. We found that the incidence of PE was significantly higher in older patients, females, those with higher BMI, hypertensive patients, diabetics, and patients with co-morbidities. Also, PE was significantly higher in patients presented with dyspnea, chest pain, longer duration of symptoms at hospital admission, and lower oxygen concentration. The mean serum Hb level, platelet count, TLC and absolute lymphocytic count were markedly reduced in those who had PE. All the patients who developed PE had a CO-RADS scale five on their CT chest scan. Age > 65, BMI > 25, DM, and associated co-morbidities were the independent patients’ characteristics associated with the development of PE after the multivariate regression analysis.
Conclusion: PE is a common complication of Covid 19 infection. PE is associated with a variety of clinical and laboratory parameters in univariate analysis, but age > 65, BMI > 25, DM, and associated co-morbidities were the independent patients’ characteristics associated with the development of PE in those infected with Covid-19.
Keywords: Covid-19, SARS-CoV-2, CO-RADS, pulmonary embolism
Introduction
The Covid-19 pandemic, which has affected millions of people globally and spread to more than 200 nations,1 is the most egregiously horrible in the past 100 years.2 Covid-19 is brought on by the severe acute respiratory syndrome coronavirus (SARS-CoV-2) and one of its cellular receptors is the angiotensin-converting enzyme 2 (ACE2).3 From asymptomatic or mild symptoms in about 80% of patients to a case mortality rate of about 2% in hospitalised patients, Covid-19 exhibits a variety of clinical manifestations.2–4 Utilizing laboratory tests for early risk stratification is depending on the reasoning that predictive biomarkers of disease severity enable the timely recognition of patients at more risk of progression towards adverse outcomes; this helps for early appropriate therapeutic intervention, thus focusing the allocation of limited medical care resources on patients who would get the best advantages.3 Numerous studies have shown that hospitalized individuals with severe COVID-19 types have aberrant serum coagulation profiles. Additionally, the patients with COVID-19 infection who died fit the clinical requirements for disseminated intravascular coagulation (DIC).5 In a study using computed tomography (CT) scans, pulmonary thrombi were present in the patients with SARS-CoV-2-related pneumonia.6 Unambiguously, increased D-dimer levels were linked to in-hospital mortality.7 Patients with severe COVID-19 have been shown to have a decreased risk of mortality when taking anticoagulant medication.8 Compared to before the pandemic, the COVID-19 pandemic has been linked to higher incidence of PE-related mortality.9 The risk factors and indicators of pulmonary embolism (PE) in COVID-19-infected patients are poorly understood.
Methods
Design of the Study
We conducted a retrospective cohort study on 297 patients infected with COVID-19, aged ≥ 18 years old. We collected the patient’s data records from 4 different centers at period of 3 months duration according to the illustrated flowchart (Figure 1). Based on the World Health Organization (WHO) criteria, we suspected COVID-19 infection, which was later verified by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) testing of nose and pharyngeal swabs.10 The patient was also determined to have COVID-19 when CT results that were regarded characteristic for COVID-19 (ie, significant bilateral and peripheral ground-glass opacities and/or alveolar consolidation) and clinical data were in agreement.11,12
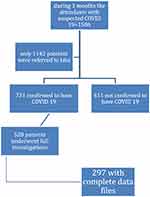 |
Figure 1 Flowchart showing the patients’ inclusion in the study. |
Due to the study’s retrospective nature, informed consent was not required. The patient’s data confidentiality was ensured. Anonymous data were taken from the recorded files after approval from the institution. The study protocol was in compliance with the 1975 Helsinki Declaration’s ethical principles, and the Beni Suef’s Faculty of Medicine ethics committee approved it.
The approval number is: FMBSUREC/01022022/Awad.
All the Patients Underwent
- A thorough medical history and examination.
- Baseline complete blood count (CBC), erythrocytic sedimentation rate (ESR), C- reactive protein (CRP), D-dimer, liver enzymes, serum creatinine, serum lactate dehydrogenase (LDH), and serum ferritin.
- At the initial visit, all patients got a triage-based unenhanced chest CT scan. A consultant radiologist who reviewed these scans determined the likelihood of COVID-19 using a 5-point scale (CO-RADS).
- Patients with suspected PE were diagnosed according to the ESC guidelines recommendations,13 and the patients underwent a computer tomography pulmonary angiography (CTPA). For severity scoring of PE using CTPA, we depended on the obstruction index assessed by the Qandali score, based on the obstruction site, percent of occlusion, and the number of peripheral branches involved, the maximum embolism score of each patient was 2×20=40.
- We categorized the patients according to their risk stratification into low, intermediate-low, intermediate-high, and high-risk according to the ESC Guidelines13 involves the evaluation of two sets of prognostic criteria: (i) the presence of clinical, imaging, and laboratory markers of PE severity, largely associated with the presence of RV dysfunction; and (ii) presence of comorbidities and any other exacerbating factors that may negatively affect early prognosis.
- A biplane Simpson’s rule-based echocardiography study evaluated the left ventricular ejection fraction (LVEF). Tricuspid annular plane systolic excursion (TAPSE) was used to measure RV function. Using the calculated trans-tricuspid systolic pressure gradient and the addition of measured right atrial pressure in accordance with inferior vena cava dimension and inspiratory distensibility, we evaluated the Systolic pulmonary artery pressure (sPAP) from the tricuspid regurgitant jet velocity.
- Resting 12 lead electrocardiogram.
- All the patients were managed according to the Egyptian COVID-19 protocol.14
Analysis of Data
SPSS v. 25 (Statistical Package for Social Science) for Windows was used to analyze the data. Mean, standard deviation (SD), and median (for non-parametric variables) were used to present the quantitative variables. The qualitative variables were reported as both percentages (%) and numbers (No.). To distinguish between cases and controls with respect to scale variables, an Independent T-test was employed. The categorical variables were compared between the two groups using the chi-Square test. Binary logistic regression analysis was used to predict different risk factors of the acquisition of pulmonary embolism.
Receiver operating characteristic (ROC) curve analysis was used to detect the optimal cut-off of different laboratory parameters associated with the development of pulmonary embolism. Youden's index was used to identify the optimal threshold value, and the area under the curve (AUC), a measure of their diagnostic efficacy, was calculated. P-values were used to evaluate the results’ significance; results were deemed significant when P-value 0.05.
Results
Baseline Characteristics of the Studied Population
The disease was found to affect females (60.6%), middle-age (the mean patient’s age was 49±15.9 years), overweight and obese patients (mean body mass index (BMI) was 28.9±5.2) more commonly. The most common patient’s symptoms at admission were fever 68.7%, cough 68.7%, and dyspnea 77.8%, with a mean symptoms’ duration of 9.3±5.3 days. We found only 27.3% of patients were presented with loss of taste and smell, and 14.1% presented with gastrointestinal (GIT) symptoms. The most common laboratory abnormalities at admission were elevated ESR, CRP, serum ferritin, and D- dimer, besides TR’s presence. PE was diagnosed in 18.2% of patients (Tables 1–3).
 |
Table 1 Patients Distribution Regarding Their Baseline Demographic Characteristics |
 |
Table 2 Patients Distribution Regarding Their Baseline Clinical Presentations |
 |
Table 3 Patients Distribution Regarding Their Laboratory Investigations |
PE and Patient’s Baseline Demographic and Clinical Characteristics
We found that the incidence of PE vs non-PE was significantly higher in older patients (mean age 55.9±13.6 vs 47.5±16.1; P= 0.004), females (25% vs 7.7%; p=0.002), those with higher BMI (33±3.8 vs 28.1±5.1; p < 0.001), hypertensive patients (31.4% vs 10.9%; p <0.001), diabetics (38.9% vs 13.6%; p = 0.001), and patients with co-morbidities (chronic obstructive pulmonary disease, atrial fibrillation, malignancy, rheumatologic diseases, chronic kidney disease, cardiovascular disease, autoimmune diseases, and hematological disorders) (38.2% vs 7.7%; p <0.001). Surprisingly, we noticed that none of the 54 smokers developed PE while 22.2% of the non-smokers developed it (Table 1).
Furthermore, the incidence of PE was significantly higher in patients presented with dyspnea (p = <0.001), chest pain (p = <0.001), longer duration of symptoms at hospital admission (p = <0.001), and lower oxygen concentration (p = <0.001). While no one presented with GIT symptoms or taste and smell loss developed PE. Patients presented with fever showed a significantly lower incidence of PE (p = 0.021) (Table 2).
PE and Patient’s Laboratory Investigations
We found that the mean serum Hb level (12.4±1.2 vs 13.1±1.5; p= 0.003), platelet count (177.2±90.1 vs 232.2±94.4; p= 0.002), TLC (4505.6±2718.4 vs 7306.1±2912.8; p< 0.001), and absolute lymphocytic count (0.8±0.3 vs 1.3±0.5; p= 0.002), were significantly lower in patients developed PE compared to those without PE. While the mean D-dimer level was significantly higher in those developed PE 1586.1±471.9 ng/mL (median=1375) vs 702.9±620.7 ng/mL (median=450); p< 0.001). All the patients who developed PE had a CO-RADS scale five on their CT chest scan.
As regards the echocardiographic parameters, the patients who developed PE had a significantly higher mean RV basal diameter (4±0.6 vs 3.3±0.5; p<0.001) and sPAP (52±13.9 vs 36.3±12; p<0.001) and significantly lower mean TAPSE (17.2±2.8 vs 19.9±2.8; p<0.001) compared to those did not developed PE (Table 3).
The Pulmonary Embolism’s Risk Stratification in Affected Patients
We found that 12 (22.2%) patients had a low-risk PE, while 9 (16.7%) patients had intermediate-low risk PE, 20 (37%) patients had intermediate-high risk PE, and 13 (24.1%) patients had a high- risk PE (Table 4). Subsegmental PE was present in nine of the patients who developed PE.
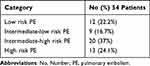 |
Table 4 Pulmonary Embolism’s Risk Stratification in Affected Patients |
Independent Predictors of PE
Age > 65 (OR 5.015, 95% CI 1.423: 17.674, p = 0.012), BMI > 25 (OR 9.053, 95% CI 2.581: 31.754, p = 0.001), DM (OR 32.537, 95% CI 6.004: 76.321, p <0.001), and associated co-morbidities (OR 46.476, 95% CI 8.689: 48.591, p <0.001) were the independent patients’ characteristics associated with the development of PE after the multivariate regression analysis (Table 5).
 |
Table 5 Multivariable Binary Logistic Regression Analysis for the Prediction of Pulmonary Embolism from Different Patients’ Characteristics |
The ROC Curve Analysis
The ROC curve analysis demonstrated that the following laboratory parameters were associated with the development of PE in univariate analysis; 1- TLC at a cut off ≤ 2800 (p- value = <0.001). 2- Absolute Lymphocytic count at a cut off ≤ 0.66 (p- value = <0.001). 3- D- dimer at a cut off >1000 ng/mL (p- value = <0.001). 4- platelet count at a cut off ≤180×103 (p- value = 0.003). 5- ESR at a cut off >35 (p- value = 0.0108) (Table 6 and Table 7).
 |
Table 6 Cut off, Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value of Different Laboratory Parameters Associated with Pulmonary Embolism Occurrence |
Discussion
We found that COVID 19 mainly affects females, middle-aged, overweight and obese patients. The most common patient’s symptoms at admission were fever, cough, and dyspnea, with a mean symptoms’ duration of 9.3±5.3 days. We found only 27.3% of patients were presented with loss of taste and smell, and 14.1% presented with GIT symptoms. The most common laboratory abnormalities at admission were elevated ESR, CRP, serum ferritin, and D- dimer, besides TR’s presence.
According to our study, 18.2% of COVID-19 hospitalised patients got PE, which is a much greater frequency than that of the general population as described in the most recent PE ESC guidelines13 and comparable to a study conducted by Scudiero et al who reported incidence of 14% of cases developed PE15 and a meta-analysis by Jiménez et al reported the incidence at 17%.16 While some studies found a decreased prevalence of PE in patients with covid 19 infection, including those by Hobohm et al, Miró et al, and Fauvel et al (1.9%, 0.5%, and 8.3%, respectively).17–19 An earlier study on diabetes patients in upper Egypt revealed a greater incidence of cardiovascular risk factors and cardiovascular risk factor clustering in those populations, which may help to explain the significantly elevated risk of PE in covid-19 infected individuals in our study.20 We tested many variables suggested to be associated with PE, including patient’s characteristics and laboratory findings, and we found that Age > 65, BMI > 25, DM, and associated co-morbidities were the independent patients’ characteristics associated with the development of PE in those infected with Covid-19 after multivariate regression analysis, while female gender, hypertension, long duration of symptoms, dyspnea, chest pain, and low oxygen concentration were significantly associated with PE in univariate analysis. The echocardiographic parameters TAPSE, sPAP, and RV basal diameter were all significantly associated with the development of PE in univariate analysis. Also, low hemoglobin level, low platelet count, low total leucocytic count, low absolute lymphocytic count, higher D-dimer, and higher CO-RADS score were strongly associated with PE in univariate analysis. TAPSE and sPAP were found to be independently associated with the development of PE by Scudiero et al,15 whereas D-dimer, cardiac injury, and late hospitalisation following the onset of symptoms showed a significant association with PE at univariable analyses, which is consistent with our findings.
Kho et al did yet another study21 suggested that patients who exhibit sudden deterioration, a protracted illness with non-resolving symptoms, increased dyspnoea, ongoing oxygen needs, or noticeably elevated D-dimer values should be checked for PE, especially if COVID-19 infection is present. In this setting, TTE and to a lesser extent the ECG are unreliable PE predictors. We discovered a D-dimer cutoff of >1000 ng/mL, which is more than double the typical threshold value (500 ng/mL) often taken into account for PE diagnosis in the general population13 while Scudiero F, et15 found a higher D-dimer cutoff (1743 ng/mL) for PE diagnosis, but Silva et al22 recommended the adoption of a D-dimer threshold of 500 ng/mL because larger thresholds reduce this strategy’s utility as a screening test while increasing specificity. D-dimer is frequently abnormal in COVID-19 due to the inflammatory response brought on by the SARS-CoV-2 infection and the hypoxia-inducible transcription factor-dependent signalling cascade.23 These results highlight the significance of clinical features, echocardiographic measures, computed tomography, and lab data for the diagnosis and prognostic classification of PE in COVID 19-infected patients.24 We discovered that 61% of patients with covid-19 infection who developed PE were at intermediate-high or high risk (37% and 24.1%, respectively). Our findings corroborated those of Hobohm et al, Miró et al, and Hajra et al17,18,25 As we know, Patients who have COVID 19 infection make it difficult to do echocardiographic evaluations, necessitating special safety measures.26 However, the most recent ESC recommendations suggested echocardiography as the primary imaging modality for early PE detection.13
Limitations of Our Study
There are some restrictions to be aware of with our study. First, the study’s retrospective design. Secondly, the comparative analysis of the data gathered in the current study was restricted by the relatively small number of patients.
Conclusion
A common consequence of COVID 19 infection is PE. Several clinical and laboratory factors are connected to PE in univariate analysis, but age > 65, BMI > 25, DM, and associated co-morbidities were the independent patients’ characteristics associated with the development of PE in those infected with Covid-19.
Abbreviations
ACE, angiotensin converting enzyme; AUC, area under the curve; Covid-19, coronavirus disease 2019; CT, computerized tomography; CTPA, computerized tomography pulmonary angiography; DIC, disseminated intravascular coagulopathy; DM, diabetes mellitus; PE, pulmonary embolism; ROC curve, receiver operating characteristic curve; RV, right ventricle; BMI, body mass index; sPAP, systolic pulmonary artery pressure; TAPSE, Tricuspid annular plane systolic excursion; TLC, total leucocytic count; WHO, World Health Organization.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The need for informed consent was waived due to the retrospective design of the study. The study protocol was approved by: Beni Suef’s Faculty of Medicine ethics committee. The approval number is: FMBSUREC/01022022/Awad. Date of approval, February 2022.
Acknowledgments
We thank all the hospitals’ team who provided us with the required data and registries.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in All these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
There are no conflicts of interest among the authors in this study.
References
1. World Health Organization. Coronavirus disease (COVID-2019) situation reports; 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
2. World Health Organization. Director-general’s opening remarks at the media briefing on COVID-19, 11 March 2020; 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-The-media-briefingon-covid-19---11-march-2020.
3. Li F, Li W, Farzan M, et al. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi:10.1126/science.1116480
4. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi:10.1183/13993003.00547-2020
5. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi:10.1111/jth.14817
6. Caruso D, Zerunian M, Polici M, et al. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020;3:201237.
7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
8. Paranjpe I, Fuster V, Lala V, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi:10.1016/j.jacc.2020.05.001
9. Farmakis IT, Valerio L, Bikdeli B, et al. Annual mortality related to pulmonary embolism in the U.S. before and during the COVID-19 pandemic. J Am Coll Cardiol. 2022;80(16):1579–1581. PMID: 36038034; PMCID: PMC9412135. doi:10.1016/j.jacc.2022.08.721
10. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance; 2020. Available from: https://www.who.int/docs/defaultsource/coronaviruse/clinical-management-of-novel-cov.pdf.
11. Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–796.
12. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi:10.1016/S1473-3099(20)30086-4
13. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. doi:10.1093/eurheartj/ehz405
14. The Egyptian protocol for management of COVID-19. Available from: https://www.researchgate.net/publication/345813633_Management_Protocol_for_COVID-19_Patients_MoHP_Protocol_for_COVID19_November_2020.
15. Scudiero F, Silverio A, Di Maio M, et al. Pulmonary embolism in COVID-19 patients: prevalence, predictors and clinical outcome. Thromb Res. 2021;198:34–39. doi:10.1016/j.thromres.2020.11.017
16. Jiménez D, García-Sanchez A, Rali P, et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159(3):1182–1196. doi:10.1016/j.chest.2020.11.005
17. Hobohm L, Sagoschen I, Barco S, et al. COVID-19 infection and its impact on case fatality in patients with pulmonary embolism. Eur Respir J. 2023;61(1):2200619. PMID: 35981745; PMCID: PMC9411730. doi:10.1183/13993003.00619-2022
18. Miró Ò, Jiménez S, Mebazaa A, et al. Pulmonary embolism in patients with COVID-19: incidence, risk factors, clinical characteristics, and outcome. Eur Heart J. 2021;42(33):3127–3142. PMID: 34164664; PMCID: PMC8344714. doi:10.1093/eurheartj/ehab314
19. Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020;41(32):3058–3068. PMID: 32656565; PMCID: PMC7528952. doi:10.1093/eurheartj/ehaa500
20. Hussein A, Mahmoud SED, Awad MS, Mahmoud HEM. Assessment of cardiovascular risk factors in patients with type 2 diabetes in upper Egypt villages. Diabetes Metab Syndr Obes. 2020;13:4737–4746. PMID: 33311991; PMCID: PMC7725276. doi:10.2147/DMSO.S282888
21. Kho J, Ioannou A, Van den Abbeele K, et al. Pulmonary embolism in COVID-19: clinical characteristics and cardiac implications. Am J Emerg Med. 2020;38(10):2142–2146. doi:10.1016/j.ajem.2020.07.054
22. Silva BV, Jorge C, Plácido R, et al. Pulmonary embolism and COVID-19: a comparative analysis of different diagnostic models performance. Am J Emerg Med. 2021;50:526–531. doi:10.1016/j.ajem.2021.09.004
23. Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. doi:10.1016/j.thromres.2019.07.013
24. Garcia-Olivé I, Sintes H, Radua J, et al. D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir Med. 2020;169:106023. doi:10.1016/j.rmed.2020.106023
25. Hajra A, Goel A, Malik AH, et al. Impact of COVID-19 on patients hospitalized with deep vein thrombosis and/or pulmonary embolism: a nationwide analysis. Curr Probl Cardiol. 2023;48(4):101553. PMID: 36528208; PMCID: PMC9749377. doi:10.1016/j.cpcardiol.2022.101553
26. Skulstad H, Cosyns B, Popescu BA, et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21(6):592–598.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

