Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Patient and physician perceptions of disease management in Parkinson’s disease: results from a US-based multicenter survey
Authors Hermanowicz N , Castillo-Shell M, McMean A, Fishman J, D’Souza J
Received 4 December 2018
Accepted for publication 4 April 2019
Published 30 May 2019 Volume 2019:15 Pages 1487—1495
DOI https://doi.org/10.2147/NDT.S196930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Neal Hermanowicz,1 Michelle Castillo-Shell,2 Angela McMean,3 Jesse Fishman,2 Joseph D’Souza2
1Movement Disorders Program and Department of Neurology, University of California-Irvine, Irvine, CA, USA; 2UCB Pharma, Smyrna, GA, USA; 3Ashfield Insight & Performance, part of Ashfield Healthcare Communications Group Ltd, Macclesfield, Cheshire, UK
†Dr. Joseph D’Souza passed away on February 16, 2019
Background: Clinical care for patients with Parkinson’s disease (PD) is complex, and disconnect may exist between patient and physician perceptions of treatment, disease awareness, and impact on quality of life (QoL). Relatively few studies have analyzed patient and physician perspectives of disease management concurrently, and even fewer have compared responses between corresponding patients and their physicians. This study aimed to characterize these aspects and identify opportunities to improve alignment.
Methods: This cross-sectional study used an online survey and chart review. Participating physicians completed a profiling survey, followed by patient record forms (PRFs) for their next five patients with PD. Patients completed paper questionnaires. PRFs were matched with patient questionnaires, and patient and physician responses compared.
Results: Of 107 participating physicians, 70 completed 350 PRFs. Patients completed 71 questionnaires; 66 were matched to PRFs. From a physician perspective, there was alignment between the motor symptoms that were most bothersome for patients and those that were most discussed (physicians felt tremor was most bothersome for most patients [71%]; 77% of physicians included tremor among top three most discussed), but disconnect between the most bothersome and most discussed nonmotor symptoms (physicians felt fatigue was most bothersome for most patients [35%]; cognitive impairment was the most discussed nonmotor symptom, with 52% of physicians including it in top three most discussed). Patients and physicians reported moderate satisfaction with current PD medication. Patients considered form of delivery more important than did physicians. Physicians showed a strong level of awareness of PD’s impact on patient QoL, although validated QoL instruments were not widely used. Physicians were more confident than patients about patients’ awareness of support resources for patients with PD.
Conclusion: Nonmotor symptoms, form of medication delivery, and awareness of support services are areas where PD physician and patient alignment could be increased to improve outcomes.
Keywords: Parkinson’s disease, survey, disease awareness, quality of life, patients, physicians
Introduction
Parkinson’s disease (PD) is a common and progressive neurodegenerative disorder, characterized by heterogeneous motor and nonmotor symptoms.1 In the US, significant barriers exist to providing high-quality care for patients with PD, including limited access to subspecialist neurologists and lack of clinical expertise of primary care physicians.2 For each patient, the complexity of PD leads to clinical challenges; integrating the patient perspective as part of shared decision-making is essential to improving care.3
The management of chronic diseases such as PD typically represents a feedback loop composed of five steps shared by patients and physicians: patient identification, diagnosis, choice of therapy, disease and drug information, and patient monitoring (Figure 1).4 Effective navigation through this patient–physician partnership is fundamental to improving patient outcomes; however, there can be a disconnect between patient and physician perceptions of disease management, leading to suboptimal treatment outcomes.4
 | Figure 1 The five stages of the patient–physician partnership.
Note: Reproduced from Groenewegen A, Tofighy A, Ryvlin P, Steinhoff BJ, Dedeken P. Measures for improving treatment outcomes for patients with epilepsy – results from a large multinational patient-physician survey. Epilepsy Behav. 2014;34:58–67. Creative Commons license and disclaimer available from: |
Many surveys have evaluated the perceptions of patients living with PD across a varied range of domains, including symptoms,5–7 experience and expectations of therapy,8–12 and psychosocial factors such as stigma.13 Few studies have analyzed patient and physician perspectives of disease management concurrently,14–16 and even fewer have compared perspectives between patients and their own physicians; however, the results can reveal points of divergence. For instance, a survey evaluating patient and physician perceptions of telemedicine consultations for PD indicated that physicians believed overall quality of care to be compromised by their limited ability to examine patients virtually, but patients rarely commented on this point.17 Furthermore, one large prospective study into the reasons behind treatment modifications in patients with PD (the REASON study) found that patients placed more significance than neurologists on nonmotor symptoms as the basis for treatment changes.15,16 This highlights another area of disease management where patient and neurologist perceptions diverge. Characterizing areas of disconnect may provide the opportunity to improve outcomes through increasing alignment between patients and physicians, particularly in areas where more research is needed, such as awareness of support groups, information, and resources.
The objectives of our study were to provide an understanding of current clinical practice and PD information/resource preferences among physicians treating patients with PD, and to quantify any areas of disconnect between patient and physician perceptions of clinical management, treatment satisfaction, disease awareness, and educational needs.
Methods
Study design
The study used a cross-sectional design based on a quantitative online survey and chart review. The survey was administered in the US by the research agency Ashfield Healthcare Communications Group Ltd, United Kingdom (Macclesfield, Cheshire, UK).
The survey included two sections: physician profiling and patient record forms (PRFs) (Figure 2). Physician demographics were assessed using a 70-min online survey, after which each physician completed PRFs for the next five patients with PD whom they saw. Patients were asked to complete a paper questionnaire along with their caregiver, if present.
 | Figure 2 Structure of the study survey.Abbreviations: PD, Parkinson’s disease; PRF, patient record form; QoL, quality of life. |
The questionnaire was developed using steps 2 through 5 of the patient–physician partnership framework; step 1, identification of new patients, was not included because all participating patients had already received a diagnosis of PD. The questions focused on perceptions across several key domains: symptoms and treatment, impact of PD on patient quality of life (QoL), and information and resources/knowledge about PD.
Participants
The physicians included in the study were members of a physician panel who accepted an invitation to participate. They were US-based neurologists and movement disorder specialists who had been in clinical practice for 3–60 years, saw at least 10 patients with PD in a month, and were responsible for initiating or altering drug therapy for patients with PD. Participating patients were US-based patients with PD who were seen by the physicians in the study.
Data collection and analysis
All data were collected between July 21 and December 9, 2016. Data entry and analysis were carried out by Ashfield Healthcare Communications Group Ltd. Physician data were recorded automatically through the online system, and patient data were recorded on paper questionnaires, which were mailed back to the research agency and manually entered into the online system. All data were quality checked during and after fieldwork; any erroneous respondents were removed and replaced. Quality checking included examining online survey completion times for those completing the survey very quickly, and checking for consistent selection of “Don’t know” where that option was available, choosing the same response to each question, inconsistent responses, nonsensical open-ended responses, or unrealistic numeric responses; any respondents fitting the above criteria were double-checked and removed and replaced if necessary. PRF records were matched with patient questionnaires by cross-checking patient survey identity, year of birth, sex, and year of diagnosis of PD.
Analyses included comparisons between the physician group and the patient group and between matched PRFs and patient questionnaires. Independent t-tests with a 95% confidence level (SPSS 22TM) were used for comparisons between the physician group and patient group. Paired t-tests were used for the matched sample of PRFs versus patient questionnaires. Based on the formula of adjusted alpha for Bonferroni correction, for comparisons between PRFs and patients’ questionnaires, the adjusted alpha was 0.05/3=0.017. The same was true for comparisons between the physician and patient groups.
Results
Characteristics of participants and visit duration
Of the 107 physicians recruited, 70 provided complete PRFs. Of these, 52 were neurologists and 18 were movement disorder specialists. Overall, physicians completed 350 PRFs, patients completed 71 questionnaires, and 66 patient questionnaires were matched to PRFs (based on patient’s date of birth, gender, and date of diagnosis). In addition, caregivers completed 32 questionnaires. Patient characteristics from PRFs and patient questionnaires are summarized in Table 1.
The physician-reported “average visit duration for patients with early PD” was shorter (mean 31 min; range 10–120 min) than that for patients with advanced PD (mean 36 min; range 10–120 min).
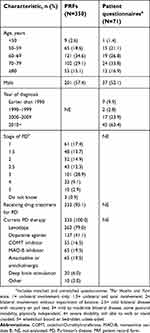 | Table 1 Patient characteristics from PRFs and patient questionnaires |
Symptoms and treatment
Physicians reported motor symptoms to be the most bothersome symptoms for patients with PD (N=350 PRFs), most commonly tremor (71%), bradykinesia (54%), and rigidity (52%), followed by postural instability (36%) and freezing of gait (21%). For patients with severe PD (defined as Hoehn and Yahr stage 4 or 5; N=42), physicians identified postural instability as the most bothersome motor symptom (76%). Regarding nonmotor symptoms, fatigue/loss of energy (35%), depression (30%), and sleep disturbances (29%) were reported by physicians as the most bothersome for patients, followed by cognitive impairment (19%) and fear/anxiety (15%).
From a physician perspective (N=107), there was alignment between the most bothersome motor symptoms for patients and the motor symptoms discussed most during patient visits, with physicians most commonly ranking tremor (77%), postural instability (55%), and bradykinesia (53%) in their top three most discussed motor symptoms. However, there was some disconnect between the nonmotor symptoms physicians identified as most bothersome for patients and the nonmotor symptoms that physicians reported were discussed most at patient visits. Physicians (N=107) most commonly ranked cognitive impairment (52%), psychosis symptoms (38%), and sleep disturbances (36%) in their top three most discussed nonmotor symptoms. Cognitive impairment was the most discussed nonmotor symptom, but as indicated above, it was identified by physicians as the most bothersome nonmotor symptom in only 19% of cases.
Overall, physicians (N=107) reported spending a greater proportion of each visit discussing motor symptoms compared with nonmotor symptoms (64% vs 36%). However, patients (N=71) perceived that motor and nonmotor symptoms were discussed with a similar frequency, with 87% and 73% reporting discussion at every visit, respectively. With respect to nonmotor symptoms, 61% of patients reported discussing these for a quarter of the visit time, and 28% of patients for half of the time.
There was also some divergence between patient and physician perceptions of the areas discussed at visits (Table 2). While patients perceived that emotional impact was discussed at almost every visit, physicians perceived that this was discussed less frequently (matched patients vs matched PRFs, P<0.001). Analysis on matched PRFs showed that patients perceived that they discussed other PD-related conditions less frequently than physicians perceived although this difference did not reach statistical significance (matched patients vs matched PRFs, P=0.017).
 | Table 2 Matched PRFs versus patients |
Overall, the levels of physician-reported (N=107) medication adherence were high, with 68% of physicians reporting that their patients adhered to their medication more than 75% of the time. The most commonly physician-reported (N=107) reasons for nonadherence were the frequency of dosing (61%), side effects of medication (57%), and patients not remembering to take their medication (51%). Patients were moderately satisfied, and physicians believed their patients to be moderately satisfied, with the current PD medication prescribed (matched patients vs matched PRFs, P=0.045) (Table 2).
Patients and physicians were in alignment that treatment efficacy (patients vs physicians, P=0.764) and safety and tolerability profile (patients vs physicians, P=0.923) are important factors guiding selection of PD therapy (Table 3). However, patients rated form of medication delivery as being significantly more important than did physicians (patients vs physicians, P=0.0169).
 | Table 3 Patient versus physician perceptions of aspects of PD |
Impact of PD on patient QoL
The majority of patients (N=71) reported being asked how they were feeling at every visit (87%), while 58% were asked about their overall QoL at every visit (58%). Patient (N=71), physician (N=350 PRFs), and caregiver (N=32) perceptions of the impact of PD on patient QoL were well aligned, with the majority in each group reporting some negative impact of PD on QoL (patients 62%, physicians 58%, caregivers 54%), and a smaller proportion reporting an extremely negative impact of PD on patient QoL (patients 22%, physicians 24%, caregivers 29%).
Regarding specific activities of daily living, patients (N=71) reported that PD had the most negative impact on their ability to drive (41%), followed by leisure activities (24%) and the ability to work or study (24%). Although physicians (N=350 PRFs) reported that the majority of their patients (74%) had not received a QoL assessment during the past 12 months, physicians showed a strong level of awareness of the impact of PD on patient QoL. Mean scores were similar across the QoL aspects evaluated (Table 2), with the exception of social relationships, where patients perceived a lower level of impact of PD compared with physicians (matched patients vs matched PRFs; P<0.001).
Disease awareness and resources
Patients (N=71) and caregivers (N=32) reported turning most frequently to their physician for information about PD, with 69% of patients and 47% of caregivers seeking information every few months. Physicians (N=107) who self-rated their overall level of knowledge as high (scoring 6 or 7, on a scale of 1–7 [1= not at all informed to 7= extremely well informed]) reported feeling most informed about the clinical profiles of drugs (67%) and national treatment guidelines (61%), and least informed about patient support groups/resources (33%) and patient coping strategies (40%). Furthermore, the majority of physicians (72%) advised patients to attend support groups, despite reporting feeling least informed about these compared with other key aspects of PD management.
Physicians were more confident about patients’ knowledge than the patients themselves were (Table 3), with physician ratings being significantly higher than patient ratings regarding awareness of patient support services (patients vs physicians, P=0.005). Uptake of patient support services was low, with only 4% of 71 patients, and 3% of 32 caregivers reporting using patient websites or support groups.
Discussion
The results of this US-based multicenter survey provide important insights into the perspectives of patients living with PD and their treating physicians. The survey responses reveal areas of disconnect between patient and physician perceptions of PD therapy, impact on QoL, and disease awareness, which represent potential opportunities to improve care.
The survey revealed that the focus of discussions between patients and physicians tends to be on motor symptoms, especially tremors, as these were the most bothersome symptoms for patients with PD in this study. This perception has been previously observed in patients with earlier-stage PD,5 despite the relatively low impact of tremor on patients’ overall health status18,19 and the inconsistent response of tremor to current medical therapies.1 The REASON study also found that the presence/worsening of motor symptoms such as tremor and bradykinesia was the main reason driving neurologists to change PD therapy and for patients with PD to request a change in therapy due to treatment dissatisfaction.15,16 There is less alignment between the most bothersome and the most discussed nonmotor symptoms. This may be related to the relatively low PD severity of the study group, with approximately 30% of patients being staged as 1 or 1.5 on the Hoehn and Yahr scale, as nonmotor symptoms may be perceived as more troublesome by patients with advanced PD compared with patients with earlier-stage PD.5 Divergence between neurologists and patients in the relevance they place on nonmotor symptoms as the basis for treatment modifications was previously reported in the REASON study;15,16 neurologists may place more emphasis on motor than nonmotor symptoms because they are more familiar with them, motor symptoms are easier to assess and more responsive to changes in therapy.15 Regardless of disease stage, the impact of nonmotor symptoms on patient QoL is at least as significant as that of motor symptoms,18,20 and it is important for physicians to fully understand each patient’s experience of living with nonmotor impairments to plan and optimize care.
Both patients and physicians expressed a moderate level of satisfaction with PD medication, indicating that further improvements should be made in this area. Physician-reported levels of medication adherence were high, although the factors influencing compliance were not evaluated from a patient perspective. While physicians and patients were aligned on the importance of medication efficacy, safety, and cost, patients considered the form of medication delivery to be more important than did physicians. This finding is supported by a survey of patients with PD and practicing neurologists in Germany, in which 12.3% of patients reported feeling impaired by the form of delivery of their medication, but only 3.7% of physicians reported that their patients were negatively affected by this aspect.21 Patients with PD often feel that treatment regimens are overly complex, and pill burden may be especially high when additional medication is required for comorbidities.22,23 In addition, adherence to complex treatment regimens is a particular concern for patients with cognitive impairment.23 Physicians could give more consideration to the form of medication delivery when making treatment decisions, which could have a positive impact on aspects such as treatment adherence, and improve overall patient satisfaction with therapy.
In this study, physicians showed a strong level of awareness of the impact of PD on their patients’ QoL, although there was scope to improve alignment regarding its impact on social relationships, as well as its emotional impact. Importantly, three-quarters of patients had not received a QoL assessment in the past 12 months, which suggests that physicians are using other subjective or patient-reported measures to determine the level of impact on patient QoL. Many PD-specific QoL assessment tools have been developed, and can be used in combination to gain a comprehensive profile of patient QoL and how this changes over time.24 QoL measures may be patient-based or require input from a health care professional; they may be generic, such as the 39-item Parkinson’s Disease Questionnaire (PDQ-39)25 and the Patient-Reported Outcomes in Parkinson’s Disease,26 or focused on a specific domain, for instance function (Self-Assessment Parkinson’s Disease Disability Scale)27 or expectations of therapy (Patient-Reported Outcome Tool for Advanced Parkinson’s Disease).9 Although a standardized approach has not been established, many of these tools are designed to complement clinical assessment and support physicians seeking to improve or maintain patient QoL as a central goal of care.24 Although QoL is not currently a reimbursable measure for physicians in the US, validation of the association between QoL and patient satisfaction could encourage more widespread use of QoL tools or other patient activation measures.
In general, physicians were more confident about patients’ knowledge of various aspects of PD than were patients, particularly regarding the availability of patient support resources. Patients and caregivers most commonly turn to their physician for information; however, the majority of physicians do not feel well informed about patient coping strategies, an area some patients indicated they would like to discuss more frequently, or patient support groups and services. Furthermore, only a small minority of patients and caregivers are currently using support groups or websites, which may be because of physicians’ lack of knowledge and awareness of these tools. Physicians could focus more on these educational needs during consultations, and aim to provide educational materials and signpost support services to patients and caregivers. For instance, in the US, organizations such as the Michael J. Fox Foundation provide access to downloadable resources, educational webinars, and information about local patient groups.
Study limitations
This study reports subjective perceptions and did not use standardized scales, which are not currently available to evaluate the research questions posed in this study. The sample size was relatively small, and the majority of patients had relatively mild disease, which may not be representative of the general population of patients with PD. Patients were not stratified based on factors such as disease stage or time since diagnosis, which may have affected aspects of the survey such as QoL and level of disease awareness, nor were they stratified based on whether their care was provided by a general neurologist or a movement disorder specialist. In addition, potential confounding factors such as patient age and level of education were not taken into account. The findings might have differed depending on which medication patients were taking and any associated differences in adherence. Furthermore, some findings, such as the low uptake of patient support services, may also have differed if the study had been conducted in other countries. These would be interesting avenues for future research.
Conclusions
In conclusion, nonmotor symptoms, form of medication delivery, and awareness of support services are areas where PD physician and patient alignment could be increased to potentially improve patient outcomes, with physicians focusing more on these areas during consultations. Most patients had not received a QoL assessment in the past 12 months, representing another area for improvement.
Ethics approval and informed consent
This research was compliant with the European Pharmaceutical Market Research Association Code of Conduct and the Health Insurance Portability and Accountability Act of 1996. Ethical approval was not required. Informed consent was obtained from all physicians at the start of the online survey, and from all patients on the paper questionnaire they completed.
Abbreviations
PD, Parkinson’s disease; PDQ-39, 39-item Parkinson’s Disease Questionnaire; PRF, patient record form; PRO-PD, Patient-Reported Outcome Tool for Parkinson’s Disease; PRO-APD, Patient-Reported Outcome Tool for Advanced Parkinson’s Disease; QoL, quality of life.
Acknowledgments
The authors thank the physicians, patients, and their caregivers who took part in the survey.
The authors would like to acknowledge the outstanding contribution of their late co-author Joseph D’Souza to this research, for his dedication to science, and for his efforts to help improve the lives of people with Parkinson’s disease.
Medical writing and editorial assistance was provided by Lauri Arnstein, MA, MBBS (Evidence Scientific Solutions, London, UK) and Nicole Meinel, PhD, CMPP (Evidence Scientific Solutions, London, UK), funded by UCB Pharma (Smyrna, GA, USA), and publication coordination was provided by Helen Ysak, PharmD (Publication and Data Dissemination Lead – Neurology, UCB Pharma, Smyrna, GA, USA). This survey was funded by UCB Pharma, Smyrna, GA, USA. UCB Pharma was responsible for the design and conduct of the survey, the collection, analysis, and interpretation of the data, and was involved in the preparation and review of the manuscript. The authors made the final decision to submit the manuscript for publication.
Author contributions
All authors were involved in designing the study and interpretation. Neal Hermanowicz is a physician who took part in this study. Angela McMean conducted the study and was involved in data analysis. All authors were involved in the decision to submit the article for publication, drafting or critically revising the manuscript and approved the final version for submission to this journal. Joseph D’Souza was unable to approve the final revised version which addressed peer reviewer comments; however, all of his co-authors approved the final revised version for publication and agree to be accountable for all aspects of the work.
Disclosure
Neal Hermanowicz was on the UCB Speakers’ Bureau and received honoraria in 2016. Neal Hermanowicz also reports personal fees from UCB Pharma, outside the submitted work. Michelle Castillo-Shell is a salaried employee of UCB Pharma and has received stock options from her employer. Angela McMean is a salaried employee of Ashfield Healthcare Communications Group Ltd. Jesse Fishman is a former employee of UCB Pharma and is a current employee of Janssen Pharmaceuticals. Jesse Fishman also reports personal fees from Johnson and Johnson and UCB Pharma, during the conduct of the study. Joseph D’Souza was an employee of UCB Pharma when the study was conducted and the manuscript developed. The authors report no other conflicts of interest in this work.
References
1. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3
2. Racette BA, Willis AW. Time to change the blind men and the elephant approach to Parkinson disease? Neurology. 2015;85(2):2015190–2015196. doi:10.1212/WNL.0000000000001739
3. Lim SY, Tan AH, Fox SH, Evans AH, Low SH. Integrating patient concerns into Parkinson’s disease management. Curr Neurol Neurosci Rep. 2017;17(1):3. doi:10.1007/s11910-017-0717-2
4. Groenewegen A, Tofighy A, Ryvlin P, Steinhoff BJ, Dedeken P. Measures for improving treatment outcomes for patients with epilepsy – results from a large multinational patient-physician survey. Epilepsy Behav. 2014;34:58–67. doi:10.1016/j.yebeh.2014.02.033
5. Politis M, Wu K, Molloy S, Bain PG, Chaudhuri KR, Piccini P. Parkinson’s disease symptoms: the patient’s perspective. Mov Disord. 2010;25(11):1646–1651. doi:10.1002/mds.23135
6. Hung SW, Adeli GM, Arenovich T, Fox SH, Lang AE. Patient perception of dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81(10):1112–1115. doi:10.1136/jnnp.2009.173286
7. Breen KC, Drutyte G. Non-motor symptoms of Parkinson’s disease: the patient’s perspective. J Neural Transm. 2013;120(4):531–535. doi:10.1007/s00702-012-0928-2
8. Weermink MG, van Til JA, van Vugt JP, Movig KL, Groothuis-Oudshoorn CG, IJzerman MJ. Involving patients in weighting benefits and harms of treatment in Parkinson’s disease. PLoS One. 2016;11(8):e0160771. doi:10.1371/journal.pone.0160771
9. Reddy P, Martinez-Martin P, Brown RG, et al. Perceptions of symptoms and expectations of advanced therapy for Parkinson’s disease: preliminary report of a Patient-Reported Outcome tool for Advanced Parkinson’s disease (PRO-APD). Health Qual Life Outcomes. 2014;12:11. doi:10.1186/1477-7525-12-11
10. Wüllner U, Fuchs G, Rekatat N, Randerath O, Kassubek J. Requirements for Parkinson’s disease pharmacotherapy from the patients’ perspective: a questionnaire-based survey. Curr Med Res Opin. 2012;28(7):1239–1246. doi:10.1185/03007995.2012.702101
11. Mathers J, Rick C, Jenkinson C, et al. Patients’ experiences of deep brain stimulation for Parkinson’s disease: a qualitative systematic review and synthesis. BMJ Open. 2016;6(6):e011525. doi:10.1136/bmjopen-2016-011525
12. Schrag A, Khan K, Hotham S, Merritt R, Rascol O, Graham L. Experience of care for Parkinson’s disease in European countries: a survey by the European Parkinson’s Disease Association. Eur J Neurol. 2018;25(12):1410-e120. doi:10.1111/ene.13738
13. Maffoni M, Giardini A, Pierobon A, Ferrazzoli D, Frazzitta G. Stigma experienced by Parkinson’s disease patients: a descriptive review of qualitative studies. Parkinsons Dis. 2017;2017:7203259. doi:10.1155/2017/7203259
14. Fargal M, Grobe B, Oesterle E, Hastedt C, Rupp M. Treatment of Parkinson’s disease: a survey of patients and neurologists. Clin Drug Investig. 2007;27(3):207–218. doi:10.2165/00044011-200727030-00004
15. Tinazzi M, Abbruzzese G, Antonini A, et al. Reasons driving treatment modification in Parkinson’s disease: results from the cross-sectional phase of the REASON study. Parkinsonism Relat Disord. 2013;19(12):1130–1135. doi:10.1016/j.parkreldis.2013.08.006
16. Abbruzzese G, Barone P, Ceravolo R, et al. Clinical variables associated with treatment changes in Parkinson's disease: results from the longitudinal phase of the REASON study. Neurol Sci. 2015;36(6):935–943. doi:10.1007/s10072-014-2060-6
17. Mammen JR, Elson MJ, Java JJ, et al. Patient and physician perceptions of virtual visits for Parkinson’s disease: a qualitative study. Telemed J E Health. 2018;24(4):255–267. doi:10.1089/tmj.2017.0119
18. Duncan GW, Khoo TK, Yarnall AJ, et al. Health-related quality of life in early Parkinson’s disease: the impact of nonmotor symptoms. Mov Disord. 2014;29(2):195–202. doi:10.1002/mds.25664
19. Qin Z, Zhang L, Sun F, et al; Chinese Parkinson Study Group. Health related quality of life in early Parkinson’s disease: impact of motor and non-motor symptoms, results from Chinese levodopa exposed cohort. Parkinsonism Relat Disord. 2009;15(10):767–771. doi:10.1016/j.parkreldis.2009.05.011
20. Hinnell C, Hurt CS, Landau S, Brown RG, Samuel M; PROMS-PD Study Group. Nonmotor versus motor symptoms: how much do they matter to health status in Parkinson’s disease? Mov Disord. 2012;27(2):236–241. doi:10.1002/mds.23961
21. Jost WH, Bausch J. Patients’ perspective on current treatment options for Parkinson’s disease. Basal Ganglia. 2017;9:7–11. doi:10.1016/j.baga.2017.05.001
22. Malek N, Grosset DG. Medication adherence in patients with Parkinson’s disease. CNS Drugs. 2015;29(1):47–53. doi:10.1007/s40263-014-0220-0
23. Fleisher JE, Stern MB. Medication nonadherence in Parkinson’s disease. Curr Neurol Neurosci Rep. 2013;13(10):382. doi:10.1007/s11910-013-0382-z
24. Martinez-Martin P. What is quality of life and how do we measure it? Relevance to Parkinson’s disease and movement disorders. Mov Disord. 2017;32(3):382–392. doi:10.1002/mds.26885
25. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4(3):241–248. doi:10.1007/BF02260863
26. Mischley LK, Lau RC, Weiss NS. Use of a self-rating scale of the nature and severity of symptoms in Parkinson’s disease (PRO-PD): correlation with quality of life and existing scales of disease severity. NPJ Parkinsons Dis. 2017;3:20. doi:10.1038/s41531-017-0021-5
27. Biemans MA, Dekker J, van der Woude LH. The internal consistency and validity of the self-assessment Parkinson’s disease disability scale. Clin Rehabil. 2011;15(2):221–228. doi:10.1191/026921501667641185
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
