Back to Journals » Patient Preference and Adherence » Volume 13
Patient And Nurse Experience Of Using Somatostatin Analogues To Treat Gastroenteropancreatic Neuroendocrine Tumors: Results Of The Somatostatin Treatment Experience Trial (STREET)
Authors Ström T , Kozlovacki G, Myrenfors P, Almquist M
Received 25 April 2019
Accepted for publication 18 September 2019
Published 23 October 2019 Volume 2019:13 Pages 1799—1807
DOI https://doi.org/10.2147/PPA.S213472
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Torbjörn Ström,1 Gordana Kozlovacki,2 Peter Myrenfors,1 Martin Almquist3
1Medical Department, Ipsen AB, Stockholm, Sweden; 2Department of Medical Sciences, Section of Endocrine Oncology, Uppsala University, Uppsala, Sweden; 3Department of Endocrine and Sarcoma Surgery, Lund University, Lund, Sweden
Correspondence: Martin Almquist
Department of Endocrine and Sarcoma Surgery, Lund University, Lund 221 85, Sweden
Tel +46 46 17 62 45
Fax +46 46 14 72 98
Email [email protected]
Purpose: Evaluate patients’ and nurses’ experiences, including injection problem frequency, with the somatostatin analogues (SSAs) lanreotide autogel® (Somatuline® autogel®, deep subcutaneous) and octreotide long-acting release (LAR) (Sandostatin® LAR®, intramuscular) when treating gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Methods: An observational, cross-sectional study across 2 NET centers in Sweden. Questionnaires based on participants’ most recent injection experience were sent to patients with GEP-NETs treated with octreotide or lanreotide, and to nurses administering these treatments. Nurses were identified via patients completing their questionnaires. Resource use was sourced from Swedish prescription registry records. The planned sample size was 200, based on an estimated proportion of 0.50 and ±7% precision.
Results: 119/156 patients (n=53, lanreotide; n=66, octreotide) and 43/53 nurses (n=22, lanreotide; n=21, octreotide) completed questionnaires. Despite smaller recruitment than planned, the endpoint precision was ±9% with 119 participants, and still considered reasonable. More octreotide-treated patients reported problems (18% vs none; P=0.001) and experienced moderate-to-high anxiety pre-injection (11% vs 2%). Patients had similar physical HRQoL scores overall (Short Form-12 mean composite scores: physical: 39.4 vs 37.6; mental: 50.7 vs 49.6). The mean number of lanreotide and octreotide doses dispensed per year were 11.1 and 12.6, respectively (P<0.05). In the lanreotide group, 28% self-injected, while 29% were not aware they could self-inject. In the octreotide group, 3% self-injected and 73% were unaware of the availability of an SSA for self-injection. Most patients (61%) felt well-informed about their disease and treatment. Nurses were generally experienced and felt confident and well-informed about giving SSA injections; however, only 12% felt well-informed about the disease and treatment.
Conclusion: Those treated with lanreotide reported fewer injection problems and experienced less pre-injection anxiety than those treated with octreotide. SSA choice did not appear to affect patients’ HRQoL. Some patients treated with octreotide were unaware of an SSA with the flexibility of self-injection.
Keywords: gastroenteropancreatic neuroendocrine tumors, self-administration, somatostatin analogues
Background
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are a rare group of malignancies with an annual incidence of five cases per 100,000 people.1 They can cause various hormone-related symptoms, such as flushing and diarrhea, as a result of secretion of serotonin and vasoactive hormones.2 These symptoms can be treated with somatostatin analogues (SSAs), which suppress excess hormone secretion.2
Two long-acting SSA formulations, with different indications, have been shown to effectively slow the progression of metastatic NETs in previous studies,3,4 and both are currently available for this indication. Octreotide long-acting release (LAR) (Sandostatin® LAR®, Novartis, Basel, Switzerland5) – referred to herein as octreotide – is provided as a powder for suspension for intramuscular injection.5 Lanreotide autogel® (Somatuline® Autogel®, Ipsen Pharma, Slough, UK6) – referred to herein as lanreotide – is manufactured as a prefilled syringe for deep subcutaneous (SC) injection, and is approved for self-injection.6 Johanson et al showed in a randomized crossover study that 88% of patients who try self-administration of lanreotide prefer it to administration by a healthcare professional.7 In a survey of nurses evaluating SSA device attributes, lanreotide scored higher than octreotide for 15 of the 16 attributes and had a significantly higher overall preference score.8 However, there is a general lack of observational research on the practicalities of SSA administration in clinical use, the treatment experience of patients and nurses, factors that may influence this experience, and health-related quality of life (HRQoL) among patients with GEP-NETs.
Here, we report patients’ and nurses’ experiences of lanreotide and octreotide for treating GEP-NETs. The primary objective was to determine whether patients treated with lanreotide had fewer injection problems than those treated with octreotide. We also investigated the HRQoL of patients treated with SSA, resource use associated with treatment, and patients’ knowledge about the disease and its treatment options. Nurses’ experience with SSA treatment was also evaluated.
Methods
Study Design And Participants
The Somatostatin Treatment Experience Trial (STREET) was an observational, retrospective, cross-sectional and non-interventional study among patients and nurses from two Swedish NET centers: Lund University Hospital and Uppsala University Hospital.
Patients were eligible for inclusion if they were ≥18 years old with a histopathologically confirmed diagnosis of GEP-NET (Ki 67 <10%) and had been treated with an SSA for between 3 months and 3 years. Due to the non-interventional and retrospective study design, the decision to prescribe an SSA was made prior to and independently of the decision to enroll patients. There were no specific exclusion criteria. Overall, 156 patients were identified by searching medical records at the study centers; to avoid selection bias, all patients identified by the searches were considered eligible for inclusion. In total, 53 nurses were identified by asking eligible patients to provide contact details (as part of the questionnaire) for nurses who had administered their SSA. As some patients shared the same nurse, fewer nurses were recruited than patients.
Eligible patients and nurses were contacted by mail; the letter contained information about the study, a paper questionnaire and a consent form. A reminder was sent to those who did not respond.
Responses to the questionnaires were collected by a third party (Meductus AB), who entered them into electronic case report forms (eCRFs). For patients, additional information from their medical records and from their Swedish prescription registry records was entered into the eCRF. All data in the eCRFs were anonymized.
Prior to study start, the protocol was reviewed and approved by the regional ethics committee in Lund (reference number 2015/650). The study was conducted in compliance with the recommendations of the Declaration of Helsinki (2008) and the International Ethical Guidelines for Epidemiological Studies (2008). It adhered to the recommendations of the International Epidemiological Association Guidelines for Proper Conduct in Epidemiologic Research and the International Society for Pharmacoepidemiology Good Pharmacoepidemiological Practices Guidelines. The study was registered with clinicaltrials.gov (NCT02788565). All patients and nurses provided their written informed consent prior to participation in the study.
Assessments And Outcome Measures
Patient Questionnaire
The patient questionnaire contained questions on the patient’s experience of SSA treatment (problems with SSA injection, anxiety before injection), knowledge about the disease and treatment options, resource use (exploratory analysis), and HRQoL. Problems with injection were assessed by asking patients to provide information, based on their most recent injection, on any problems they had experienced with the syringe, including whether only a partial amount or none of the SSA dose had been injected. The primary endpoint of the study was the proportion of patients who had problems with their most recent SSA injection.
Anxiety levels before the injection were rated using the following categories: not at all, some, moderate, much, or very much. Knowledge about the disease and treatment options was assessed by asking patients how well informed they were on these matters, whether they had been informed about the possibility of self-injection, whether they had self-administered their last injection, and reasons for choosing/not choosing to self-inject. As part of an exploratory analysis to assess resource use, patients were asked about the frequency of injection and time spent receiving assistance from healthcare professionals.
HRQoL was evaluated using the Short Form-12 (SF-12), a validated and reliable questionnaire that contains six questions on physical HRQoL and six on mental HRQoL.9 These questions do not relate directly to the injection experience, but instead cover physical functioning, physical and emotional influences on work, bodily pain, general health, vitality, social functioning, and mental health.
Nurse Questionnaire
Nurses were asked to report on the last SSA injection they performed. The nurse questionnaire included questions on: the number of SSA injections administered each year; confidence injecting the SSA (rated according to the following categories: not at all, a little, moderate, very [or very much]); any problems during administration of the most recent injection; and knowledge about the disease and its treatment. Whether nurses had experience of injecting both SSAs, or had a preference for one over the other, was not recorded.
Other Outcome Measures
The Swedish prescription registry was used to source data on the number of packets with ATC codes H01CB03 (octreotide) and H01CB02 (lanreotide) that were dispensed to each patient between 1 January 2014 and 31 December 2014. This information was used to calculate the average frequency of SSA use each year.
Safety and effectiveness were not evaluated in this non-interventional, cross-sectional study. Octreotide and lanreotide were administered and managed within routine medical care. Adverse events were reported according to the spontaneous reporting procedure. Two adverse events related to SSA treatment were reported as free text on the data collection form from the patients. Both patients were treated with lanreotide. One patient reported occasional severe pain during the injection due to administration with a large-gauge syringe. Another patient reported anxiety before the injection: ‘Feeling uncomfortable due to all side effects’. Both adverse events were evaluated as non-serious and no further follow-up was needed.
Statistical Methods
All patient- and nurse-related analyses were based on the complete study populations (ie all patients and nurses, respectively, who completed the consent forms and had data available). The target population for patients was 200, with an expected proportion of 0.50 (50%) patients treated with each SSA, which would allow for a precision of ±7% for the percentage estimates of the various categorical endpoints. However, one center dropped out of the trial, which made sufficient recruitment difficult, so patient enrolment was terminated prematurely. Based on the achieved population of 119 patients, with an expected proportion of 50%, precision was ±9% which was still considered reasonable and exploratory analysis results with a P<0.05 may still be interpreted as indicative results.
Descriptive statistics are presented for all data. For categorical or discrete variables, the absolute and relative (percentage) numbers were based on the non-missing number of observations for each category. There was no imputation for missing continuous data. Exploratory statistical comparisons between the lanreotide and octreotide patient groups were conducted using either a Chi-square test (categorical data) or a Mann–Whitney U-test (continuous data).
Results
Patients
In total, 156 eligible patients were identified at the two participating clinics; of these, 119 (76%) returned a valid questionnaire together with the informed consent form. Overall, 66 (55%) patients were treated with octreotide and 53 (45%) were treated with lanreotide.
Patients’ demographic and clinical characteristics in the overall population and in the lanreotide and octreotide subgroups are summarized in Table 1. Most patients (60%) were male and the mean (SD) age was 63.9 (10.22) years (Table 1). All patients were treated for GEP-NETs. A chromogranin blood test was performed in all patients except one. In most patients (74%), the primary tumor was located in the small intestine. Surgery of the primary tumor had been conducted in 77% of patients. There were some imbalances between the lanreotide and octreotide groups; in the former, the male-to-female ratio was lower, patients were less likely to have a primary tumor in the small intestine, and the ratio of Grade 1 to Grade 2 tumors was higher.
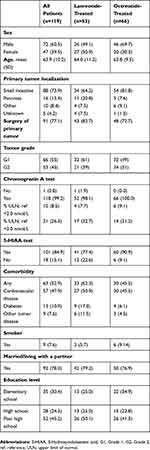 |
Table 1 Baseline Demography And Clinical Characteristics |
Injection intervals of less than 4 weeks were reported more often in octreotide- than lanreotide-treated patients, with 17/65 (26%) patients in the octreotide group receiving their injection every 2 or 3 weeks, compared with 5/53 (9%) patients in the lanreotide group.
Patient Experience
The proportion of patients who had experienced a problem with the most recent SSA injection was significantly higher in the octreotide group (n=12 [18%]) than in the lanreotide group (n=0) (P=0.001, Chi-square).
A total of seven (11%) patients treated with octreotide reported that they felt moderate-to-high anxiety before the injection, compared with one (2%) patient treated with lanreotide (Figure 1).
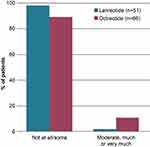 |
Figure 1 Patient anxiety levels before injection with lanreotide and octreotide. Answer to question: To what extent do you feel anxiety before the injection? |
Patient HRQoL
The mean SF-12 physical composite score in the overall population was 38.4. Mean scores were similar in the subgroups evaluated (by SSA treatment, sex and age) (Table 2). The mean SF-12 mental composite score in the overall population was 50.1. Women and younger patients had lower mental composite scores (Table 2).
 |
Table 2 SF-12 Mean Physical And Mental Composite Scores (Levene's Test for Equality of Variances) |
Resource Use
The exploratory analysis of resource use resulted in the following findings: the mean (95% confidence interval) number of doses dispensed per year was lower in the lanreotide group (11.1 [10.04, 12.16] doses) than in the octreotide group (12.6 [11.36, 13.84]). Corresponding treatment intervals were shorter in octreotide-treated patients. Furthermore, the dosing intervals (<4 weeks) indicated that 22 patients were above label dose; 17 patients (26%) in the octreotide group and five patients (10%) in the lanreotide group.
Overall, 100 patients required assistance from healthcare professionals to administer the injection (39 in the lanreotide group and 61 in the octreotide group); in 93% of cases, the treatment visit lasted less than 2 hrs (92% in the lanreotide group and 93% in the octreotide group). Overall, 15 patients in the lanreotide group (28%) administered treatment at home (with injections performed by themselves or their families), compared with two patients (3%) in the octreotide group. Conversely, more octreotide patients (17 [26%]) received treatment in hospital (compared with 6 [11%] in the lanreotide group). The main reason for choosing self-injection in the lanreotide group was that it “saves time”, the reason stated by 7/12 patients (59%).
Patient Knowledge
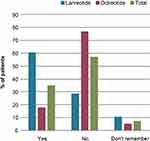
Overall, 43/56 (77%) patients in the octreotide group were not aware of the availability of an SSA that could be self-injected (ie lanreotide) and 11/38 (29%) in the lanreotide group were not aware that their treatment could be self-injected (Figure 2). Of the 43 octreotide-treated patients who did not know about the availability of self-injected SSAs, 7 (16%) said that they would consider starting self-administration (for most [n=6] this response was on the proviso that they received good instructions), while 10 (23%) stated they would be hesitant to start self-injections. Two patients did not state any preference for self-injections.
Overall, 73 patients (61%) felt well informed about their disease and its treatment, 33 (28%) felt moderately informed, 12 (10%) somewhat informed, and 1 (1%) not at all informed.
Nurses’ Experience
Overall, 43/53 nurses responded to the questionnaire, 22 (51%) of whom had administered lanreotide and 21 (49%) of whom had administered octreotide as their most recent injection. Most nurses were experienced in administering SSA treatment. Eight nurses (19%) gave fewer than five injections per year, 22 (51%) gave more than 10, and five (12%) gave more than 20 per year. Most nurses stated they felt confident when giving the most recent injection; for those who administered lanreotide, 18 (82%) felt very confident, and for those who administered octreotide, 18 (85%) felt very confident. Only five nurses (12%) felt that they were well informed about the disease and its treatment. However, they felt much better informed about the practicalities of treatment injection; overall, 19 nurses (86%) who administered lanreotide and 20 who administered octreotide (95%) felt moderately or well informed about the practicalities.
Of the 21 nurses injecting octreotide, two (10%) stated that problems had occurred during the most recent injection. Problems included syringe malfunction and not being able to inject any of the dose, leading to several injection attempts. As a result, the patient had to return to the pharmacy and come back the same or the following day. None of the nurses injecting lanreotide stated they had any problems with their most recent injection.
When the nurses were asked to recall the time taken to administer their most recent SSA injection, the mean (SD) was 15.4 (6.1) minutes and the median (range) was 15 (5–30) minutes. The mean (SD) time for injection was similar between the two SSAs: lanreotide 15.2 (6.0) minutes; octreotide 15.7 (6.4) minutes. Patients self-administering treatment were excluded from this comparison.
Discussion
Lanreotide and octreotide have several practical differences in terms of the formulation provided and the route of administration; the former is provided as a prefilled syringe for deep SC injection, while the latter is supplied as a powder for reconstitution for intramuscular injection. Although patients’ general HRQoL (evaluated using the SF-12) was similar in the two treatment groups, moderate-to-severe anxiety before injections (analyzed independently to HRQoL) was reported more frequently by octreotide-treated patients (11%) than lanreotide-treated patients (2%). This may be partly due to 18% of patients treated with octreotide experiencing problems during their most recent injection, compared with no patients in the lanreotide group. Interestingly, only two nurses (10%) who had most recently administered octreotide reported problems with the injection. Furthermore, the discrepancy between patients’ and nurses’ responses may reflect the fact that nurses in the study were experienced in injecting SSAs and displayed high levels of confidence; their interpretation of a ‘problem’ may therefore differ from patients’ interpretation.
Patients with GEP-NETs treated with SSAs had a considerably lower SF-12 physical score than that expected for a healthy population. These results are consistent with studies conducted in patients with NETs in Norway10 and the USA,11 which showed that these patients had lower physical HRQoL than the general population.12 Effective management of the patient’s disease and optimal symptom control is necessary to maximize HRQoL. In the current study, results for the mental component score of the SF-12 in the overall population were in line with those for a healthy population.12 However, women with GEP-NETs had lower (worse) scores than men, and younger patients (<65 years) had lower scores than those aged ≥65 years. It is reasonable to deduce that daily life is more affected by the disease for younger patients who are still working. In a study from 2017, Singh et al showed that 49% of patients with NETs had to take time off work because of their disease, and a further 27% had to ask their employer to make adjustments to accommodate their condition (eg a flexible working schedule).13 These measures may lead to stress and a negative impact on mental HRQoL. Singh et al also reported that 45% of patients with NETs felt that the disease affected their ability to perform everyday household chores, and 39% felt it affected their ability to care for their family.13
Time spent on each treatment administration may also affect the treatment experience. The results of this exploratory analysis show that more than 90% of patients receiving assistance with injection at a healthcare institution reported that their last injection had consumed up to 2 hrs of their time. It is therefore likely that much time could be saved if more patients were better informed on the possibility of self-injecting, provided that some time is invested initially to educate patients on the procedure.
The exploratory analysis of data from the Swedish pharmacy register indicate that the injection interval was <4 weeks in considerably more of the octreotide- (26%) than lanreotide-treated (10%) patients. This finding was confirmed by self-reported data from the patients in the study. It seems unlikely that physicians would have shortened the injection interval before increasing the dose to the recommended maximum. Thus, these patients were likely receiving higher-than-recommended dosages. The fact that higher doses were administered to more patients in the octreotide group than in the lanreotide group could be explained by the fact that a dose of octreotide 30 mg may not be equivalent to a dose of lanreotide 120 mg, although a literature search found no studies comparing clinical efficacy (in terms of NET symptoms or NET-relevant hormones) of octreotide 30 mg versus lanreotide 120 mg. It is also possible that the use of a higher dose of octreotide was a result of differences in symptoms and clinical status between the two groups of patients. For example, patients in the octreotide group had a higher rate of small intestine tumor and Grade 2 tumors, whereas pancreatic tumors were more common in the lanreotide group. Registry data have shown that patients with small intestinal tumors have a higher prevalence of SSA use compared with those with pancreatic tumors.14
In general, patients on SSA treatment felt well informed about their disease and its treatment. However, 29% of lanreotide-treated patients were not aware that it could be self-injected, and 73% of octreotide-treated patients were unaware of the availability of an SSA product (ie lanreotide) that could be self-injected. Amongst the patients that were previously unaware of self-injecting, almost one-fifth reported that they would consider self-injecting if they received good instructions. It is possible that this number could be higher if information about the possibility of self-injecting SSAs was given in a more structured way, rather than as a single question within a much longer questionnaire. In the lanreotide group, 28% (15/53) of patients self-injected. Of those who were aware that self-administration was possible, the proportion of patients who had actively chosen to self-inject was 38% (15/42). These results are consistent with the findings of Johanson et al, who found that 35% of lanreotide-treated patients who were offered self/partner injection preferred this to administration by a healthcare professional.7 Allowing patients to take part in decisions relating to treatment may increase satisfaction with treatment, and the knowledge that self-injection (or injection by a partner) is possible may be associated with feelings of freedom from the disease (eg allowing more flexible planning). Self-injections may also relieve some of the burden patients feel about taking time off work for treatment. However, it is important to note that self-injection at home is not standard practice in all countries, particularly in the USA where some insurance companies do not allow it.
The current study also assessed nurses’ experience of administering SSAs and their knowledge of the disease and its treatment. The nurses were relatively experienced, with more than half having administered 10 or more injections per year. Most nurses reported feeling confident about administering lanreotide and octreotide, and well informed about the practicalities of their administration; however, only five nurses (12%) felt well informed about the disease and treatment, indicating that there is a lack of education about this relatively rare disease. Addressing this knowledge gap could lead to an improvement in patients’ overall treatment experience.
One limitation of this cross-sectional study is the relatively small number of participants, recruited from only two centers in Sweden. Furthermore, the planned sample size was not reached, so these findings should be interpreted with caution. The broader applicability of the results is therefore unclear. In addition, many of the data were self-reported, relying on accuracy of participant recall, and absolute doses were not recorded. Strengths of the study are the high response rate and inclusion of real-world data from the Swedish prescription registry.
Conclusions
In this study, patients with GEP-NETs were well-informed about their disease and, in comparison with the general population, had a relatively good mental HRQoL. Physical HRQoL was, however, considerably lower than in the general population. The choice of SSA (lanreotide versus octreotide) did not appear to affect patients’ general HRQoL, as evaluated using the generic SF-12 questionnaire, but those treated with lanreotide reported fewer injection problems and experienced less anxiety before injections than those treated with octreotide. Some patients treated with octreotide were not aware of the availability of another SSA with a different route of administration, and the flexibility of self-injection. Although the patient response rate was high, the study did not recruit the target number of patients, hence the statistical precision was somewhat reduced and results should be interpreted with caution.
Abbreviations
eCRF, electronic case report form; GEP-NET, gastroenteropancreatic neuroendocrine tumor; HRQoL, health-related quality of life; LAR, long-acting release; NET, neuroendocrine tumor; SC, subcutaneous; SF-12, short form-12; SmPC, summary of product characteristics; SSA, somatostatin analogue; STREET, Somatostatin Treatment Experience Trial.
Medical Writing Support
The authors thank Priya Joshi, MSc, of Watermeadow Medical an Ashfield Company, for providing medical writing and editorial support, which was funded by Ipsen in accordance with Good Publication Practice guidelines.
Data Sharing Statement
Where patient data can be anonymized, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to [email protected] and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.
Acknowledgments
The authors thank all patients involved in the study, as well as their caregivers, care team, investigators and research staff in participating institutions. We would also like to thank Meductus AB for input into the design of the study and eCRf, and handling of data, and JK Biostatistics AB for design of the eCRF and statistical analysis. This study was sponsored by Ipsen.
Author Contributions
TS: Designed the study; was involved in data review and interpretation; drafted the manuscript following input from all the authors. GK: Conceived the study. PM: Designed the study; was involved in data review and interpretation. MA: Conceived and designed the study; was involved in data review and interpretation; drafted the manuscript following input from all the authors. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
TS and PM are employees of Ipsen. MA reports grants, personal fees, non-financial support from IPSEN, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi:10.1200/JCO.2007.15.4377
2. Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2018. doi:10.3322/caac.21493
3. Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. doi:10.1056/NEJMoa1316158
4. Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol. 2009;27(28):4656–4663. doi:10.1200/JCO.2009.22.8510
5. Novartis. Sandostatin® LAR Summary of Product Characteristics. Available from: https://www.ema.europa.eu/documents/referral/sandostatin-lar-article-30-referral-annex-iii_en.pdf. 2018.
6. Ipsen Ltd. Somatuline® Autogel® Summary of Product Characteristics. Available from: https://www.medicines.org.uk/emc/product/8257/smpc. 2018.
7. Johanson V, Wilson B, Abrahamsson A, et al. Randomized crossover study in patients with neuroendocrine tumors to assess patient preference for lanreotide Autogel® given by either self/partner or a health care professional. Patient Prefer Adherence. 2012;6:703–710. doi:10.2147/PPA.S34337
8. Adelman DT, Burgess A, Davies PR. Evaluation of long-acting somatostatin analog injection devices by nurses: a quantitative study. Med Devices (Auckl). 2012;5:103–109. doi:10.2147/MDER.S37831
9. Ware J, Kosinski M, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Boston, MA, USA: The Health Institute, New England Medical Center; 1998.
10. Haugland T, Vatn MH, Veenstra M, Wahl AK, Natvig GK. Health related quality of life in patients with neuroendocrine tumors compared with the general Norwegian population. Qual Life Res. 2009;18(6):719–726. doi:10.1007/s11136-009-9487-x
11. Beaumont JL, Cella D, Phan AT, Choi S, Liu Z, Yao JC. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas. 2012;41(3):461–466. doi:10.1097/MPA.0b013e3182328045
12. Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171–1178. doi:10.1016/s0895-4356(98)00109-7
13. Singh S, Granberg D, Wolin E, et al. Patient-reported burden of a neuroendocrine tumor (NET) diagnosis: results from the first global survey of patients with NETs. J Glob Oncol. 2017;3(1):43–53. doi:10.1200/JGO.2015.002980
14. Lesén E, Granfeldt D, Houchard A. et al. Cost‐of‐illness of metastatic gastroenteropancreatic neuroendocrine tumours in Sweden—a population‐based register-linkage study. Eur J Cancer Care. 2019. e12983. doi:10.1111/ecc.12983
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.