Back to Journals » Clinical Ophthalmology » Volume 8
Patient adherence and persistence with topical ocular hypotensive therapy in real-world practice: a comparison of bimatoprost 0.01% and travoprost Z 0.004% ophthalmic solutions
Authors Campbell J, Schwartz G, LaBounty B, Kowalski J, Patel V
Received 5 June 2013
Accepted for publication 13 October 2013
Published 14 May 2014 Volume 2014:8 Pages 927—935
DOI https://doi.org/10.2147/OPTH.S49467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Joanna H Campbell,1 Gail F Schwartz,2 Britni LaBounty,3 Jonathan W Kowalski,1 Vaishali D Patel1
1Allergan, Inc., Irvine, CA, USA; 2Greater Baltimore Medical Center and Wilmer Eye Institute, Johns Hopkins University, Baltimore, MD, USA; 3Principled Strategies, Inc., Encinitas, CA, USA
Background: Effective control of intraocular pressure is predicated upon patient compliance with pharmacotherapy. We compared patient adherence and persistence with two new ocular hypotensive formulations, using real-world utilization data.
Methods: This observational cohort study employed pharmacy claims data from the Source® Lx (Wolters Kluwer Pharma Solutions) database. Patients with an initial (index) prescription for topical bimatoprost 0.01% or travoprost Z (April to June 2011) and no claim for ophthalmic prostaglandin or prostamide analogs within the previous 18 months were identified. Treatment adherence was expressed as proportion of days covered with study medication during the first 365 days after the index prescription. Treatment persistence with study medication was assessed over the first 12 months using Kaplan–Meier survival analyses, allowing a maximum 30-day gap for prescription refill. Treatment status was determined monthly over this period.
Results: A total of 12,985 patients were assessed for treatment adherence, and 10,470 for treatment persistence. Adherence was better with bimatoprost 0.01% than with travoprost Z (mean proportion of days covered 0.540 versus [vs] 0.486, P<0.001), and more patients showed high adherence (proportion of days covered >0.80) with bimatoprost 0.01% than travoprost Z (29.1% vs 22.3%, P<0.001). Continuous 12-month persistence was higher with bimatoprost 0.01% than with travoprost Z (29.5% vs 24.2%, P<0.001). At month 12, more patients were on treatment with bimatoprost 0.01% than travoprost Z (48.8% vs 45.7%, P<0.01). Similar findings were demonstrated in cohorts of ocular hypotensive treatment-naïve patients, branded latanoprost switchers, and older patients (age ≥65 years), and after inclusion of patient characteristics as covariates.
Conclusion: For patients with glaucoma or ocular hypertension, bimatoprost 0.01% offers compliance advantages over travoprost Z.
Keywords: ocular hypotensive, bimatoprost, travoprost, treatment compliance
Introduction
Glaucoma, a chronic, progressive optic neuropathy, is the second-leading cause (after cataract) of blindness worldwide,1,2 affecting over 60 million adults.2 In addition to advanced age and genetic predisposition, elevated intraocular pressure (IOP) is a well-established risk factor for primary open-angle glaucoma (POAG), the predominant form of the disease.3,4 Treatment focuses on lowering IOP, because effective IOP control can delay or halt progression of ocular hypertension to glaucoma5 and progression of glaucomatous damage.6–10 Topical prostaglandin/prostamide analogs (PGAs), such as latanoprost, travoprost, and bimatoprost, offer improved efficacy compared with older classes of ocular hypotensives, such as carbonic anhydrase inhibitors, α2 adrenergic agonists, and β-blockers,11–13 and are considered the first-line choice for medical management of POAG and ocular hypertension along with β-blockers.14–16 These products have fewer contraindications, and thus may be safer than other agents, (such as β-blockers in patients at risk of cardiopulmonary side effects), and require fewer instillations, which is an advantage for the patient.16,17
In practice, to achieve effective IOP control, patients need to remain closely compliant with ocular hypotensive therapy. For chronic and typically asymptomatic conditions, such as ocular hypertension and glaucoma, treatment adherence (consistent daily use of medication in accordance with dosage recommendations)18 and persistence (continued use of medication over time)17 pose a particular challenge.19 Although persistence with topical PGAs is superior to that achieved with carbonic anhydrase inhibitors, α2 adrenergic agonists, and β-blockers,20–24 it is nevertheless suboptimal, and <50% of patients who start therapy are likely to remain on treatment 1 year later.23,25,26 Patient persistence with topical PGAs compares unfavorably with that of many chronic medications, including statins, bisphosphonates, and oral hypoglycemics.27
Several head-to-head randomized controlled trials, as well as a recent meta-analysis, have demonstrated that the original formulation of bimatoprost (0.03%) has superior IOP-lowering efficacy to travoprost.28–30 However, a number of real-world utilization studies indicate that patient adherence and persistence may be lower with this formulation of bimatoprost than with travoprost, presumably because of the higher incidence of conjunctival hyperemia associated with bimatoprost 0.03%.30,31 Bimatoprost 0.01% ophthalmic solution (Lumigan® 0.01%; Allergan, Inc., Irvine, CA, USA) is a new formulation of bimatoprost that offers similar IOP-lowering activity to bimatoprost 0.03% combined with a lower rate of conjunctival hyperemia.32 A recent head-to-head randomized controlled trial comparing the tolerability of topical PGAs found that conjunctival hyperemia rates were comparable for bimatoprost 0.01% and travoprost Z,33 a new formulation of travoprost 0.004% with SofZia® preservative (Travatan Z®; Alcon Laboratories, Fort Worth, TX, USA). The objective of this pharmacy claims analysis was to compare patient adherence and persistence with these two new topical formulations, bimatoprost 0.01% and travoprost Z, using real-world pharmacy claims data.
Methods
Study design and patient selection
We performed a retrospective observational cohort study using pharmacy claims data derived from the Source® Lx (Wolters Kluwer Pharma Solutions) database. This integrated, patient-level data source covers physician practices, hospitals, and pharmacies, and includes outpatient and hospital medical claims and pharmacy prescription claims. The Source Lx database represents the health care services of more than 115 million US patients with commercial, Medicare Part D, and Medicaid insurance coverage. All patient information collected from the database was deidentified in compliance with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act.
The Source Lx database was screened to identify patients who had a prescription for bimatoprost 0.01% or travoprost Z dispensed between April and June 2011. The date of first supply of study medication during this period was defined as the “index” date. For study eligibility, patients were required to have ≥18 months’ continuous database enrollment before the index date. Patients included in the adherence analysis were also required to have ≥12 months’ continuous database enrollment after the index date. Patients included in the persistence analysis had to be continuously enrolled in the database until discontinuation of study medication or until completion of 12 months’ postindex follow-up (whichever occurred earlier). Patients were excluded from the study if they had a prescription for any ophthalmic PGA dispensed during the 18 months preceding the index date. Concomitant prescription of an ocular hypotensive other than the study medication was permitted during the postindex follow-up period. Information collected from the pharmacy claims records included patient demographics (age, sex, and payer status), prior ocular hypotensive treatment, the date of supply of study medication, the number of bottles supplied, and the bottle size.
Study outcomes
Treatment adherence
As a measure of treatment adherence, we estimated the number of days in the first therapy year from prescription fills for which supply of study medication was available (days covered). Accordingly, the proportion of days covered (PDC) with study medication is:
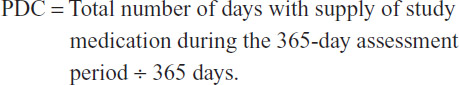
Claims-based estimates of days’ supply for nondiscrete drug formulations such as eyedrops are known to be inaccurate,34 and for this reason we calculated the number of days’ drug supply provided by each prescription fill directly, using data from a drop-count study that measured the number of drops per bottle. This drop-count study determined the actual volume of drug solution contained in commercially available bottles of bimatoprost 0.01% (2.5, 5.0, and 7.5 mL sizes) and travoprost Z (2.5 and 5.0 mL sizes) and the number of drops dispensed from each bottle. For both products, four bottles of each available size were tested to quantify the average drug volume and average number of dispensed drops. Based on these data, and assuming that the study medications were administered according to the product label and that all doses were applied bilaterally, a more accurate estimate of the days’ supply represented by each bottle size was obtained:
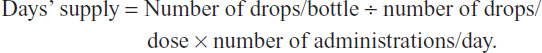
The results indicated that each 2.5, 5.0, and 7.5 mL bimatoprost 0.01% bottle provided 46.8, 91.4, and 131.3 days’ supply of medication, whereas each 2.5 and 5.0 mL travoprost Z bottle provided 45.9 and 84.3 days’ supply, respectively. In the event that a prescription was refilled before the previous days’ supply was exhausted, the fill date of the subsequent prescription was adjusted to coincide with the last day covered by supply from the previous prescription. Mean and median PDC values were calculated for each study medication over the 365-day assessment period, as well as the frequency distribution of patients across the following PDC categories: 1) PDC ≤0.20 (low adherence), 2) 0.20< PDC ≤0.80 (moderate adherence), and 3) PDC >0.80 (high adherence).
Treatment persistence
Persistence with study medication was assessed over a period of up to 12 months from the index date, using a gap-analysis method whereby patients were permitted a 30-day grace period to obtain their next prescription refill. The use of a grace period for prescription refill prevents minor gaps in prescription coverage counting as treatment discontinuations, and is standard in these types of analyses.35 Patients were regarded as remaining on treatment if they refilled their prescription ≤30 days after exhaustion of their existing drug supply, and deemed to have discontinued treatment if they did not refill their prescription within this time frame. Accordingly, the date of treatment discontinuation was set at 30 days after the date of exhaustion of the drug supply from the latest prescription fill. Time to treatment failure (ie, discontinuation of medication) for at-risk patients in the two study groups was assessed using Kaplan–Meier survival analysis.
Treatment status
In order to compare the treatment status of the two study groups over the 12-month postindex period, patients were classified at the end of each month as either 1) “treatment continuers” (ie, with drug supply in the previous month and a prescription refill or carryover drug supply for the current month), 2) “treatment discontinuers” (no prescription fill or carryover drug supply in the current month), or 3) “treatment restarters” (prescription fill in the current month but no drug supply in the previous month). Patients continuing treatment from the previous month or restarting treatment in the current month (categories 1 and 3 combined) were defined as being “on treatment.”36
Base-case and sensitivity analyses
The base-case analysis was conducted using the full study population, which was naive to topical PGA therapy. Covariate-adjusted sensitivity analyses were performed to control for patient characteristics that were unbalanced between treatment cohorts at baseline, namely treatment-naive status and insurance type (commercial, Medicare, Medicaid, or other). Additional sensitivity analyses were conducted to assess the robustness of the compliance findings in different patient cohorts: 1) the subpopulation of patients who had not received any ocular hypotensive therapy during the preindex period (“treatment-naive patients”), 2) the subpopulation of patients ≥65 years of age, and 3) a separate sample of patients who had switched from branded latanoprost (Xalatan®; Pfizer, New York, NY, USA) therapy. The base-case analysis allowed a 30-day grace period for prescription refill; this was also varied in sensitivity analyses to 15 and 60 days.
Statistical analysis
Statistical analyses were conducted using R software version 2.12.2 (Vienna University of Economics and Business; http://www.R-project.org). Intergroup comparisons of mean and median PDC values were performed using Student’s t-test and Wilcoxon’s signed-rank test, respectively. Covariate-adjusted analysis of mean PDC values was performed using a linear regression model (lm procedure). Intergroup comparisons of the proportions of patients with low, moderate, and high treatment adherence, and proportions of patients on treatment (continuers plus restarters) were performed using the χ2 test. Covariate-adjusted analysis of adherence and treatment status was performed using logistic regression models (glm [family = binomial {logit}] procedure). Kaplan–Meier survival analysis was used to generate treatment-persistence curves, and intergroup comparisons were performed using the log-rank test for homogeneity. Covariate-adjusted analysis of persistence was performed using Cox proportional hazard models. A P-value≤0.05 was considered statistically significant.
Results
A total of 12,985 patients met the study inclusion criteria and were assessed for treatment persistence (5,099 on bimatoprost 0.01%, 7,886 on travoprost Z) in the base-case analysis. Of these, 10,470 patients who had continuous 12 months’ post-index follow-up were additionally assessed for treatment adherence (4,131 on bimatoprost 0.01%, 6,339 on travoprost Z). Baseline demographic and clinical characteristics of the persistence and adherence study populations are summarized in Table 1. Sensitivity analyses were performed in subgroups of treatment-naive patients (9,585 and 7,461 were assessed for persistence and adherence, respectively), in patients ≥65 years of age (8,989 and 7,265 were assessed for persistence and adherence, respectively), and in a sample of branded-latanoprost switchers (4,012 and 3,334 were assessed for persistence and adherence, respectively).
In both the persistence and adherence studies (base-case analysis), the two treatment groups shared similar age and sex distributions. However, in comparison with the travoprost Z group, the bimatoprost 0.01% group contained a higher proportion of commercially insured patients (persistence study, 56.5% versus [vs] 52.1%, P<0.001; adherence study, 55.0% vs 51.2%, P<0.001) and treatment-experienced patients (persistence study, 27.5% vs 25.3%, P<0.01; adherence study, 30.1% vs 27.9%, P<0.05). Similar proportions of patients in the bimatoprost 0.01% and travoprost Z groups received adjunctive ocular medication either at baseline or during postindex follow-up (persistence study, 29.5% vs 29.2%; adherence study, 28.4% vs 28.2%).
Base-case analysis
Patient adherence with study medication over the complete 12-month period after the index prescription was significantly better with bimatoprost 0.01% than with travoprost Z (mean PDC 0.540 vs 0.486, P<0.001; median PDC 0.512 vs 0.460, P<0.001). During this period, a significantly greater proportion of patients in the bimatoprost 0.01% group exhibited high adherence (PDC >0.80) compared with those in the travoprost Z group (29.1% vs 22.3%, P<0.001). Conversely, significantly more patients in the travoprost Z group showed moderate adherence (0.20< PDC ≤0.80) compared with those in the bimatoprost 0.01% group (55.5% vs 50.2%, P<0.001). Similar proportions of patients showed low adherence (PDC ≤0.20) with bimatoprost 0.01% and travoprost Z (20.7% vs 22.2%, not significant) (Table 2).
  | Table 2 Patient adherence with study medication over the 12-month postindex period |
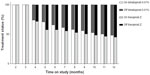
Kaplan–Meier survival curves, based on a 30-day grace period for prescription refill, revealed differences in treatment persistence in favor of bimatoprost 0.01% over travoprost Z (Figure 1). A significantly greater proportion of patients starting on bimatoprost 0.01% remained on continuous treatment for the full 12 months compared with those starting on travoprost Z (29.5% vs 24.2%, P<0.001) (Table 3).
  | Table 3 Proportion of at-risk patients maintaining continuous 12-month persistence with study medication |
A cross-sectional analysis of patients’ treatment status each month, allowing a 30-day grace period for prescription refill, indicated that the proportion of patients on index therapy (treatment continuers plus restarters) was consistently higher with bimatoprost 0.01% compared with travoprost Z from month 4 onward (Figure 2). At 12 months postindex, a significantly greater proportion of patients was on treatment (continuing or restarting treatment) with bimatoprost 0.01% than with travoprost Z (48.8% vs 45.7%, P<0.01) (Table 4).
  | Table 4 Proportion of at-risk patients on study medication at month 12 after the index prescription claim |
Sensitivity analyses
Covariate-adjustment for treatment-naive status and insurance type at index fill had minimal impact on the unadjusted estimates of the magnitude or statistical significance of treatment adherence and persistence with bimatoprost 0.01% provided by the various regression models (Table 5). For the cohorts of treatment-naive patients, patients ≥65 years of age, and patients switching from latanoprost, levels of adherence and 12-month persistence with study medication (based on a 30-day refill gap) closely mirrored those observed in the base-case analysis of the full study population (Tables 2–4). For all three patient cohorts, treatment adherence and 12-month persistence rates, based on a 30-day refill gap, were significantly higher with bimatoprost 0.01% than with travoprost Z (Tables 2–4).
As anticipated, estimates of treatment persistence with study medication fell when the allowed refill gap was reduced to 15 days and increased when it was extended to 60 days (Tables 3 and 4). These variations in refill gap affected persistence rates similarly across the cohorts of treatment-naive patients, patients ≥65 years of age and patients switching from latanoprost, and the full study population (Tables 3 and 4). Nevertheless, the advantage noted with bimatoprost 0.01% versus travoprost Z in terms of continuous 12-month treatment persistence and overall treatment persistence (as indicated by “on treatment” status) at month 12, based on a 30-day refill gap, was consistently replicated across the three patient cohorts and the full study population when the refill gap was reduced to 15 days or extended to 60 days (Tables 3 and 4).
Discussion
The results of this study, which is the first to compare patient adherence and persistence with bimatoprost 0.01% and travoprost Z, lend support to previous reports of suboptimal compliance with ocular hypotensive medications as a class.23,25,26,37 However, the results indicate that within this class of drugs, there are possibly clinically relevant differences in persistence and adherence between individual PGAs. Thus, in this large population of patients new to PGA therapy, more patients achieved uninterrupted 12-month persistence with bimatoprost 0.01% than with travoprost Z ophthalmic solutions (29.5% vs 24.2% of patients). Results from the restart analysis, which took into account the substantial number of patients who discontinued and subsequently restarted study medication, demonstrated a similar persistence benefit, with more patients “on treatment” with bimatoprost 0.01% than with travoprost Z 12 months after initiating therapy (48.8% vs 45.7%). Similarly, the PDC covered with study medication over the first therapy year was significantly higher with bimatoprost 0.01% than with travoprost Z (mean PDC 0.54 vs 0.49). Findings from the sensitivity analyses reinforce those from base-case analysis, and suggest that the persistence advantage with bimatoprost 0.01% relative to travoprost Z is real, since it was consistently demonstrated after covariate adjustment for unbalanced factors across treatment cohorts, and across a range of grace periods (15–60 days), as well as in separate cohorts of elderly patients, treatment-naive patients, and patients switching from branded latanoprost.
Previous compliance studies have compared older formulations of bimatoprost to older formulations of travoprost, and have provided mixed results. A retrospective pharmacy claims analysis (during the period 2001–2004) of patients (n=6271) naive to ocular hypotensive treatment who were receiving topical PGAs (bimatoprost, travoprost, and latanoprost) reported continuous 12-month prescription-refill rates of ≤10% for the individual agents. Rates of 58% (travoprost) to 68% (latanoprost) after 12 months were reported for “on treatment” (combined treatment continuer plus restarter).26 Another retrospective pharmacy claims analysis (during the period 2004–2005) of treatment-naive patients (n=7873) who received topical PGAs (bimatoprost, travoprost, and latanoprost) reported PDC values of 0.64–0.66 over the first therapy year, based on imputed days’ supply from the claims data.37 None of these studies included as a comparator agent bimatoprost 0.01%, which is better tolerated than the older formulation, bimatoprost 0.03%,31 and therefore has the potential for superior adherence and persistence.
Several factors, including treatment efficacy, the frequency and nature of adverse ocular effects (whether drug- or preservative-related), and the cost of medication, may impact patient willingness to continue ocular hypotensive therapy.26 However, sensitivity analyses that included type of insurance – a proxy for treatment affordability – as a covariate did not change the tenor of the study findings. The most common adverse effect of topical PGAs (hyperemia) is recognized as a risk factor for interruption of topical PGA therapy.26,38 The contribution of preservatives to ocular adverse effects, especially benzalkonium chloride (BAK), the most widely used preservative in ocular hypotensive formulations (including bimatoprost), remains in question. Although in vitro and in vivo animal studies have shown dose-dependent, BAK-induced epithelial cellular damage,39–41 the low BAK concentrations in ophthalmic solutions are thought to be unlikely to cause clinically significant adverse corneal effects.42,43 Likewise, whether SofZia offers any tolerability advantage over BAK is uncertain. While in vitro studies in corneal epithelial cells demonstrate that SofZia has lower toxicity than BAK,44 clinical studies suggest equivalent ocular tolerability between travoprost formulations with and without BAK.45
Strengths of the present study include its use of a data source (Source Lx) appreciably larger than that used in previous investigations of compliance with topical PGA therapy.46–48 The large study population, its broad demographic base, the nonselective patient-eligibility criteria, and the real-world setting described by the claims data allow the study findings to be readily generalized. The use of drop-count data controlled for variations in volume of dispensed medication and provided a more accurate estimate of days’ supply than that available from prescription claims alone.
As a retrospective observational study, this analysis is subject to several limitations. Administrative claims data are often incomplete, and subject to possible coding errors. The clinical information provided in an administrative claims database is limited, and undocumented factors, such as treatment motivation, glaucoma diagnosis, disease severity, and physical or mental frailty, may potentially influence patient compliance. Distribution of free drug samples to patients, resulting in potential underestimation of adherence,21 was not captured by the database. In addition to these unknown putative confounders, the study made no adjustment for imbalances between the two treatment groups in known baseline characteristics, such as age, sex, and insurance type. Allowance of adjunctive glaucoma medication during the follow-up period may have adversely influenced patient compliance with study medication. While our use of drop-count data provided some measure of control for variations in bottle size and medication volume, it should be noted that the drop-count technique may not simulate patients’ use (and wastage) of eyedrops in the real-world setting. Furthermore, prescription refill and drug supply does not necessarily equate to treatment adherence, since a patient may use the medication in his/her possession inconsistently or inappropriately, resulting in inadequate IOP control.49 Finally, the claims data provide no information on possible reasons for the compliance advantage observed with bimatoprost 0.01%.
In conclusion, patient compliance with topical PGA therapy is suboptimal. The results of this study indicate that bimatoprost 0.01% offers advantages in adherence and persistence over travoprost Z. For the prescriber, this is a potentially important consideration in selecting appropriate ocular hypotensive medication.
Acknowledgments
Editorial support, including medical writing assistance with development of the first draft of the manuscript, revision of the manuscript based on author feedback, and styling the manuscript for journal submission, was provided by Andrew Fitton, PhD, and Diann Glickman, PharmD, of Evidence Scientific Solutions, and was funded by Allergan, Inc., Irvine, CA, USA. The sponsor participated in the study design, data analysis and interpretation, and preparation and review of the manuscript. Drop-count study data for estimation of days’ supply of study medication were kindly provided by Richard Fiscella, Allergan, Inc., and Sami Labib and Brian Maynard, Department of Pharmacy Practice, University of Illinois, Chicago, IL, USA.
Disclosure
This study was sponsored by Allergan, Inc., Irvine, CA, USA. JHC, JWK, and VDP are employees of Allergan, Inc. GFS has acted as a clinical expert consultant to Allergan, Inc., for this project, but has not received any compensation. GFS does receive ongoing research support from Allergan, Inc., on other projects, as well as from Tissue Banks International. BL is an employee of Principled Strategies, Inc.
References
Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. | |
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. | |
Leske MC, Connell AM, Wu SY, Hyman LG, Schachat AP. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol. 1995;113(7):918–924. | |
Le A, Mukesh BN, McCarty CA, Taylor HR. Risk factors associated with the incidence of open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci. 2003;44(9):3783–3789. | |
Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713; discussion 829–830. | |
Mao LK, Stewart WC, Shields MB. Correlation between intraocular pressure control and progressive glaucomatous damage in primary open-angle glaucoma. Am J Ophthalmol. 1991;111(1):51–55. | |
[No authors listed]. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. | |
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120(10):1268–1279. | |
Parrish RK 2nd, Feuer WJ, Schiffman JC, Lichter PR, Musch DC. Five-year follow-up optic disc findings of the Collaborative Initial Glaucoma Treatment Study. Am J Ophthalmol. 2009;147(4):717–724. e1. | |
Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118(9):1766–1773. | |
Hedman K, Alm A. A pooled-data analysis of three randomized, double-masked, six-month clinical studies comparing the intraocular pressure reducing effect of latanoprost and timolol. Eur J Ophthalmol. 2000;10(2):95–104. | |
van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology. 2005;112(7):1177–1185. | |
Stewart WC, Konstas AG, Nelson LA, Kruft B. Meta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicines. Ophthalmology. 2008;115(7):1117–1122. e1. | |
Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline Expert Committee, Canadian Ophthalmological Society. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of glaucoma in the adult eye. Can J Ophthalmol. 2009;44(Suppl 1):S7–S93. | |
European Glaucoma Society. Terminology and Guidelines for Glaucoma. 3rd ed. Savona, Italy: DOGMA; 2008. | |
American Academy of Ophthalmology Glaucoma Panel, Hoskins Center for Quality Eye Care. Preferred practice pattern guidelines: primary open-angle glaucoma PPP – October 2010. Available from: http://one.aao.org/preferred-practice-pattern/primary-openangle-glaucoma-ppp--october-2010. Accessed October 15, 2013. | |
Zhang WY, Po AL, Dua HS, Azuara-Blanco A. Meta-analysis of randomised controlled trials comparing latanoprost with timolol in the treatment of patients with open angle glaucoma or ocular hypertension. Br J Ophthalmol. 2001;85(8):983–990. | |
Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. | |
Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113(3):431–436. | |
Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–606. | |
Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci. 2007;48(11):5052–5057. | |
Reardon G, Schwartz GF, Mozaffari E. Patient persistency with pharmacotherapy in the management of glaucoma. Eur J Ophthalmol. 2003;13(Suppl 4):S44–S52. | |
Rait JL, Adena MA. Persistency rates for prostaglandin and other hypotensive eyedrops: population-based study using pharmacy claims data. Clin Experiment Ophthalmol. 2007;35(7):602–611. | |
Dasgupta S, Oates V, Bookhart BK, Vaziri B, Schwartz GF, Mozaffari E. Population-based persistency rates for topical glaucoma medications measured with pharmacy claims data. Am J Manag Care. 2002; 8(Suppl 10):S255–S261. | |
Reardon G, Kotak S, Schwartz GF. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adherence. 2011;5:441–463. | |
Zimmerman TJ, Hahn SR, Gelb L, Tan H, Kim EE. The impact of ocular adverse effects in patients treated with topical prostaglandin analogs: changes in prescription patterns and patient persistence. J Ocul Pharmacol Ther. 2009;25(2):145–152. | |
Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728–740. | |
Cantor LB, Hoop J, Morgan L, Wudunn D, Catoira Y. Intraocular pressure-lowering efficacy of bimatoprost 0.03% and travoprost 0.004% in patients with glaucoma or ocular hypertension. Br J Ophthalmol. 2006;90(11):1370–1373. | |
Kammer JA, Katzman B, Ackerman SL, Hollander DA. Efficacy and tolerability of bimatoprost versus travoprost in patients previously on latanoprost: a 3-month, randomised, masked-evaluator, multicentre study. Br J Ophthalmol. 2010;94(1):74–79. | |
Aptel F, Denis P. Balancing efficacy and tolerability of prostaglandin analogues and prostaglandin-timolol fixed combinations in primary open-angle glaucoma. Curr Med Res Opin. 2011;27(10):1949–1958. | |
Arias A, Schargel K, Ussa F, Canut MI, Robles AY, Sánchez BM. Patient persistence with first-line antiglaucomatous monotherapy. Clin Ophthalmol. 2010;4:261–267. | |
Katz LJ, Cohen JS, Batoosingh AL, Felix C, Shu V, Schiffman RM. Twelve-month, randomized, controlled trial of bimatoprost 0.01%, 0.0125%, and 0.03% in patients with glaucoma or ocular hypertension. Am J Ophthalmol. 2010;149(4):661–671. e1. | |
Crichton AC, Vold S, Williams JM, Hollander DA. Ocular surface tolerability of prostaglandin analogs and prostamides in patients with glaucoma or ocular hypertension. Adv Ther. 2013;30(3):260–270. | |
Grymonpre RE, Didur CD, Montgomery PR, Sitar DS. Pill count, self-report, and pharmacy claims data to measure medication adherence in the elderly. Ann Pharmacother. 1998;32(7–8):749–754. | |
Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449–457. | |
Schwartz GF, Platt R, Reardon G, Mychaskiw MA. Accounting for restart rates in evaluating persistence with ocular hypotensives. Ophthalmology. 2007;114(4):648–652. | |
Reardon G, Schwartz GF, Kotak S. Persistence on prostaglandin ocular hypotensive therapy: an assessment using medication possession and days covered on therapy. BMC Ophthalmol. 2010;10:5. | |
Wirta D, Vandenburgh AM, Weng E, Whitcup SM, Kurstjens S, Beddingfield FC 3rd. Long-term safety evaluation of bimatoprost ophthalmic solution 0.03%: a pooled analysis of six double-masked, randomized, active-controlled clinical trials. Clin Ophthalmol. 2011;5:759–765. | |
Epstein SP, Chen D, Asbell PA. Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009;25(5):415–424. | |
Epstein SP, Ahdoot M, Marcus E, Asbell PA. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009;25(2):113–119. | |
Ichijima H, Petroll WM, Jester JV, Cavanagh HD. Confocal microscopic studies of living rabbit cornea treated with benzalkonium chloride. Cornea. 1992;11(3):221–225. | |
Khoh-Reiter S, Jessen BA. Evaluation of the cytotoxic effects of ophthalmic solutions containing benzalkonium chloride on corneal epithelium using an organotypic 3-D model. BMC Ophthalmol. 2009;9:5. | |
Lass JH, Eriksson GL, Osterling L, Simpson CV. Comparison of the corneal effects of latanoprost, fixed combination latanoprost-timolol, and timolol: a double-masked, randomized, one-year study. Ophthalmology. 2001;108(2):264–271. | |
Kahook MY, Ammar DA. In vitro toxicity of topical ocular prostaglandin analogs and preservatives on corneal epithelial cells. J Ocul Pharmacol Ther. 2010;26(3):259–263. | |
Lewis RA, Katz GJ, Weiss MJ, et al. Travoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacy. J Glaucoma. 2007;16(1):98–103. | |
Reardon G, Schwartz GF, Mozaffari E. Patient persistency with ocular prostaglandin therapy: a population-based, retrospective study. Clin Ther. 2003;25(4):1172–1185. | |
Reardon G, Schwartz GF, Mozaffari E. Patient persistency with topical ocular hypotensive therapy in a managed care population. Am J Ophthalmol. 2004;137(Suppl 1):S3–S12. | |
Wilensky J, Fiscella RG, Carlson AM, Morris LS, Walt J. Measurement of persistence and adherence to regimens of IOP-lowering glaucoma medications using pharmacy claims data. Am J Ophthalmol. 2006; 141(Suppl 1):S28–S33. | |
Stone JL, Robin AL, Novack GD, Covert DW, Cagle GD. An objective evaluation of eyedrop instillation in patients with glaucoma. Arch Ophthalmol. 2009;127(6):732–736. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.