Back to Journals » OncoTargets and Therapy » Volume 11
Pathological complete response induced by neoadjuvant treatment using BRAF and MEK inhibitors in a patient with unresectable BRAF V600E-mutant malignant melanoma of the gallbladder
Authors Yu Z , Quiroz E, Shen Y, Jaiyesimi IA
Received 13 June 2018
Accepted for publication 16 August 2018
Published 4 December 2018 Volume 2018:11 Pages 8723—8728
DOI https://doi.org/10.2147/OTT.S177111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Zhou Yu,1 Elisa Quiroz,2 Yulei Shen,3 Ishmael A Jaiyesimi1
1Department of Hematology and Oncology, William Beaumont Hospital, Oakland University – William Beaumont School of Medicine, Royal Oak, MI, USA; 2Department of Internal Medicine, William Beaumont Hospital, Oakland University – William Beaumont School of Medicine, Royal Oak, MI, USA; 3Department of Pathology and Laboratory Medicine, William Beaumont Hospital, Oakland University – William Beaumont School of Medicine, Royal Oak, MI, USA
Abstract: Historically, patients with locally advanced or metastatic melanoma have an extremely poor prognosis. In recent years, major breakthroughs in cutaneous melanoma treatment have led to remarkable improvements in patient outcomes. However, there are limited published data on the efficacy of these novel therapies in the treatment of mucosal melanoma due to rarity of the disease. We report a case of successful neoadjuvant targeted therapy with BRAF and MEK inhibitors followed by radical surgical excision in a patient with advanced malignant melanoma of the gallbladder.
Keywords: melanoma, gallbladder, BRAF V600E mutation, neoadjuvant, targeted therapy
Introduction
Over the past decades, the incidence of malignant melanoma has increased. Melanoma has been reported as the fifth and sixth most common cancer types in the United States in men and women, respectively. In 2017, 87,110 new malignant melanoma cases and 9,730 malignant melanoma deaths were projected to occur in the United States.1 In recent years, major breakthroughs in cutaneous melanoma treatments including immunotherapy and targeted therapies have led to remarkable improvements in patient outcomes. Mucosal melanomas are rare and account for ~1% of all melanomas. Melanoma arising from the mucosal epithelium of the gallbladder is extremely rare. Due to the rarity of this neoplasm, management of both primary and metastatic melanoma of the gallbladder is not well defined.2,3 Published data on the efficacy of these novel therapies in the treatment of gallbladder melanoma are limited. We report a case of successful neoadjuvant targeted therapy followed by radical surgical excision in a patient with previously unresectable advanced malignant melanoma of the gallbladder.
Case report
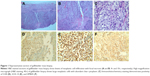
A 58-year-old male presented with nausea, vomiting, and abdominal pain. Laboratory studies showed significantly elevated direct bilirubin and alkaline phosphatase levels as well as mildly elevated transaminases, consistent with bile duct obstruction. Abdominal ultrasound revealed cholelithiasis with distention of the gallbladder (12.1×5.5×6.7 cm) and common bile duct dilation (10 mm). The patient underwent endoscopic retrograde cholangiopancreatography, and common biliary duct cannulation was unsuccessful. A laparoscopic intraoperative cholangiogram was attempted and a firm mass was found involving the infundibulum of gallbladder, and the decision was made to convert an open cholecystectomy. Due to the extensive tumor burden including multiple fixed lymph nodes within the hepatoduodenal ligament, further dissection of the gallbladder was not attempted. A cholecystostomy was placed and an incisional biopsy of the gallbladder mass was obtained. The specimen was remarkable for high-grade malignant melanoma (Figure 1). Immunohistochemical staining was positive for S100, HMB-45, and SOX-10, supporting the diagnosis of malignant melanoma (Figure 1). Computed tomography (CT) revealed thickening of the gallbladder wall with enlarged retrocrural lymph node as well as multiple periportal and peripancreatic lymph nodes (Figure 2). Magnetic resonance imaging of the brain was negative for metastatic disease. To rule out the diagnosis of metastatic melanoma from a primary cutaneous site, an extensive skin examination was performed by a dermatologist and no primary cutaneous melanoma was found. Positron emission tomography (PET) was not obtained due to limited utility in the immediate postoperative setting.
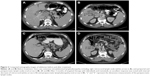
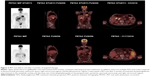
The patient was started on single-agent nivolumab (Opdivo; Bristol-Myers Squibb, New York, NY, USA) at 3 mg/kg intravenously every 2 weeks. After three cycles of nivolumab treatment, the patient developed grade 2 liver toxicity and the treatment was discontinued. A baseline PET scan was obtained prior to the initiation of other therapies and revealed extensive lymph nodular metastasis involving the upper abdomen, the retroperitoneum, and the left supraclavicular region (Figure 3). The patient’s B-Raf (BRAF) mutation analysis was positive for a BRAF V600E mutation (RT-PCR was done at William Beaumont Hospital Advanced Diagnostic Molecular Laboratory, using primers and probes from BRAF mutation detection kit; Entrogen, Tarzana, CA, USA). The patient was started on targeted therapy with the BRAF inhibitor dabrafenib (Tafinlar; Novartis International AG, Basel, Switzerland) 150 mg twice daily in combination with the mitogen-activated protein kinase (MEK) inhibitor trametinib (Mekinist; Novartis) 2 mg daily. A CT scan after 2 months of targeted therapies showed an interval decrease in size of the gallbladder mass and associated lymphadenopathy (Figure 2). A PET scan after 5 months of treatment showed significant decrease in the metabolic activity and size of the lymphadenopathy (Figure 3, lower panel). The patient experienced recurrent episodes of fever which were considered to be therapy-related after infectious etiologies were ruled out. The therapy-related pyrexia was managed by the use of acetaminophen, steroids, and temporary drug dose interruption, and eventually dose reduction. Another follow-up CT scan after 8 months of treatment showed complete resolution of abdominal lymphadenopathy and no evidence of metastasis. The patient underwent cholecystectomy and central hepatectomy with portal lymphadenectomy. Pathological analysis revealed no evidence of residual melanoma in gallbladder, and all dissected lymph nodes were negative for melanoma involvement. No adjuvant targeted therapy was given. At the time of this report, the patient remains in good condition and has survived 26 months after his melanoma diagnosis and has been disease-free for 14 months.
Our study received approval from the ethics committee of the Beaumont Health. The patient also provided written informed consent to publish this case report details.
Discussion
Melanomas of the gallbladder are extremely rare. The vast majority of melanoma involvement of the gallbladder is secondary to metastatic disease from a primary cutaneous site and is usually part of extensive metastasis. Primary melanoma of the gallbladder remains a debatable clinical entity. Most melanocytes are located in the epidermis and dermis of the skin. During embryologic development, melanocytes can migrate from the neural crest to endodermal derivatives such as the esophagus, intestine, rectum, vagina, and gallbladder; this explains the presence of melanocytes within their mucosa and supports the potential for rise of primary melanoma from these sites.4 Heath and Womack established the diagnostic criteria of primary gallbladder melanoma: the tumor must principally be solitary and arises from the gallbladder mucosa and must either display junctional activity or have any other primary sites excluded by thorough investigation.4 However, distinguishing between a primary mucosal gallbladder melanoma and a metastatic melanoma to gallbladder can still be challenging. We believe that our patient has primary gallbladder melanoma based on solitary gallbladder tumor characteristics and that no other primary sites were identified. Unfortunately, his initial intraoperative core needle biopsy specimen did not include gallbladder mucosa and junctional activity could not be evaluated. We cannot completely exclude a potential metastatic origin as the primary cutaneous site could have regressed or have been too small to be identified by conventional clinical and laboratory investigations.
From a clinical point of view, both primary gallbladder melanoma and metastatic melanoma involving the gallbladder have poor prognosis. Survival typically ranges from a few weeks to several years. Primary cases and isolated gallbladder metastatic disease have a better prognosis than disseminated disease.
Data regarding the management of gallbladder melanoma are limited and include mostly single-case reports or small number of case series. In a series of 19 cases reported by Dong et al,3 two patients who had metastatic disease confined to the gallbladder and underwent cholecystectomy had favorable outcomes. One of the two patients died of recurrent disease 9.2 years later. The other remained alive 13.8 years post-resection at the time of that report. Among the other 17 patients who had gallbladder involvement as part of systematic metastatic disease, patients who had disease amendable to complete resection achieved 100% survival at 1 year compared to 0% for those with unresectable disease.3 Katz et al reported a median survival of 16 months after palliative cholecystectomy in 13 patients with metastatic melanoma of the gallbladder.5 Two out of the three patients who had laparoscopic cholecystectomy later developed port site recurrence even if great care was taken to avoid dissemination of cancerous cells during surgery. Christou et al reported the successful management of a metastatic melanoma case with open cholestectomy and wedge resection.6 Martel et al reported a survival of 18 months at the date of the publication in a patient who underwent radical open cholecystectomy.7 Giannini et al reported two patients with gallbladder metastases from cutaneous melanoma survived at least 6 months after surgical excision.8 Although these data are limited, it appears that unresectable gallbladder melanoma has the worst prognosis. Surgical resection, if possible, is the key to a better outcome, even in disseminated cases. All these case reports were published before the era of the novel therapies.
An improved understanding of the molecular pathways in melanoma has given rise to new treatment options. Approximately 50% of melanomas have been found to carry BRAF mutations that lead to the constitutive activation of the MAP kinase/ERK signaling pathways.9 These signaling pathways regulate cell proliferation, differentiation, and survival. Constitutively activated pathways result in melanoma tumorigenesis. The most common BRAF mutation involves a substitution of glutamate for valine at codon 600 (BRAF V600E). The second most common mutation is BRAF V600K, substituting lysine for valine.9 Phase III clinical trials have confirmed that inhibition of BRAF alone,10,11 or the combined inhibition of BRAF and its downstream target, MEK,12,13 results in robust response rates (from 48% to 68%) and improved the overall survival of patients with advanced or metastatic BRAF-mutant melanoma. The BRAF inhibitors dabrafenib and vemurafenib (Zelboraf; Genentech, Inc., South San Francisco, CA, USA) as single agents, and BRAF and MEK inhibitor combinations (dabrafenib/trametinib or vemurafenib/cobimetinib) have been approved for the treatment of metastatic BRAF mutant melanoma. While the benefit of adjuvant vemurafenib monotherapy was inconclusive,14 adjuvant dabrafenib/trametinib has been shown to reduce the risk of disease recurrence or death by 53% in stage III BRAF mutant melanoma and was approved by the Food and Drug Administration recently.15
Although promising, patients with mucosal (including gallbladder site) or ocular melanoma were always excluded from all the clinical studies. Unlike ocular melanoma, which does not express BRAF mutation, about 10% of mucosal melanoma cases have BRAF mutation.16 In the literature, Giannini et al reported a case in which BRAF/MEK inhibitors were used after surgical excision of gallbladder metastasis from cutaneous melanoma.8
Here we presented a case of presumed primary gallbladder melanoma. The patient was judged to have inoperable disease as a result of locally advanced disease with multiple lymph node metastases. The presence of a BRAF V600E mutation allowed preoperative treatment with BRAF/MEK inhibitors. The patient had an excellent response and was converted to a surgical candidate. To our knowledge, the complete pathological remission achieved with the use of BRAF/MEK inhibitors has not been previously reported in the literature of gallbladder melanoma. The impressive response suggests that presurgical targeted therapy may be combined with surgery to achieve long-term survival.
It is difficult to judge the efficacy of immunotherapy in this case due to the lack of a baseline PET scan before the immunotherapy. The fact that there was still significant tumor burden after three cycles of nivolumab is consistent with the observation that the onset of response to immunotherapy is usually slower than the targeted therapy. It is widely accepted that if tumor burden is high, or there is an urgent need to reduce tumor size, targeted therapy is preferred. Our patient also did not tolerate immunotherapy due to liver toxicity.
Conclusion
Malignant melanoma of the gallbladder is associated with an aggressive course and poor prognosis. Here we report our successful experience in managing a patient with primary gallbladder melanoma using a combination of BRAF and MEK inhibitors followed by surgical resection and achieved pathological complete remission. Targeted therapy may be used in combination with surgical resection to improve the outcome of patients with BRAF mutant gallbladder melanoma.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. | ||
Haskaraca MF, Ozsoy M, Ozsan I, Kurt K. Primary malignant melanoma of the gallbladder: a case report and review of the literature. Case Rep Surg. 2012;2012:693547. | ||
Dong XD, Dematos P, Prieto VG, Seigler HF. Melanoma of the gallbladder: a review of cases seen at duke university medical Center. Cancer. 1999;85(1):32–39. | ||
Heath DI, Womack C. Primary malignant melanoma of the gall bladder. J Clin Pathol. 1988;41(10):1073–1077. | ||
Katz SC, Bowne WB, Wolchok JD, Busam KJ, Jaques DP, Coit DG. Surgical management of melanoma of the gallbladder: a report of 13 cases and review of the literature. Am J Surg. 2007;193(4):493–497. | ||
Christou D, Katodritis N, Decatris MP, et al. Melanoma of the gallbladder: appropriate surgical management and review of the literature. Clin Case Rep. 2014;2(6):313–318. | ||
Martel JP, McLean CA, Rankin RN. Melanoma of the gallbladder. Radiographics. 2009;29(1):291–296. | ||
Giannini I, Cutrignelli DA, Resta L, Gentile A, Vincenti L. Metastatic melanoma of the gallbladder: report of two cases and a review of the literature. Clin Exp Med. 2016;16(3):295–300. | ||
Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239–1246. | ||
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. | ||
Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. | ||
Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. | ||
Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. | ||
Maio M, Lewis K, Demidov L, et al. Adjuvant vemurafenib in resected, BRAF. Lancet Oncol. 2018;19(4):510–520. | ||
Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. | ||
Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–2147. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.