Back to Journals » OncoTargets and Therapy » Volume 13
Pathological Complete Response After a Single Dose of Anti-PD-1 Therapy in Combination with Chemotherapy as a First-Line Setting in an Unresectable Locally Advanced Gastric Cancer with PD-L1 Positive and Microsatellite Instability
Authors Jin H, Li P, Mao C, Zhu K, Chen H, Gao Y, Yu J
Received 21 December 2019
Accepted for publication 16 January 2020
Published 26 February 2020 Volume 2020:13 Pages 1751—1756
DOI https://doi.org/10.2147/OTT.S243298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Hailong Jin,1 Peijie Li,2 Chenyu Mao,3 Kankai Zhu,1 Hai Chen,1 Yuan Gao,1 Jiren Yu1
1Department of Gastrointestinal Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, People’s Republic of China; 2Department of Pathology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, People’s Republic of China; 3Department of Oncology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, People’s Republic of China
Correspondence: Yuan Gao; Jiren Yu
Department of Gastrointestinal Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Qingchun Road 79, Hangzhou 310003, People’s Republic of China
Tel +86 571 8723 6147
Fax +86-571-8707 2577
Email [email protected]; [email protected]
Abstract: Programmed cell death-1 (PD-1) immune checkpoint inhibitors have exhibited promising efficacy in various types of tumors. Here, we report an unresectable locally advanced gastric cancer (GC) with programmed cell death ligand-1 (PD-L1) positive and microsatellite instability (MSI), which exhibiting an unexpected efficacy of pathological complete response (pCR) after a single dose of anti-PD-1 therapy in combination with chemotherapy as a first-line setting. A 66-year-old man diagnosed with gastric cancer and was clinically staged as cT4aN+M0. After one cycle treatment (oxaliplatin plus capecitabine plus anti-PD-1antibody), repeated computed tomography (CT) showed tumor shrinkage and the plasma carcinoembryonic antigen decreased dramatically. Curative distal gastrectomy with D2+ lymphadenectomy was performed and the pathological staging was pT0N0M0. Immunohistochemistry and polymerase chain reaction-based method revealed that the patient had a PD-L1 positive and MSI-high GC, while the Epstein-Barr virus was absent. Furthermore, much CD8+ tumor-infiltrating lymphocytes (TILs) were observed within the tumor and invasion front, which were responsible for tumor shrinkage, due to the recognition of abundant tumor neoantigens and their corresponding immune checkpoints. Our case report indicates that a combination of immunotherapy plus chemotherapy as a first-line treatment might offer a promising treatment option for locally advanced gastric adenocarcinoma with PD-L1 positive and MSI-high.
Keywords: gastric cancer, immune checkpoint inhibitor, programmed cell death-1, microsatellite instability, chemotherapy
Introduction
Recently, programmed cell death-1 (PD-1) immune checkpoint inhibitors (ICIs) have exhibited promising efficacy in various types of tumors.1 Taking the results of the ATTRACTION-2 study and KEYNOTE-059 study, PD-1 ICIs were approved for the third-line (or higher) treatment of advanced and recurrent or metastatic gastric cancer (GC) in Asian and Western countries.2–6 Several studies have indicated that patients with programmed cell death ligand-1 (PD-L1) positive or microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) or high tumor mutation burden (TMB) or Epstein-Barr virus (EBV) positive may drive greater benefit from ICIs.7 Here, we report the case of an unresectable locally advanced GC patient with PD-L1 positive and MSI-H, who exhibited an unexpected efficacy of pathological complete response (pCR) after a single dose of anti-PD-1 therapy in combination with chemotherapy as a first-line setting.
Case Presentation
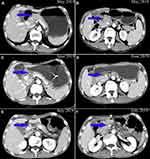
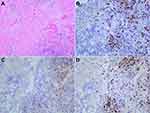
A 66-year-old man with weight loss visited our hospital in April, 2019. Gastroduodenoscopy revealed a giant ulcer in the antrum and the pathology examination confirmed poor differentiated adenocarcinoma and Helicobacter pylori infection was positive. On the basis of computed tomography (CT), the patient was clinically staged as cT4aN+M0, stage III, which was considered unresectable with highly suspicious bulky N3 lymph nodes metastasis and encasement of major vascular structures (including hepatic artery and superior mesenteric vein), (Figure 1A and B) according to American Joint Committee On Cancer (AJCC), the 8th edition.8 An elevated carcinoembryonic antigen (CEA) with 33.0 ng/mL was observed. Immunohistochemically, tumor cells were negative for human epidermal growth factor receptor-2 (HER2). This patient was enrolled in a clinical trial.9 He received HX008 (a recombinant humanized PD-1 monoclonal antibody, 3mg/kg on D1) in combination with chemotherapy (130mg/m2 oxaliplatin on D1 and 1000mg/m2 capecitabine twice a day on D1-14, every 3 weeks, namely XELOX regimen). 3 weeks later, he was readmitted due to frequently vomit, then he was fasted and treated with nasogastric tube decompression. A nasal jejunum tube was failed to implant through endoscopy for enteral nutrition due to completely pyloric obstruction and total parenteral nutrition support was given. However, repeated CT showed tumor shrinkage (Figure 1C and D) and the CEA decreased to 4.5 ng/mL. The patient was well tolerant to the treatment without severe side effects. Curative distal gastrectomy with D2+ lymphadenectomy was performed in July, 2019 and the pathological staging was pT0N0M0, pathological complete response (pCR), with 41 lymph node achieved. Histological findings demonstrated prominent interstitial fibrosis and abundant lymphocyte infiltration with no residual tumor cells (Figure 2A). Although after surgery, the patient suffered from lymphatic leakage, he was recovered smoothly. Repeated CT after surgery showed curative dissection of lymph nodes along hepatic artery and superior mesenteric vein (Figure 1E and F). In September 2019, the patient started to receive XELOX regimen without ICIs as an adjuvant treatment.
To investigate the association of tumor factors with the efficacy of PD-1 blockade, we used immunohistochemistry to assess the expression of mismatch repair proteins and a polymerase chain reaction-based method to assess DNA mismatch repair across 6 mononucleotide repeat markers (NR21, NR24, NR27, BAT25, BAT26, MONO27) in previous biopsy specimens. The results showed that two mismatch repair proteins (MLH1 and PMS2) were negative, while MSH2 and MSH6 were positive (Figure 3). In addition, all 6 markers were unstable and the tumor was classified to be MSI-H GC. EBV was absence in the tumor by using EBV-encode small RNA (EBERs) in situ hybridization (Figure 4A). Expression of PD-L1 was assessed in tumor biopsy samples by immunohistochemistry assay (PD-L1 IHC 22C3, 1:100, Dako). The tumor proportion score (TPS) was 40%, which is the percentage of tumor cells showing partial or complete membrane staining (Figure 4B). Additionally, we performed immunohistochemistry (IHC) of CD3, CD4, and CD8 to analyze the tumor-infiltrating lymphocytes (TILs), much CD8+ TILs were observed within the tumor and invasion front (Figure 2B–D).
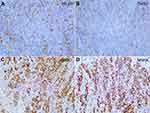 |
Figure 3 Immunohistochemistry of mismatch repair proteins. The tumor cells were negative for MLH1 (A), PMS2 (B) and positive for MSH2 (C), MSH6 (D); original magnification: x100. |
Discussion
GC is the fifth most common malignancy and the third leading cause of cancer deaths worldwide.10 Cytotoxic chemotherapy containing fluoropyrimidine plus a platinum agent is a standard first-line treatment for locally advanced GC with or without the combination of trastuzumab depending on the expression of HER2, however, the 5-year over survival remains poor.11,12 An exploratory analysis of MAGIC trial revealed that patients with MSI-H or dMMR GC could not benefit from perioperative chemotherapy.13 Recently, ICIs have led to a paradigm shift for treatment in advanced gastric cancer and several biomarkers were identified to help guide selection of patients who were most likely to benefit from PD-1 blockade.14 In KEYNOTE-059, the objective response rate (ORR) was 15.5% in PD-L1 positive tumors, while the ORR was 6.4% in PD-L1 negative tumors.3 In KEYNOTE-061, patients with a PD-L1 combined positive score of 10 or higher benefit more from anti-PD-1 therapy.15 Also, subgroup analysis of KEYNOTE-059 and KEYNOTE-061 showed that the ORR was 57.1% and 46.7% in patients with MSI-H tumors. Based on these evidences, pembrolizumab was approved for the treatment of third-line (or higher) advanced PD-L1 positive GC and MSI-H GC.5,6 However, the efficacy of ICIs combining with chemotherapy as a first-line setting for the treatment of advanced GC remains unknown. Several clinical trials were ongoing, including KEYNOTE-062 and KEYNOTE-585.16,17 Our case showed a rare pCR after a single dose of anti-PD-1 therapy in combination with chemotherapy as a first-line setting in a locally advanced GC patient with PD-L1 positive and MSI-H. MSI-H tumors have common histopathological characteristics, including lymphocytic infiltration, numerous somatic mutations and abundant neoantigens formation.6 These neoantigens are targets for the immune system and MSI-H tumors may be sensitive to immunotherapy. In addition, increased expression of PD-1 and PD-L1 can also be observed in MSI-H tumors.18 Nonetheless, prevalence of MSI-H tumors was were very low in previous studies, ranging from 3% to 22%.3,15,19 Our case was very well treated with PD-1 blockade and the property of MSI-H might contribute to success in response. Numerous infiltrated CD8+ T lymphocytes were observed within the tumor and invasion front in our case which were responsible for tumor shrinkage, due to the recognition of abundant tumor neoantigens and their corresponding immune checkpoints. It has been reported that 8% to 10% GC were associated with EBV infection.20 The Cancer Genome Atlas project also revealed that amplification of JAK2, CD274 (also known as PD-L1) and PDCD1LG2 (also known as PD-L2) was frequently observed in EBV-positive gastric adenocarcinoma.19 However, in our patient, EBV was absence in tumor cells.
In conclusion, we report a case of an unresectable locally advanced gastric adenocarcinoma with PD-L1 positive and MSI-H, who exhibited an unexpected efficacy of pCR after a single dose of anti-PD-1 therapy in combination with chemotherapy as a first-line setting. Our case report indicates that a combination of immunotherapy plus chemotherapy as a first-line treatment might offer a promising treatment option for advanced GC. Depending on the results of previous studies and combining with our findings, we speculate that advanced GC with MSI-H was suitable for immunotherapy with or without chemotherapy, while advanced GC with microsatellite instability-low (MSI-L) and microsatellite stable (MSS) were suitable for chemotherapy or chemoradiotherapy (CRT) on the future standard treatment. This kind of molecular profile might have the potential to improve patient selection and spare a significant proportion of patients with GC unnecessary treatment.
Consent for Publication
The institutional review board of The First Affiliated Hospital, Zhejiang University School of Medicine approved the study. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgments
HL Jin was supported by the grant of The General Program of Science and Technology in Medical Health of Zhejiang Province (grant number: 2018259351) and JR Yu was supported by the grant of The Major Science and Technology Project of Zhejiang Province (2014C03040-1). The authors thank the patient for his participation and his agreement to publication of the report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi:10.1056/NEJMoa1200690
2. Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi:10.1016/S0140-6736(17)31827-5
3. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013.
4. Bang Y-J, Kang Y-K, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the Phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22(4):828–837. doi:10.1007/s10120-018-00909-5
5. Muro K, Van Cutsem E, Narita Y, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(1):19–33. doi:10.1093/annonc/mdy502
6. Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. 2017;377(15):1409–1412. doi:10.1056/NEJMp1709968
7. Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–1458. doi:10.1038/s41591-018-0101-z
8. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): gastric cancer. (Version 2). 2019.
9. Study of HX008 as a combination with chemotherapy for treatment of locally advanced or metastatic gastric cancer including gastroesophageal junction carcinoma. (CTR20181270). Available from: http://www.chinadrugtrials.org.cn.
10. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi:10.1002/ijc.29210
11. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. doi:10.1007/s10120-016-0622-4
12. Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38–49. doi:10.1093/annonc/mdw350
13. Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3(9):1197–1203. doi:10.1001/jamaoncol.2016.6762
14. Joshi SS, Maron SB, Catenacci DV. Pembrolizumab for treatment of advanced gastric and gastroesophageal junction adenocarcinoma. Future Oncol. 2018;14(5):417–430. doi:10.2217/fon-2017-0436
15. Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–133. doi:10.1016/S0140-6736(18)31257-1
16. Study of pembrolizumab (MK-3475) as first-line monotherapy and combination therapy for treatment of advanced gastric or gastroesophageal junction adenocarcinoma (MK-3475-062/KEYNOTE-062). Available from: https://clinicaltrials.gov/ct2/show/NCT02494583.
17. Study of pembrolizumab (MK-3475) plus chemotherapy versus placebo plus chemotherapy in participants with gastric or gastroesophageal junction (GEJ) adenocarcinoma (MK-3475-585/KEYNOTE-585). Available from: https://clinicaltrials.gov/ct2/show/NCT03221426.
18. Dudley JC, Lin M-T, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22(4):813–820. doi:10.1158/1078-0432.CCR-15-1678
19. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi:10.1038/nature13480
20. Jacome AA, Lima EM, Kazzi AI, et al. Epstein-Barr virus-positive gastric cancer: a distinct molecular subtype of the disease? Rev Soc Bras Med Trop. 2016;49(2):150–157. doi:10.1590/0037-8682-0270-2015
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.