Back to Journals » Cancer Management and Research » Volume 13
Pathological Analysis and Endoscopic Characteristics of Colorectal Laterally Spreading Tumors
Authors Li DH, Liu XY, Huang C, Deng CN, Zhang JL, Xu XW, Xu LB
Received 9 October 2020
Accepted for publication 13 January 2021
Published 9 February 2021 Volume 2021:13 Pages 1137—1144
DOI https://doi.org/10.2147/CMAR.S286039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chien-Feng Li
Da-Huan Li,1,* Xue-Ying Liu,1,* Chao Huang,2 Chao-Nan Deng,3 Jia-Lu Zhang,1 Xiao-Wen Xu,1 Liang-Bi Xu1
1Department of the Digestive Endoscopy, The Affiliated Hospital of Guizhou Medical University, Guiyang, 550000, People’s Republic of China; 2Department of the Emergency, The Affiliated Hospital of Guizhou Medical University, Guiyang, 550000, People’s Republic of China; 3Department of the Pathology, The Affiliated Hospital of Guizhou Medical University, Guiyang, 550000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liang-Bi Xu
Department of the Digestive Endoscopy, The Affiliated Hospital of Guizhou Medical University, No. 28 of Guiyi Street, Yunyan District, Guiyang, 550000, People’s Republic of China
Tel +8615685178740
Fax +86085186774297
Email [email protected]
Objective: This study aims to analyze the endoscopic and pathological characteristics of colorectal laterally spreading tumors (LSTs) to assist malignant risk stratification to inform selection of the appropriate treatment strategy.
Methods: Patients with colorectal LST were selected as retrospective study objects. Characteristics, including endoscopic findings and the most common site of LSTs of different diameters and histological types, were analyzed. The risk factors for malignancy in colorectal LST were explored by multivariate logistic regression analysis.
Results: LSTs with diameters of ≥ 20 mm were found mainly in the rectum and mainly with granular-mixed (G-M) morphology (36% and 44.6%, respectively; p < 0.05), while LSTs with diameters of < 20 mm were found mainly in the ascending colon and mainly with granular-homogenous (G-H) morphology (40.9% and 46.2%, respectively; p < 0.05). Adenoma was the main histological type in patients with tumors of all diameters. However, the cancerization rate of LSTs was 31% in patients with tumor diameter ≥ 20 mm, while there was no invasive cancer in patients with tumor diameter < 20 mm. In the low-grade dysphasia (adenoma) group, most of the lesions were located in the ascending colon and most had the morphology LST-G-H (35.8% and 39.2%, respectively; p < 0.05). In the cancerization group, most of the lesions were located in the rectum, with the morphology LST-G-M (51.6% and 67.2%, respectively; p < 0.05), and the diameter was larger than that of the adenoma group (33.84 ± 17.99 mm vs 21.68 ± 8.99 mm).
Conclusion: The rectum was the most common site for an LST with a diameter ≥ 20 mm and cancerization, of which the morphology was mainly LST-G-M (endoscopic submucosal dissection is the preferred treatment for this type of LST). LST malignancy was found to be correlated with lesion diameter, location, and morphological appearance.
Keywords: colorectal laterally spreading tumors, colonic mucosa, colonoscopy, pathological features, malignant tumor
Introduction
A colorectal laterally spreading tumor (LST) is a particular type of flat adenomatous polypoid lesion of the large intestine, which grows laterally, close to the surface of the mucosa of the large intestine.1 Because of their unique growth mode, LSTs are often misdiagnosed under colonoscopy.2,3 The morphology of LSTs is diverse under endoscopy;4 generally, such tumors can be divided into granular LSTs (LST-G) and non-granular LSTs (LST-NG), according to whether there is a granular formation. It has been suggested under dynamic observation that LSTs can develop into advanced colorectal cancer within three years, and that LSTs with a diameter >20 mm have a cancerization rate of up to 20%.5 Due to inadequate preoperative assessment of LSTs, some patients fail to receive appropriate treatment. Better identification of the characteristics of LSTs of different sizes and properties is therefore important for their preoperative evaluation. However, there remains a lack of research into the endoscopic and pathological characteristics of LSTs according to size of lesion and histological type.
The present study therefore aims to analyze the endoscopic and pathological characteristics of colorectal LSTs of different diameters and histological types and to explore the predictive factors of the malignant pathology of LSTs. This has potential to improve the clinical understanding of LSTs and enable the formulation of precise treatment plans.
Patients and Methods
Patients
The present study is a retrospective study. Patients with colorectal LST in the Affiliated Hospital of Guizhou Medical University from January 2014 to December 2019 were selected as study objects. The clinical and pathological data of all patients included in the study were complete. A total of 268 patients were enrolled in this study, all of whom underwent endoscopic or surgical treatment in our hospital. Endoscopic treatment was performed by four endoscopists, each with more than five years’ experience. Of the total number of patients, 245 underwent endoscopic treatment and 23 patients underwent surgery. LSTs were removed under operation, and all postoperative specimens were accurately measured with a ruler. LSTs were grouped and analyzed according to different diameters and histological types. Taking a lesion diameter of 20 mm as the boundary, lesions were divided into two groups: one of lesion diameter ≥20 mm and one of lesion diameter <20 mm. Taking the postoperative pathological diagnosis as the grouping standard, LSTs were also divided between a low-grade dysplasia group and a cancerization group.
The characteristics, including the endoscopic appearance and the most common site of LSTs with different diameters and histological types, were also analyzed. The present study was run in accordance with the World Medical Association’s Helsinki Declaration and was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University. All patients signed informed consent forms.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) a definite diagnosis of LST;1,4 (2) a lesion diameter of >10 mm; (3) a lateral expansion tumor growth pattern, rather than a vertical growth pattern; and (4) morphological characteristics of LST-G or LST-NG.
The exclusion criteria were as follows: (1) hereditary non-polyposis colorectal cancer (HNPCC); (2) familial adenomatous polyposis (FAP); (3) inflammatory bowel disease (IBD); and (4) incomplete clinical and pathological data (Figures 1 and 2).
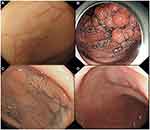 |
Figure 1 LST morphological type. (A) LST-G-H; (B) LST-G-M; (C) LST-NG-F; (D) LST-NG-PD. |
 |
Figure 2 A flow chart to illustrate inclusion and exclusion. |
Main Outcome Measures
Growth Pattern Classification of Tumor
1) Granular (G) (tumor surface with granules and nodules): a) G-homogenous (G-H), ie, uniform surface granules and nodules; and b) G-mixed (G-M), ie, coarse surface nodules.
2) Non-granular (NG) (tumor surface flat and smooth): a) pseudo-depressed (PD); and b) flat elevated.
Pathological Classification
Following the 2019 WHO classification of digestive tract tumors,6 the postoperative pathological diagnosis was classified as low-grade dysplasia, high-grade dysplasia, or adenocarcinoma. The histological groupings used for analysis were: (1) a low-grade dysplasia (adenoma) group, ie, patients with a pathological diagnosis of tubular adenoma, villous adenoma, villous tubular adenoma, or serrated lesions; and (2) a cancerization group, ie, patients with a diagnosis of high-grade dysplasia or invasive carcinoma (cancer cells breaking through the basement membrane of the mucosa and infiltrating the submucosa).
Treatment Methods
Each patient underwent either endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD) or surgery. ESD was selected when the lesions were larger than 20 mm in size and the morphology was LST-NG-PD or LST-G-M. Surgery was selected when endoscopic evaluation of the infiltration depth exceeded the deep submucosa, or when the disease was not suitable for endoscopic treatment due to the location or size of the lesion. In some cases, where the lesion was located in a difficult position (eg, between the plica, or in the sigmoid colon) or its size was close to 20 mm, it was judged too difficult to completely remove by EMR, and ESD was chosen.
Statistical Analysis
SPSS 23.0 software was used for statistical analysis. Measurement data was represented by the mean ± standard deviation (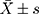 ). Countable data were expressed in percentages (%). The W-test was used as a normality test, and the F-test was used to test the homogeneity of variance. A t-test was used to compare pairs of groups, and the nonparametric test was used to compare groups that did not conform to the normal distribution. The chi-squared test was used to assess data. Logistic regression was used to analyze the risk factors for LST cancerization. p < 0.05 was regarded as statistically significant.
). Countable data were expressed in percentages (%). The W-test was used as a normality test, and the F-test was used to test the homogeneity of variance. A t-test was used to compare pairs of groups, and the nonparametric test was used to compare groups that did not conform to the normal distribution. The chi-squared test was used to assess data. Logistic regression was used to analyze the risk factors for LST cancerization. p < 0.05 was regarded as statistically significant.
Results
General Characteristics
In our hospital from January 2014 to December 2019, 436 patients were diagnosed with colorectal LST by colonoscopy; according to the inclusion and exclusion criteria, 268 patients with colorectal LST were finally included in the study.
Included patients were grouped according to lesion diameter, as follows: (1) a group of 175 with LSTs of diameter ≥20 mm, including 80 males (45.7%) and 95 females (54.3%) with an age range of 63.34 ± 10.85 years; and (2) a group of 93 with LSTs of diameter <20 mm, including 48 males (51.6%) and 45 females (48.4%) with an age range of 63.61 ± 11.13 years. There was no statistical difference between the two groups in terms of gender or age (Table 1).
 |
Table 1 Comparison of Clinical, Endoscopic and Pathological Characteristics of LST Groups with Different Diameters |
A histological grouping was also made, as follows: (1) a low-grade dysplasia (adenoma) group of 204, including 94 males (46.1%) and 110 females (53.9%) with an age range of 63.18 ± 11.27 years; and (2) a cancerization group of 64, comprising 34 males (53.1%) and 30 females (46.9%) with an age range of 64.23 ± 9.77 years. There was no statistical difference between the two groups in terms of gender or age (Table 2).
 |
Table 2 Endoscopic Characteristics in Different Histological Types of LST |
Comparison of Endoscopic and Pathological Characteristics of LSTs of Different Diameters
In the group with LSTs of diameter ≥20 mm, the rectum (36.0%) was the most common lesion site; in the group with LSTs of diameter <20 mm, the ascending colon (40.9%) was the most common site. The difference in lesion sites between the two groups was statistically significant (p = 0.000, ie, <0.05).
In the group with LSTs of diameter ≥20 mm, LST-G-M (44.6%) was the main type of morphological appearance; LST-G-H (46.2%) was the main type in the group with LSTs of diameter <20 mm. The difference in growth pattern between the two groups was statistically significant (p = 0.000, ie, <0.05).
Low-grade dysplasia was the main type of histological classification in both groups, and the difference between the two groups was statistically significant. Moreover, there was no invasive cancer in patients in the group with LSTs of diameter <20 mm (Table 1).
Comparison of Endoscopic Characteristics in Different Histological Types of LST
In the low-grade dysplasia group, lesions were mainly located in the ascending colon (35.8%); lesions in the cancerization group were mostly located in the rectum (51.6%). The difference between the two groups was statistically significant (p = 0.000, ie, >0.05). A growth pattern of LST-G-H predominated in the low-grade dysplasia group (39.2%); tumors in the cancerization group mainly manifested as LST-G-M (67.2%). The difference in growth pattern classifications between the two groups was statistically significant (p = 0.000, ie, < 0.05) (Table 2).
Multivariate Analysis of Risk Factors Related to LST Carcinogenesis
Five factors were introduced into the logistic regression model for multivariate analysis: gender, age, and lesion diameter, location, and growth pattern. The results revealed that lesion diameter, location, and growth pattern were risk factors for LST cancerization. The possibility of malignancy increased with lesion diameter. Most of the malignant LSTs were found in the rectum, with the majority displaying an LST-G-M growth pattern (Table 3).
 |
Table 3 Multivariate Analysis of Risk Factors Related to LST Carcinogenesis |
Discussion
Laterally spreading tumors (LSTs) were first reported and named by the Japanese researcher Kudo.1 They have higher malignant potential than polypoid adenomas, with unique pathological morphology and development mode.7,8 The detection rate for LSTs has increased in recent years due to the ongoing development of endoscopic diagnosis and therapeutic technology. Since LSTs are closely correlated with colorectal cancer,9 it is of great significance to fully understand the clinical endoscopic and pathological characteristics of LSTs in order to formulate accurate treatment plans.
The therapeutic strategy for LST is based on the diameter and nature of the lesion, and the depth of invasion,10–12 according to which EMR, ESD, or surgical operation can be selected. With the extensive development of ESD, more and more LSTs can be treated under endoscopy, avoiding the risks of surgical trauma and recurrence caused by inadequate endoscopic resection. However, ESD makes greater demands on surgeons and has higher treatment costs than EMR. It is therefore crucial to understand the characteristics of an LST before operating, in order to choose the most appropriate therapeutic strategy. With this in mind, the present study was designed to investigate the characteristics of LSTs regarding location distribution and morphological appearance from multiple aspects, including diameter and histological type. The aim was to aid the preoperative malignant risk stratification of LSTs and assist in the formulation of accurate treatment plans.
Regarding the age of onset, the present study found that LST is more likely to occur in the middle-aged and the elderly, which is consistent with the findings reported in the literature for LST and colorectal adenoma.13,14 However, our findings regarding gender differences are contrary to the conclusion of a previous multicenter study, that LST was more common in males:15 we found that LST was more common in females than in males, and there were more female patients than males in the group with lesions of diameter ≥20 mm. It can be conjectured that such differences are due to two factors: first, the present study was a single-center study with a relatively small sample size; second, previous multicenter studies did not group and compare LSTs according to lesion size.
It has been reported that the rectum and the right half of the colon are the most common sites of LST.16 However, literature discussing lesion site with reference to lesion diameter is scarce. In the present study, in both the group with lesion diameter of ≥20 mm and the cancerization group, the most common site of LST was the rectum; the ascending colon was the main lesion site in the low-grade dysplasia (adenoma) group and the group with lesion diameter of <20 mm. This indicates certain differences in the most common lesion sites for LSTs of different diameters and histological types and adds reliable data to support understanding of the relationship between LST lesion diameter and lesion site.
A previous study has suggested that the morphological appearance of LSTs is closely correlated with the most common site and histopathological type.17 In Miyamoto et al,18 LST-G was most likely to be detected in the rectum, and LST-NG was most likely to be found in the colon, while LST-G-M and LST-NG-PD had higher malignant potential than the other two subtypes;19 malignant potential was correlated with lesion diameter. In the present study, LST-G-M was mainly found in the group with lesion diameter of ≥20 mm and in the cancerization group, while the majority of LSTs in the group with lesion diameter of <20 mm and in the low-grade dysplasia (adenoma) group manifested as LST-G-H. This helps us understand the relationship between LST lesion diameter and morphological appearance, and is helpful for the treatment of LST.
Categorized by histopathological type, LSTs in the two groups with lesions of different diameters were mainly adenomas; this was particularly evident (91.4%) in the group with lesions of diameter <20 mm. In the 268 cases of LST included in the present study, 23.9% were diagnosed as high-grade dysplasia or invasive cancer; of cases in the group with lesion diameter of ≥20 mm, 32% were diagnosed as high-grade dysplasia or invasive cancer, indicating that lesion diameter is closely correlated with cancerization of LST. This is supported by another finding of the present study: that in the group with lesions of diameter <20 mm (N = 93), only eight cases (approximately 8.6%) were high-grade dysplasia. Thus, differences were found in the location and appearance of LST lesions of different diameters and histological types. Moreover, the results of the present study indicate that the diameter, location, and morphological appearance of LST lesions are significantly correlated with malignant transformation. The larger the diameter of the lesion, the higher the risk of malignancy, which is most common in the rectum, with LST-G-M as the main morphological type. Thus, when the diameter of an LST is larger than 20 mm, particular care should be taken in diagnosis and preoperative evaluation.
In conclusion, differences are evident in the most common sites and growth patterns of LSTs of different diameters and histological types. In this study, LSTs with diameter ≥20 mm and LSTs in the cancerization group were most likely to occur in the rectum, with LST-G-M as the main morphological appearance. In addition, the cancerization rate of LSTs with diameter ≥20 mm was much higher than that of LSTs with diameter <20 mm. LST cancerization was closely correlated with the diameter, location, and morphological appearance of the lesion. The larger the diameter of the lesion, the greater the possibility of malignancy, and LSTs located in the rectum, most of which (in the present study) were LST-G-M, may be more malignant than those located elsewhere. These findings provide new understanding for the preoperative diagnosis and evaluation of LSTs. New breakthroughs in endoscopic technology, such as artificial intelligence (AI), may also support diagnosis; Zhou20 et al report using AI technology to help diagnose LST.
Limitations
The present study has some limitations. First, as a retrospective study (rather than a randomized controlled trial), it contains a risk of bias. Second, this was a single-center clinical study, including only a small number of samples. Future research should include a multi-center clinical study with increased sample size and should further explain the characteristics of LSTs with different diameters and histological types at the molecular and cellular levels.
Conclusion
LSTs with different diameters and histological types displayed differences in lesion location and appearance. The rectum was the most common site for LSTs of diameter ≥20 mm and for lesions with cancerization, with the morphology of such lesions mainly manifest as LST-G-M (the preferred treatment for this type of LST is ESD). The malignancy of LSTs was found to be correlated with lesion diameter, location, and morphological appearance.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article. Da-Huan Li and Xue-Ying Liu are co-first authors for this study.
Disclosure
The authors declare that they have no competing interests.
References
1. Kudo S. About laterally spreading tumor. Early Colorectal Cancer. 1998;2(5):477.
2. Zhu DL, He YS, Xiao BX, et al. Experience of endoscopic submucosal dissection in the treatment of lateral colorectal neoplasms. J Clin Digest Dis. 2016;28(2):115–117.
3. Rotondano G, Bianco MA, Buffoli F, Gizzi G, Tessari F, Cipolletta L. The cooperative Italian FLIN study group: prevalence and clinico-pathological features of colorectal laterally spreading tumors. Endoscopy. 2011;43(10):856–861. doi:10.1055/s-0030-1256639
4. Xu MD, Wang XY, Zhou PH, et al. Clinical and pathological study of endoscopic submucosal dissection in the treatment of different subtypes of colorectal lateral developmental tumors. Chin J Dig Endosc. 2012;29(8):422–428.
5. Kudo S, Lambert R, Allen JI, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68(4 Suppl):S3–S47. doi:10.1016/j.gie.2008.07.052
6. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi:10.1111/his.13975
7. Facciorusso A, Antonino M, Di Maso M, Barone M, Muscatiello N. Non-polypoid colorectal neoplasms: classification, therapy and follow-up. World J Gastroenterol. 2015;21(17):5149–5157. doi:10.3748/wjg.v21.i17.5149
8. Zhang YF. Progress in the diagnosis and endoscopic therapy of lateral colorectal neoplasms. Chin J Minim Invasive Surg. 2017;17:1117–1120.
9. Kobayashi K, Tanaka S, Murakami Y, et al. Predictors of invasive cancer of large laterally spreading colorectal tumors: a multicenter study in Japan Kiyonori Kobayashi. JGH Open. 2020;4(1):83–89. doi:10.1002/jgh3.12222
10. Russo P, Barbeiro S, Awadie H, Libânio D, Dinis-Ribeiro M, Bourke M. Management of colorectal laterally spreading tumors: a systematic review and meta-analysis. Endosc Int Open. 2019;7(2):E239–E259.
11. Son DJ, Kweon SS, Lee J, et al. Risk factors associated with clinical outcomes of endoscopic mucosal resection for colorectal laterally spreading tumors: a Honam Association for the Study of Intestinal Diseases (HASID) multicenter study. Turk J Gastroenterol. 2019;30(4):350–356. doi:10.5152/tjg.2019.18393
12. Uraoka T, Saito Y, Matsuda T, et al. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55(11):1592–1597. doi:10.1136/gut.2005.087452
13. Kudo T, Kudo SE, Wakamura K, et al. Diagnostic performance of endocytoscopy for evaluating the invasion depth of different morphological types of colorectal tumors. Dig Endosc. 2015;27(7):754–761. doi:10.1111/den.12469
14. Li SS, Wang F, Gu ZF. Pathological features and diagnosis and treatment of lateral colorectal neoplasms in elderly patients before and after surgical resection. J Pract Clin Med. 2017;21:184–185.
15. Saito T, Kobayashi K, Sada M, et al. Comparison of the histopathological characteristics of large colorectal laterally spreading tumors according to growth pattern. J Anus Rectum Colon. 2019;3(4):152–159. doi:10.23922/jarc.2018-036
16. Kim BC, Chang HJ, Han KS, et al. Clinicopathological differences of laterally spreading tumors of the colorectum according to gross appearance. Endoscopy. 2011;43(2):100–107. doi:10.1055/s-0030-1256027
17. Goto SP, Sakamoto N, Mitomi H, et al. Histological distinction between the granular and nongranular types of laterally spreading tumors of the colorectum. Gastroenterol Res Pract. 2014;2014:153935. doi:10.1155/2014/153935
18. Miyamoto H, Ikematsu H, Fujii S, et al. Clinicopathological differences of laterally spreading tumors arising in the colon and rectum. Int J Colorectal Dis. 2014;29(9):1069–1075. doi:10.1007/s00384-014-1931-x
19. Soliman H, Brieau B, Guillaumot MA, et al. Invasive pit pattern, macronodule and depression are predictive factors of submucosal invasion in colorectal laterally spreading tumours from a Western population. United European Gastroenterol J. 2018;6(10):1569–1577. doi:10.1177/2050640618804713
20. Zhou G, Xiao X, Tu M, et al. Computer aided detection for laterally spreading tumors and sessile serrated adenomas during colonoscopy. PLoS One. 2020;15(4):e0231880. doi:10.1371/journal.pone.0231880
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
