Back to Journals » Clinical Ophthalmology » Volume 15
Pars Plana Vitrectomy Outcomes for Rhegmatogenous Retinal Detachment Qualifying for Pneumatic Retinopexy
Authors Kurochkin P, Huang N, Petrela R, Rosenberg KI, Brown JS, Oellers P
Received 20 January 2021
Accepted for publication 24 February 2021
Published 19 March 2021 Volume 2021:15 Pages 1207—1214
DOI https://doi.org/10.2147/OPTH.S302413
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Philip Kurochkin, Natalie Huang, Redion Petrela, Kevin I Rosenberg, Jamin S Brown, Patrick Oellers
Retina-Vitreous Surgeons of CNY, Liverpool, NY, USA
Correspondence: Patrick Oellers
Retina-Vitreous Surgeons of CNY, 200 Greenfield Pkwy, Liverpool, NY, 13088, USA
Tel +1 315-445-8166
Email [email protected]
Purpose: To investigate real-world outcomes of pars plana vitrectomy (PPV) for eyes with primary rhegmatogenous retinal detachments (RRD) eligible for pneumatic retinopexy (PnR).
Methods: This was a single center retrospective case series looking at consecutive patients with primary RRDs. A database was created on all patients with a primary RRD from 2010 to 2018 based on billing code 67108. Eyes anatomically eligible for PnR were reviewed for preoperative, intraoperative and postoperative characteristics. The main outcome assessed was single surgery anatomical success (SSAS), final anatomical success (FAS), and postoperative LogMAR vision.
Results: A total of 720 eyes (age, 62.9 ± 9.1 years; 61.7% were male) met inclusion criteria for PnR and underwent PPV. SSAS was 94.0% and FAS was 99.9%. Preoperative and final LogMAR vision was 0.853 and 0.293 (P< 0.001) in eyes with SSAS vs 0.714 and 0.648 (P=0.686) in eyes with primary failure. PVR was the most common etiology of primary surgical failure (n=21, 49%). Patients who failed primary repair had a mean of 1.12 additional surgeries with a median time of 45 days between surgeries.
Conclusion: A robust single surgery success rate with good visual outcomes was achieved across 8 years and multiple surgeons utilizing PPV to treat primary RRDs in eyes which anatomically qualified for pneumatic retinopexy in a real-world setting.
Keywords: rhegmatogenous retinal detachment, pars plana vitrectomy, pneumatic retinopexy
Introduction
Rhegmatogenous retinal detachment (RRD) remains one of the leading causes of vision loss world-wide.1 The incidence of RRD has been reported to be 7.98–18.2 per 100,000 with higher rates in males and older patients.2–6 Despite advances in technology and surgical techniques, rates of vision loss remain high with 42% reaching 20/40 vision and only 28% of eyes with macular detachment.7 Patients with primary RRD require a secondary surgery approximately 10–40% of the time.8,9
There are various treatments for repair of RRD, which include pars plana vitrectomy (PPV), scleral buckle (SB), pneumatic retinopexy (PnR) and laser or cryopexy in select cases. Presenting features of RRD as well as surgeon preference drive decision making for treatment selection. Numerous reports indicate that PPV and SB may have better primary anatomical success than PnR. In contrast to PnR, PPV and SB however carry standard risks associated with anesthesia and require a readily available operating room. In addition, PPV is associated with worsening of cataract and SB can induce myopia among other potential side effects.10,11 In general terms, retinal detachments with superior break(s) within 1–2 clock hours and without significant media opacity may be eligible for PnR. In these RRDs, surgeon choice of PPV versus PnR remains controversial. Recently, a randomized prospective clinical trial named PIVOT reported favorable outcomes for PnR versus PPV.10 While there is abundant real-world data on PnR,12–16 knowledge about PPV outcomes for eyes that specifically meet anatomical PnR criteria is limited to the PIVOT trial. Prospective clinical trials are the gold-standard for research, yet results are not always reproduced in clinical practice.17 In order to help surgeons and patients make best informed decisions, it is important to elucidate real-world evidence of how PPV performs in this specific subset of RRDs. At our center, the vast majority of eyes that would be eligible for PnR based on anatomic criteria undergo PPV due to surgeon preference and operating room availability. Here, we present a single-institution, retrospective real-world dataset for PPV outcomes of eyes with RRD anatomically eligible for PnR.
Methods
This study is a retrospective consecutive case-series of patients with primary RRDs between 12/30/2010 −12/31/2018 at Retina-Vitreous Surgeons of Central New York, Liverpool, NY. Patients were identified by auditing billing common procedural terminology (CPT) code 67108. Institutional review board approval was obtained from Crouse Hospital in Syracuse, NY (IRB# 2019.0102). The study further adhered to the tenets of the Declaration of Helsinki.
A total of 8 fellowship trained vitreoretinal surgeons at a single private practice repaired all the retinal detachments. For vitrectomy, a standard 23- or 25-gauge setup with either the Alcon Constellation Vitrectomy System (Alcon, Fort Worth, TX) or DORC EVA (Dutch Ophthalmic Research Center, VN Zuidland, The Netherlands) was used and visualization was performed with a Zeiss Resight Wide Angle Viewing System (Zeiss, Dublin, CA). Use of endolaser, method of subretinal fluid drainage and tamponade agent was performed at the discretion of each surgeon.
All patient charts were reviewed. The study included patients that met the following inclusion criteria of primary ipsilateral RRD and PnR criteria as previously described by the PIVOT study investigators: a single break or group of breaks smaller than 1 clock hour in the detached retina, all breaks located in the superior retina above the 8 and 4 o’clock meridian and breaks in attached retina in any location.10 Exclusion criteria were evidence of significant media opacity with inability for a complete retinal examination, inferior breaks within the detached retina, proliferative vitreoretinopathy (PVR) grade B or worse as well as prior RRD in the ipsilateral eye.
Data was collected on conventional demographics (age, gender, sex), best-corrected visual acuity (BCVA) (pre-/postoperative), lens status (pre-/postoperative), clock hours localizing RRD, number of retinal tears, macular attachment status, PVR (pre-/postoperative), macular hole (MH) (pre-/postoperative). Surgical variables recorded included gauge used for PPV, adjuvant SB, and type of gas or oil used during surgery. Any additional surgical procedures required to achieve anatomical success as well as time elapsed between primary and secondary repair were recorded. Postoperative variables recorded were presence of cystoid macular edema, vitreous hemorrhage, increased intraocular pressure (defined as pressure above 25 mmHg, within 30 days of surgery), hypotony (defined as pressure below 7 within one day of surgery), postoperative epiretinal membrane and postoperative corneal failure.
Logarithm of the Minimum Angle of Resolution (LogMAR) conversion was performed as previously described.18 Primary outcomes were single surgery success (SSAS; defined as retinal attachment achieved at final follow up with a single surgery) and final anatomical success (FAS; defined as retinal attachment achieved at final follow up irrespective of number of surgeries). Secondary outcomes were BCVA at final follow up in LogMAR as well as BCVA change in LogMAR between preoperative visit and last follow up.
Baseline characteristics (age and gender) were summarized descriptively using mean and standard deviations. Preoperative findings and postoperative findings were analyzed using statistical hypothesis tests for corresponding categories relating to SSAS or SSAF. A chi-square or a Fisher exact test was used, with a Freeman-Halton extension when appropriate. LogMAR measurement averages were individually analyzed for statistical significance using Standard T-test for categories of SSAS or SSAF.
Results
A total of 1797 consecutive charts were reviewed within this study, of which 1607 with sufficient data were analyzed. Of those, 720 eyes were anatomically eligible for PnR (44.8%). The gender distribution was 61.7% male and 38.3% female. Mean age and standard deviation (SD) of patients was 62.2 ± 10.1, 63.3 ± 8.6 and 62.9 ± 9.1 for females, males and total patients respectively in patients meeting PnR criteria. Four-hundred and thirty-nine (61.0%) eyes were phakic, 279 (38.8%) pseudophakic and 2 (0.3%) were aphakic. Lattice degeneration was observed in 194 (26.9%) eyes. Most eyes had a single retinal tear 621 (86.3%) eyes. A macula on detachment was present in 427 (59.3%) eyes and a macula off detachment in 293 (40.7%) eyes (Table 1).
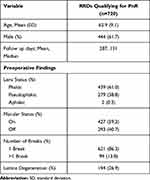 |
Table 1 Baseline Preoperative Characteristics |
SSAS was seen in 95.3%, 93.2%, among females, males, respectively and 94.0% overall (P=0.260) in patients qualifying for PnR. Final anatomical surgical success (FAS) was seen in 99.9%. Among all patients qualifying for PnR, LogMAR BCVA preoperative and postoperative vision was 0.845 and 0.314 respectively with a delta of 0.531 between preoperative to final vision (P<0.001) (Table 2).
 |
Table 2 Postoperative Outcomes |
Preoperative and postoperative LogMAR in eyes with SSAS were 0.853 and 0.293 respectively with a delta of 0.574 (P<0.001). In eyes with SSAF, preoperative LogMAR was 0.714 with an improvement to 0.648 at final postoperative vision, and the delta did not reach statistical significance (delta of 0.066; P=0.686). There was a significant difference in postoperative LogMAR (P=0.006) and LogMAR delta (P=0.005) between eyes with SSAS and SSAF. In terms of Snellen visual acuity, final BCVA was 20/40 or better in 70.3%, 71.5% and 51.2% of patients in all eyes, SSAS group, the SSAF group, respectively (P<0.001). BCVA was 20/200 or worse in 7.2%, 6.2% and 23.3% of patients in all eyes, SSAS and the SSAF groups, respectively (P<0.001) (Table 3).
 |
Table 3 Preoperative and Postoperative Visual Acuity in Patients with Single Surgery Anatomical Success and Failure |
No preoperative variable had a significant effect on primary surgical success. Preoperative lens status in patients who achieved SASS were as follows; 413 (61.0%) phakic eyes, 262 (38.7%) pseudophakic eyes and 1 (0.3%) aphakic eyes, in patients with SSAF there were 26 (60.5%) phakic eyes, 17 (39.5%) pseudophakic eyes, and 0 (0.0%) aphakic eyes (P=0.925 phakic vs pseudophakic). A macula on detachment was present in 400 cases (59.1%) with SSAS and 27 (62.8%) cases with SSAF (P=0.631). The number of observed tears and lattice did not have a significant difference between cases with SSAS and SSAF (P=0.106 and P=0.118, respectively); (Table 4).
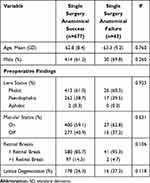 |
Table 4 Preoperative Characteristics in Patients with Single Surgery Anatomical Success and Failure |
Notable surgical characteristics were tamponade agent and adjuvant SB. The PPV tamponade agents used in surgery were distributed as follows, 584 eyes (81.6%) had sulfur hexafluoride gas (SF6), 116 (16.2%) had octafluoropropane gas (C3F8), 14 (2.0%) had ambient air and 2 (0.3%) had silicone oil (SO). Eyes with air or SO had SSAS in all cases; and eyes that had SF6 and C3F8 had 94.7% and 89.7% SSAS, respectively (P=0.039). Use of an adjuvant SB was generally low with 4 cases (0.6%) in the SSAS group compared to 2 cases (4.7%) in the SSAF group (P=0.045), as seen in Table 5.
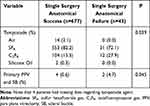 |
Table 5 Intraoperative Characteristics in Patients with Single Surgery Anatomical Success and Failure |
Several postoperative findings were found to be significantly associated with primary surgical failure. Postoperative vitreous hemorrhage was present in 13 eyes (1.8%) and showed no association to SSAS (P=0.554). Cystoid macular edema (CME) was seen in 40 (5.9%) eyes with SSAS and in 9 (20.9%) eyes with SSAF (P<0.001). PVR was overall the most common etiology for primary surgical failure. PVR was present in 2 (0.3%) eyes in the SSAS group and 21 (48.8%) eyes in the SSAF group (P<0.001). Postoperative epiretinal membrane (ERM) was present in 103 (15.2%) eyes with SSAS and 12 (27.9%) eyes with SSAF (P=0.028), (Table 6).
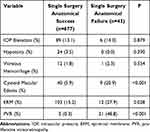 |
Table 6 Postoperative Characteristics in Patients with Single Surgery Anatomical Success and Failure |
In general, few secondary surgeries were performed to achieve final anatomical success in eyes with single surgery failure. Of the 43 eyes with SSAF, a mean number of 1.12 additional surgeries was performed, and 42 had an attached retina at final follow up. One patient did elect not to pursue further intervention despite physician recommendation for additional surgery. The mean number of days until secondary surgery was 94 days and the median was 45 days. Of all secondary surgeries, 16 (37.2%) were carried out with PPV/SB, 3 (7.0%) had a lensectomy, 20 (46.5%) had a membrane peel, and 9 (20.9%) utilized SO for tamponade (Table 7).
 |
Table 7 Surgical Characteristics of Patients Who Did Not Have Primary Surgical Success |
Discussion
Retinal detachments can be repaired by a wide array of in-office procedures or surgical techniques. Pneumatic retinopexy is a viable in-office treatment option for RRD in instances where anatomical criteria met, yet maybe associated with limited primary surgical success compared to vitrectomy and scleral buckle.10 Knowledge of real-world outcomes of PPV specifically for RRDs that meet anatomical criteria for PnR is missing in the current literature, yet is crucial to facilitate best practices and surgical decision making for vitreoretinal specialists and patients alike. Here we report favorable outcomes on 720 eyes that underwent PPV and qualified for PnR based on anatomical criteria.
Primary anatomical success rates of PPV range from 78% to 97% in the literature.6,18–22 Recently, the PIVOT trial prospectively investigated PnR versus PPV in retinal detachment with the same anatomical criteria applied in this study. The PIVOT study reported primary anatomical success rate for PPV of 93.2%; versus 80.8% in PnR. In this regard, it may be important to understand that prospective trials carry selection bias and that outcomes often fare better compared to the real-world.17 Indeed, retrospective data indicate that real-world outcomes of PnR may be diverse with primary anatomical success rates reported between 54–87%.12–15 A recent retrospective study looked at patients in a real-world setting who qualified for the PIVOT criteria and found a primary success rate of 76.4% in a patients that received PnR.16 It is notable in this regard that our study affirms a high degree of anatomical success of PPV in PnR eligible RRD in a real-world setting with a primary anatomical success rate of 94.0%; very similar to data reported in the PIVOT trial for PPV.
Given this, it is important to assess the impact of primary surgical failure of RRD repair by PnR. About 20% of patients who present with an initial macula on detachment develop a macula off detachment after a failed PnR.23,24 Eyes which fail primary PnR possess 20–25% of recurrent failure rate with secondary treatment (PPV, PnR, or PPV with SB).23,25 Patients who underwent primary PnR and required subsequent procedures typically have worse visual outcomes compared to those with primary success.14,25 In the authors’ opinion, it is important to weigh the impact and associated morbidity of the subsequent RD repair surgeries. As 20–25% of secondary repair after initial PnR failure are predicted to fail, potential for and impact of secondary and tertiary repair need to be considered prior to selecting this procedure.23,25 We hypothesize that absence of vitreous and reduced vitreous traction after a PPV may result in less impetus for development of severe postoperative PVR compared to failed cases of PnR with abundant compressed vitreous and potential vitreous traction. Highlighting this, our study indicates low morbidity for eyes that have failed initial PPV. Mean number of additional surgeries required to achieve final anatomic success was 1.12; with few eyes requiring more definitive procedures such as adjuvant scleral buckle or lensectomy. However, we believe that the best chance to repair a retinal detachment is the first surgery. Underscoring that, our study revealed statistically significant visual improvement only in eyes with primary success. In terms of morbidity of the primary repair in this study, single surgery success was achieved with PPV and short acting SF6 gas in most eyes, which is an acceptable intervention for most patients and surgeons.
Analysis of preoperative patient characteristics demonstrated no statistically significant impact on anatomical success. We found that preoperative macular status and lens status did not impact surgical outcomes, which is consistent with prior reports.19,22,26 Interestingly, there was no significant impact by the number of preoperatively identified retinal breaks or presence of lattice degeneration on single surgery success. Patients who underwent PPV with C3F8 gas had worse outcomes than those that used SF6. One explanation we attribute this observation to is that more complex RRDs often require the use of C3F8, which could lead to bias in this population. Surgical procedures that warranted the use of air or SO were all completed successfully, however were uncommon in this dataset which may have led to this discrepancy.
Analysis of postoperative variables revealed a number of findings that were associated with primary surgical failure. Amongst these findings, PVR was strongly associated with higher rates of failed surgical intervention; approximately 50% of patients who had recurring retinal detachment had developed PVR. This finding is consistent with reports of many other studies.27–29 We observed that postoperative CME also developed significantly more often in cases with primary failure. ERM formation was the most common postoperative finding overall and was more frequently observed in failed surgeries. Development of ERM and PVR to have been linked to recurrence of RRD and may represent a similar pathophysiological mechanism.29 Previous reports have observed similar correlations with recurrent PVR in setting of concurrent ERM and CME.30
Research studies have reported non-inferiority between PPV and PPV with SB.8,20 Our institution utilizes adjuvant SB mainly for complex RRD repairs. We observed that PPV compared to PPV with SB had worse surgical outcomes, but this is likely due to patient selection bias. Patient chart sampling did not include billing code 67113 (repair of complex retinal detachment with vitrectomy and membrane peeling), and a consideration of inclusion of both billing codes is warranted for follow-up studies.
Weaknesses of our study include its retrospective methodology, non-uniformity of surgeon approach and variable follow up, as well as lack of a PnR control group. Yet, the goal of this investigation was to provide real-world evidence of PPV in this subset of RRDs, and as such we provide a large dataset that would be difficult to collect in another type of research setting. Our study was inclusive of our entire patient population and did not exclude patients with ocular pathology or systemic comorbidities that could potentially limit their final outcomes. This study did specifically not assess primary scleral buckle, which is a different procedural code and as such was not captured in the chart review. Primary scleral buckle in our practice is mainly utilized for RRD without posterior vitreous detachment or complex forms of RRD; but there may be select cases in which scleral buckle is applied for RRDs that could be repaired by PnR alternatively. A further weakness of this study is that we were not able to collect data on secondary visual function such as metamorphopsia, and we believe that future research in this area is warranted. The study followed criteria of the PIVOT trial, and as such certain complex patients who by many physicians outside of a clinical trial context would not have been offered pneumatic retinopexy were included into the analysis. This, for example, includes patients who were aphakic or who received silicone oil tamponade. We feel that this approach avoids any potential bias and renders our results stronger and more applicable to any real-world setting.
In summary, we present real-world evidence of RRDs which underwent PPV and anatomically qualified for PnR per the PIVOT criteria. A robust single surgery success rate of 94% was achieved across 8 years and multiple surgeons utilizing PPV with overall low procedure associated morbidity. Primary success in this study was associated with better visual outcomes. The most defining feature for surgeons to select retinal detachment repair techniques may be personal experience and each procedure has its own advantages. Compared to PnR, PPV requires specialized training, access to resources, and has higher rates of cataract induction. However, given the potential for high rates of primary success, vitrectomy should continue to play a major role for RRDs that are eligible for PnR based on anatomic criteria.
Ethical Approval
Ethical Approval was obtained from Crouse Hospital in Syracuse, NY Review Board approval number IRB 2019.0102. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)- compliant manner.
Acknowledgments
This paper was presented at the Retina Society Virtual Meeting, 2020.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Disclosure
PO is a consultant for Allergan, Genentech; speaker for Novartis. All other authors declare no conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye. 2002;16(4):411–421. doi:10.1038/sj.eye.6700197
2. Li X. Incidence and epidemiological characteristics of rhegmatogenous retinal detachment in Beijing, China. Ophthalmology. 2003;110(12):2413–2417. doi:10.1016/S0161-6420(03)00867-4
3. Mitry D, Charteris DG, Yorston D, et al. The epidemiology and socioeconomic associations of retinal detachment in Scotland: a two-year prospective population-based study. Investig Ophthalmol Vis Sci. 2010;51(10):4963–4968. doi:10.1167/iovs.10-5400
4. Van De Put MAJ, Hooymans JMM, Los LI. The incidence of rhegmatogenous retinal detachment in the Netherlands. Ophthalmology. 2013;120(3):616–622. doi:10.1016/j.ophtha.2012.09.001
5. Wilkes SR, Beard CM, Kurland LT, Robertson DM, O’Fallon WM. The incidence of retinal detachment in Rochester, Minnesota, 1970–1978. Am J Ophthalmol. 1982;94(5):670–673. doi:10.1016/0002-9394(82)90013-7
6. Wong TY, Tielsch JM, Schein OD. Racial difference in the incidence of retinal detachment in Singapore. Arch Ophthalmol. 1999;117(3):379–383. doi:10.1001/archopht.117.3.379
7. Pastor JC, Fernández I, Rodríguez de la Rúa E, et al. Surgical outcomes for primary rhegmatogenous retinal detachments in phakic and pseudophakic patients: the Retina 1 Project-report 2 Clinical science. Br J Ophthalmol. 2008;92:378–382. Libr Prot by Copyr. doi:10.1136/bjo.2007.129437
8. Lindsell LB, Sisk RA, Miller DM, et al. Comparison of outcomes: scleral buckling and pars plana vitrectomy versus vitrectomy alone for primary repair of rhegmatogenous retinal detachment. Clin Ophthalmol. 2017;11:47–54. doi:10.2147/OPTH.S112190
9. Wickham L, Ho-Yen GO, Bunce C, Wong D, Charteris DG. Surgical failure following primary retinal detachment surgery by vitrectomy: risk factors and functional outcomes. Br J Ophthalmol. 2011;95(9):1234–1238. doi:10.1136/bjo.2010.190306
10. Hillier RJ, Felfeli T, Berger AR, et al. The pneumatic retinopexy versus vitrectomy for the management of primary rhegmatogenous retinal detachment outcomes randomized trial (PIVOT). Ophthalmology. 2019;126(4):531–539. doi:10.1016/j.ophtha.2018.11.014
11. Han DP, Mohsin NC, Guse CE, Hartz A, Tarkanian CN. Comparison of pneumatic retinopexy and scleral buckling in the management of primary rhegmatogenous retinal detachment. Am J Ophthalmol. 1998;126(5):658–668. doi:10.1016/S0002-9394(98)00181-0
12. Zaidi AA, Alvarado R, Irvine A. Pneumatic retinopexy: success rate and complications. Br J Ophthalmol. 2006;90(4):427–428. doi:10.1136/bjo.2005.075515
13. Abecia E, Pinilla I, Olivan JM, Larrosa JM, Polo V, Honrubia FM. Anatomic results and complications in a long-term follow-up of pneumatic retinopexy cases. Retina. 2000;20(2):156–161. doi:10.1097/00006982-200002000-00008
14. Fabian ID, Kinori M, Efrati M, et al. Pneumatic retinopexy for the repair of primary rhegmatogenous retinal detachment: a 10-year retrospective analysis. JAMA Ophthalmol. 2013;131(2):166–171. doi:10.1001/2013.jamaophthalmol.361
15. Seabg J, Tang M. Pneumatic retinopexy using only AIR. Retina. 1993;13:8–12. doi:10.1097/00006982-199313010-00003
16. Juncal VR, Bamakrid M, Jin S, et al. Pneumatic retinopexy in patients with primary rhegmatogenous retinal detachment meeting ‘PIVOT’ trial criteria: real-world results. Ophthalmol Retin. 2020. doi:10.1016/j.oret.2020.07.022
17. Zarbin M. Real life outcomes vs. clinical trial results. J Ophthalmic Vis Res. 2019;14(1):88–92. doi:10.4103/jovr.jovr_279_18
18. Jackson TL, Donachie PHJ, Sallam A, Sparrow JM, Johnston RL. United Kingdom national ophthalmology database study of vitreoretinal surgery: report 3, retinal detachment. Ophthalmology. 2014;121(3):643–648. doi:10.1016/j.ophtha.2013.07.015
19. Kobashi H, Takano M, Yanagita T, et al. Scleral buckling and pars plana vitrectomy for rhegmatogenous retinal detachment: an analysis of 542 eyes. Curr Eye Res. 2014;39(2):204–211. doi:10.3109/02713683.2013.838270
20. Orlin A, Hewing NJ, Nissen M, et al. Pars plana vitrectomy compared with pars plana vitrectomy combined with scleral buckle in the primary management of noncomplex rhegmatogenous retinal detachment. Retina. 2014;34(6):1069–1075. doi:10.1097/IAE.0000000000000050
21. Mitry D, Awan MA, Borooah S, et al. Surgical outcome and risk stratification for primary retinal detachment repair: results from the Scottish Retinal detachment study. Br J Ophthalmol. 2012;96(5):730–734. doi:10.1136/bjophthalmol-2011-300581
22. Mohamed Y, Ono K, Kinoshita H, et al. Success rates of vitrectomy in treatment of rhegmatogenous retinal detachment. J Ophthalmol. 2016;2016:1–9. doi:10.1155/2016/2193518
23. Anaya JA, Shah CP, Heier JS, Morley MG. Outcomes after failed pneumatic retinopexy for retinal detachment. Ophthalmology. 2016;123(5):1137–1142. doi:10.1016/j.ophtha.2016.01.017
24. Emami-Naeini P, Deaner J, Ali FS, et al. Pneumatic retinopexy experience and outcomes of vitreoretinal fellows in the United States: a multicenter study. Ophthalmol Retin. 2019;3(2):140–145. doi:10.1016/j.oret.2018.09.010
25. Vidne-Hay O, Abumanhal M, Elkader AA, Fogel M, Moisseiev J, Moisseiev E. Outcomes of rhegmatogenous retinal detachment repair after failed pneumatic retinopexy. Retina. 2019:1.
26. Wong CW, Wong WL, Yeo IYS, et al. Trends and factors related to outcomes for primary rhegmatogenous retinal detachment surgery in a large Asian tertiary eye center. Retina. 2014;34(4):684–692. doi:10.1097/IAE.0b013e3182a48900
27. Cowley M, Conway BP, Campochiaro PA, Kaiser D, Gaskin H. Clinical risk factors for proliferative vitreoretinopathy. Arch Ophthalmol. 1989;107(8):1147–1151. doi:10.1001/archopht.1989.01070020213027
28. Ambiya V, Rani PK, Narayanan R, et al. Outcomes of recurrent retinal detachment surgery following Pars Plana vitrectomy for rhegmatogenous retinal detachment. Semin Ophthalmol. 2018;33(5):657–663. doi:10.1080/08820538.2017.1395893
29. Rachal WF, Burton TC. Changing concepts of failures after retinal detachment surgery. Arch Ophthalmol. 1979;97(3):480–483. doi:10.1001/archopht.1979.01020010230008
30. Xu K, Chin EK, Parke DW
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
