Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Palliative whole-brain radiotherapy and health-related quality of life for patients with brain metastasis in cancer
Authors Gao Y, Gao F, Ma J, Zhao D
Received 22 April 2015
Accepted for publication 30 June 2015
Published 21 August 2015 Volume 2015:11 Pages 2185—2190
DOI https://doi.org/10.2147/NDT.S87109
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Ying Gao,1,* Fei Gao,2,* Jin-lu Ma,1 Dong-li Zhao1
1Department of Radiotherapy Oncology, First Affiliated Hospital of Xi’an Jiaotong University, 2Department of Neurology, First Affiliated Hospital of Xi’an Medical University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Objective: To assess the use of palliative whole-brain radiotherapy (WBRT) in the treatment of brain metastases (BMs) and to evaluate the health-related quality of life (HRQOL) of these patients.
Materials and methods: We conducted a retrospective study of 46 patients with BMs who were treated with WBRT at the First Affiliated Hospital of Xi’an Jiaotong University between January 2013 and January 2015. External beam radiotherapy techniques were used to deliver 40 Gy in 20 fractions or 30 Gy in ten fractions with a 10 MV photon beam from a linear accelerator to the whole brain. Data were stored and analyzed using SPSS version 17.0.
Results: Of the 46 patients, the survival time of patients in our study was 10.8±0.55 months: 11.8±0.46 months in patients with WBRT, 11.75±1.00 in patients with WBRT + chemotherapy, and 3±0.79 months in patients with supportive care, respectively (P<0.01). The HRQOL scores of all the patients were 70±1.16 (before therapy) and 76.83±1.04 (after therapy) (P<0.01). The HRQOL scores of the patients with WBRT were 72.23±0.88 (before therapy) and 78.49±0.87 (after therapy) (P<0.01). There was no central nervous system toxicity; only two (4.3%) patients were found to have BM hemorrhage. Radiation necrosis happened in one patient (2.2%).
Conclusion: Effective treatment options for patients with BMs are important. WBRT was evaluated to ensure survival outcomes and QOL were enhanced after therapy for patients with BMs.
Keywords: brain metastases, whole-brain radiotherapy, health-related quality of life
Introduction
Brain metastases (BMs) may cause debilitating neurological symptoms, and slight tumor growth can be fatal.1 Most patients have presenting symptoms, such as changes in mental status, headache, nausea, and vomiting, with signs of increased intracranial pressure, or focal signs, such as seizures, hemiparesis, aphasia, ataxia, and visual field defects, although some are asymptomatic.1,2–6 The brain is sheltered from improved systemic therapy behind the blood–brain barrier, as it remains a sanctuary for neoplastic cells.7,8 BMs usually occur late in the course of a patient’s cancer, when they are widely disseminated. Up to half of patients die of intracranial progression, although many die of systemic disease.9 The prognosis for patients with BMs is poor, and whole-brain radiotherapy (WBRT) is the standard therapy in clinical practice guidelines for the management of BM;9–12 it can palliate neurological symptoms and control the local disease.
In addition, systemic chemotherapy (CT) has been used to reduce tumor burden in patients with BM originating from non-small-cell lung cancer (NSCLC). However, the role of CT concurrent with WBRT for the treatment of patients with BM originating from NSCLC is controversial. Some researchers have failed to confirm the efficacy of CT, and suggest that WBRT with concurrent CT increases the incidence of adverse events; also, the treatment’s effectiveness is limited in NSCLC patients with BM, due to the blood–brain barrier.2,6 But others have indicated that chemical drugs can infiltrate the brain tissue, while radiation destroys the blood–brain barrier. Several clinical trials have showed that WBRT combined with CT is not only more effective than WBRT alone but also improves the response rate and prolongs survival.1,6,13 The choice of treatment modalities depends on the location and number of BMs, metastatic disease in other organs, the status of the primary tumor site, and the performance status of patients. The aim of treating metastases in most cases is not to destroy cancer cells, but to achieve appropriate symptom relief and assure good quality of life (QOL).14–16 In treating patients with BMs, preservation of cognitive function and QOL are important, since survival is limited. However, in terms of neurocognitive function and QOL, the efficacy of WBRT has not been a focus of previous reports and remains unclear. Therefore, we conducted a study to assess the efficacy and safety of therapy and evaluate health-related QOL (HRQOL) of patients with BMs.
Materials and methods
Patients
The study was a retrospective analysis. Between January 2013 and January 2015, 46 patients with BMs were treated with WBRT at the First Affiliated Hospital of Xi’an Jiaotong University. The present study was approved by the ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University. All patients gave signed informed consent for WBRT.
Whole-brain radiotherapy
External beam radiotherapy (RT) techniques were used to deliver 40 Gy in 20 fractions or 30 Gy in ten fractions with a 10 MV photon beam from a linear accelerator to the whole brain. WBRT was interrupted if the white blood cell count fell below 1,000/mm3 or if platelets fell below 50,000/mm3, and was resumed once counts rose above those levels.
Health-related quality of life
To assess HRQOL, four subscales from the Functional Assessment of Cancer Therapy – general (FACT-G) were utilized:17 the physical well-being (seven items), social/family well-being (seven items), functional well-being (seven items), and emotional well-being (six items) subscales. Items are rated from 0 (not at all) to 4 (very much), with a higher score indicating better HRQOL.
Statistics
Data were stored and analyzed using SPSS version 17.0. Cronbach’s α-coefficient was used to test the internal consistency of items and domains of FACT-G. A one-way analysis of variance and Student’s t-test were used to compare survival time between defined patient groups. The association between HRQOL and patient characteristics was evaluated using paired t-tests. P<0.05 was considered significant for all statistical analyses.
Results
Patient characteristics
In these patients, diagnostic procedures included clinical examination, cerebral computed tomography/magnetic resonance imaging was performed if indicated due to the symptoms present. In the analyzed group of 46 patients, symptoms were muscle weakness in 17 (37%) patients, headache in 15 (32.6%), and loss of consciousness in two (4.3%). Among the patients, 35 (76.1%) were treated with WBRT, six (13%) with WBRT + CT, and five (10.9%) with supportive care (SC) alone. Before this decision, two physicians of our department discussed with patients the risks and benefits of the treatment methods. The studied group of 46 patients consisted of 20 (43.5%) females and 26 (56.5%) males. Patient’s age ranged from 32 to 70 years, with a mean age of 52.6 years. NSCLC was found in 32 (69.6%), breast carcinoma in eight (17.4%), and rectum cancer in six (13%). The characteristics of all these patients are shown in Table 1. Before the diagnosis of BMs, all patients had received therapy, including surgery in 18 (39.1%), RT in 40 (87%), and chemoradiotherapy in 37 (80.4%). The mean time from BM diagnosis to therapy initiation was 13.5 months.
  | Table 1 Prognostic factors for survival |
Clinical outcomes
The survival time of patients in our study was 10.8±0.55 months. Survival times were 11.8±0.46 months in patients with WBRT, 11.75±1.00 months in patients with WBRT + CT, and 3±0.79 months in patients with SC (P<0.01). In addition, there were significant associations between survival and metastasis to other organs (P<0.01). However, no significant differences were found among survival time with Karnofsky Performance Status (KPS), sex, number of BMs, primary cancer site, age, or larger tumor size, as shown in Table 1.
In no analyzed patients was there any central nervous system toxicity; only two (4.3%) patients were found to have BM hemorrhage. Radiation necrosis happened in one patient (2.2%).
HRQOL
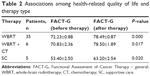
As listed in Table 2, the results identified therapy significant mediating effects on HRQOL. The HRQOL scores of all the patients were 70±1.16 (before therapy) and 76.83±1.04 (after therapy) (P<0.01). The HRQOL scores of patients with WBRT were 72.23±0.88 (before therapy) and 78.49±0.87 (after therapy) (P<0.01). Mean scores with WBRT + CT were 70.83±2.36 (before therapy) and 78.50±1.89 (after therapy) (P=0.017). HRQOL scores of patients with SC were 53.40±2.50 before therapy and 63.20±2.54 after therapy (P=0.017).
Discussion
BMs are the most common neurological complication of cancer.7 Most patients with cancer die from metastasis to vital organs or other complications, but not from the primary lesion. Therefore, the management of BMs is a health care challenge. The goal of WBRT is to alleviate neurological symptoms or prevent progression, neurological deterioration, and enhance or maintain QOL, in addition to offering a possible survival benefit by controlling intracranial metastases.2,8 However, it has been questioned whether WBRT is indicated in every case where the prognosis in patients of NSCLC with multiple BMs is very poor.18
Analyses of prognostic factors have endeavored to identify which patients would probably benefit from WBRT and which patients would not.19,20 However, it remains to be seen which patients can be managed safely and palliated without WBRT.20 In this study, the patients who received WBRT after the diagnosis of BM lived 11.8 months and achieved an appropriate relief of symptoms. Therefore, WBRT assured good QOL and enhanced HRQOL after therapy.14–16 However, the mean survival time of those with SC was only 3 months. This may suggest that the role of WBRT in the treatment of BM is important despite the fact that best SC is offered mainly to patients with KPS <50. The worst survival in best SC patients may be also explained by a bad PS of patients, which disqualifies them from RT, and the primary cancer site. However, neurological symptoms were controlled in patients who accepted the WBRT, and these patients also gained HRQOL and survival benefits, which implied that WBRT may still be a good choice for these patients with KPS <50. This is in contrast with a previous study that showed best SC can be used in patients in poor general condition if the expected survival is less than 3 months and the BMs are multiple and inoperable.21 The results were in accordance with a study that suggested KPS <70% will benefit more from WBRT compared to other types of BRT, regardless of the type of brain impairment.14 Our results also confirm clinical studies that have shown that palliative WBRT is effective with varying response rates in controlling neurological symptoms.2,9,10,22–25 A study has shown an improvement in symptoms in 64%–83% of patients after treatment with WBRT alone, and also demonstrated an increase in median overall survival from 1 month with no treatment to 3–7 months following WBRT.22 Sperduto et al showed that the addition of WBRT to radiosurgery improves local and distant brain control.23
Approximately 20%–40% of patients with cancer develop BMs during their disease course. Patients with solid tumors are at high risk for BM, such as in lung and breast cancer. In particular, it has been estimated that approximately 50% of primary lung cancers develop into BM.26 In this study, patients’ BM was from lung cancer, breast cancer, or melanoma. Among them, 70% of patients’ primary cancer site was the lungs. Furthermore, NSCLC accounts for a large percentage of lung cancer cases. It has also been estimated that 25%–30% of newly diagnosed NSCLC patients also suffer from BMs.27 It has been reported that NSCLC patients who develop BM often have poor prognoses, severe neurological symptoms, poor QOL, and dismal survival rates. The overall survival time for NSCLC patients with BM is less than 3–6 months when left untreated.28 However, the mean survival time was 10.8±0.55 months in our study, and QOL was enhanced. WBRT-induced tumor control has been correlated with better survival and improved neurocognitive function,29 which has been correlated with QOL.30,31 Therefore, effective treatment options for NSCLC patients with BM are important.
RT brings more possibilities for combined treatment. The role of CT and radiosensitizers in the treatment of BMs remains undefined, mostly because of the blood–brain barrier. WBRT can destroy the blood–brain barrier, and consequently facilitate the penetration of cytostatic drugs to the brain tumor.32,33 Research has shown that postoperative CT can also play an important role in lengthening survival. Patients who received CT after a diagnosis of BM lived longer than those without CT.34 In our study, we did not come to the same conclusion. When outcomes were compared between patients with WBRT and WBRT + CT, a marked advantage in survival could not be seen. Results did not reach statistical significance despite some patients with WBRT + CT living longer than those only with WBRT. As to survival benefit from WBRT, the response rates are encouraging, with definitive efficacy and safety of the approach.
The role of WBRT in the treatment sequence is in destroying micrometastases. The impact of WBRT on reducing the incidence of brain-tumor relapse has been demonstrated in randomized trials.14,34 The omission of WBRT results in a brain relapse risk of 70% versus 18% when WBRT is used.14,34 Metastases to other organs have a significant influence on overall survival rate. Patients who had only BMs had median survival of 12.7 months compared to those with dissemination to other organs, where median survival was 2.6 months (P=0.018).34 In a study by Han et al age, World Health Organization performance status, or the presence of metastases to organs other than the brain did not have a statistically significant influence on survival time.31 In numerous studies, KPS has emerged as a potent survival predictor in advanced-stage cancer patients.34,35–39 In gastrointestinal cancer, KPS was the only prognostic factor determining diagnosis-specific Graded Prognostic Assessment score.23,40 It has also been suggested that prognostic indexes are useful to guide tailored treatment strategies for cancer patients with BM and that the new Graded Prognostic Assessment is a valid prognostic index.40 In our study, the data were not similar to these findings. Only therapy methods and metastasis to other organs had significant associations with patients’ survival time. However, no significant difference was found among survival time with KPS, sex, number of BMs, primary cancer site, age, or larger tumor size. That the research did not gain the same conclusion may be related to the small numbers in this series. Therefore, further research is needed.
By definition, all patients with BM have stage IV disease, and treatment is palliative. Goals for the treatment of BM are alleviating neurological symptoms and preventing their progression and neurological deterioration, enhancing QOL. The limitations of this report are associated with any retrospective study, including potential referral bias, other types of selection bias, and a variety of treatments, doses, and schedules. In addition, a prospective randomized control trial comparing WBRT with various therapies (such as stereotactic radiosurgery) for BM should be considered. Our findings also suggest the need for larger studies to determine the role of WBRT in local control and to evaluate its impact on overall survival and HRQOL in advanced cancers with BM.
However, the findings of this study confirm our clinical impressions, and provide important information with which to move forward in developing better therapies for BM. Additionally, the current systemic therapy options are all associated with toxicities that are potentially detrimental to a patient’s overall QOL or well-being. Our findings point to survival benefit and enhanced HRQOL of BM after WBRT. They also imply that WBRT may be still a good choice for patients whose KPS <50. Our findings also suggest the need for larger studies looking at the role of WBRT + CT and its impact on BM.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81301937) and the International Cooperation Foundation of Shaanxi Province of China (2013KW-27-03).
Disclosure
The authors report no conflicts of interest in this work.
References
Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003;21:1–23. | ||
Qin H, Pan F, Li J, Zhang X, Liang H, Ruan Z. Whole brain radiotherapy plus concurrent chemotherapy in non-small cell lung cancer patients with brain metastases: a meta-analysis. PLoS One. 2014;9:e111475. | ||
Nguyen TD, DeAngelis LM. Brain metastases. Neurol Clin. 2007;25:1173–1192. | ||
Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78:1781–1788. | ||
Posner JB. Brain metastases: 1995. A brief review. J Neurooncol. 1996;27:287–293. | ||
Kong W, Jarvis CR, Sutton DS, Ding K, Mackillop WJ. The use of palliative whole brain radiotherapy in the management of brain metastases. Clin Oncol (R Coll Radiol). 2012;24:e149–e158. | ||
DeAngelis LM, Posner JB. Neurologic Complications of Cancer. New York: Oxford University Press; 2009. | ||
Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. | ||
Tsao MN, Lloyd N, Wong R, Chow E, Rakovitch E, Laperriere N. Whole brain radiotherapy for the treatment of multiple brain metastases. Cochrane Database Syst Rev. 2006:003869. | ||
Tsao MN, Lloyd NS, Wong RK, Rakovitch E, Chow E, Laperriere N. Radiotherapeutic management of brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2005;31:256–273. | ||
Expert Panel on Radiation Oncology-Brain Metastases, Videtic GM, Gaspar LE, et al. American College of Radiology appropriateness criteria on multiple brain metastases. Int J Radiat Oncol Biol Phys. 2009;75:961–965. | ||
Tsao MN, Lloyd NS, Wong RK. Clinical practice guideline on the optimal radiotherapeutic management of brain metastases. BMC Cancer. 2005;5:34. | ||
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. | ||
Kazda T, Pospíšil P, Doleželová H, Jančálek R, Slampa P. Whole brain radiotherapy: consequences for personalized medicine. Rep Pract Oncol Radiother. 2013;18:133–138. | ||
Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388–1395. | ||
Fernandez G, Pocinho R, Travancinha C, Netto E, Roldão M. Quality of life and radiotherapy in brain metastasis patients. Rep Pract Oncol Radiother. 2012;17:281–287. | ||
Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. | ||
Mulvenna PM. The management of brain metastases in patients with non-small cell lung cancer – is it time to go back to the drawing board? Clin Oncol (R Coll Radiol). 2010;22:365–373. | ||
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514. | ||
Nieder C, Pawinski A, Molls M. Prediction of short survival in patients with brain metastases based on three different scores: a role for ‘triple-negative’ status? Clin Oncol (R Coll Radiol). 2010;22:65–69. | ||
Tsao M, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. | ||
McTyre E, Scott J, Chinnaiyan P. Whole brain radiotherapy for brain metastasis. Surg Neurol Int. 2013;4 Suppl 4:S236–S244. | ||
Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. | ||
Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:1125–1131. | ||
Wong J, Hird A, Kirou-Mauro A, Napolskikh J, Chow E. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol. 2008;15:25–45. | ||
Seoane J, De Mattos-Arruda L. Brain metastasis: new opportunities to tackle therapeutic resistance. Mol Oncol. 2014;8:1120–1131. | ||
Berger LA, Riesenberg H, Bokemeyer C, Atanackovic D. CNS metastases in non-small-cell lung cancer: current role of EGFR-TKI therapy and future perspectives. Lung Cancer. 2013;80:242–248. | ||
Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile-diagnosis specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. | ||
Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–1266. | ||
Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70. | ||
Kraszkiewicz M, Wydmanski J. Brain metastases from stomach cancer – the role of different treatment modalities and efficacy of palliative radiotherapy. Rep Pract Oncol Radiother. 2014;20:32–37. | ||
Biswas G, Bhagwat R, Khurana R, Menon H, Prasad N, Parikh PM. Brain metastasis – evidence based management. J Cancer Res Ther. 2006;2:5–13. | ||
Addeo R, Caraglia M. Combining temozolomide with other antitumor drugs and target-based agents in the treatment of brain metastases: an unending quest or chasing a chimera? Expert Opin Investig Drugs. 2011;20:881–895. | ||
Han JH, Kim DG, Chung HT, et al. Radiosurgery for brain metastasis from advanced gastric cancer. Acta Neurochir (Wien). 2010;152:605–610. | ||
van Oorschot B, Assenbrunner B, Schuler M, Beckmann G, Flentje M. Survival and prognostic factors after moderately hypofractionated palliative thoracic radiotherapy for non-small cell lung cancer. Strahlenther Onkol. 2014;190:270–275. | ||
Rodrigues G, Macbeth F, Burmeister B, et al. International practice survey on palliative lung radiotherapy: third international consensus workshop on palliative radiotherapy and symptom control. Clin Lung Cancer. 2012;13:225–235. | ||
Gripp S, Mjartan S, Boelke E, Willers R. Palliative radiotherapy tailored to life expectancy in end-stage cancer patients: reality or myth? Cancer. 2010;116:3251–3256. | ||
Gripp S, Moeller S, Bölke E, et al. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol. 2007;25:3313–3320. | ||
Chow E, Abdolell M, Panzarella T, et al. Validation of a predictive model for survival in metastatic cancer patients attending an outpatient palliative radiotherapy clinic. Int J Radiat Oncol Biol Phys. 2009;73:280–287. | ||
Villà S, Weber C, Moretones C. Validation of the new Graded Prognostic Assessment scale for brain metastases: a multicenter prospective study. Radiat Oncol. 2011;6:23. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.