Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Paliperidone Compared with Haloperidol on the Theory of Mind Tasks in Schizophrenia: A Pilot Trial
Authors Zhong J, Zhu H, Yin D, Ning Y , Zheng S , Zhang Y, Jia H
Received 23 August 2021
Accepted for publication 22 November 2021
Published 14 December 2021 Volume 2021:17 Pages 3683—3691
DOI https://doi.org/10.2147/NDT.S335597
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Jie Zhong,1,2,* Hong Zhu,1,2,* Dongqing Yin,1,2 Yanzhe Ning,1,2 Sisi Zheng,1,2 Yanbo Zhang,3 Hongxiao Jia1,2
1The National Clinical Research Center for Mental Disorders & Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, People’s Republic of China; 3Department of Psychiatry, Faculty of Medicine, and Dentistry, College of Health Sciences, University of Alberta, Edmonton, Alberta, Canada
*These authors contributed equally to this work
Correspondence: Yanbo Zhang
Department of psychiatry, Faculty of Medicine, and Dentistry, College of Health Sciences, University of Alberta, Alberta, Edmonton, Canada
Email [email protected]
Hongxiao Jia
The National Clinical Research Center for Mental Disorders & Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, People’s Republic of China
Tel +86-010-58303065
Email [email protected]
Purpose: Theory of mind (ToM) is an important part of social cognitive function and is associated with medial prefrontal cortical (mPFC) activity. This study aimed to evaluate the efficacy of paliperidone in improving ToM task performance in patients with schizophrenia compared with haloperidol.
Patients and Methods: This study was a single-center, single-blinded (assessor), parallel-group randomized clinical trial of patients with schizophrenia randomized to paliperidone or haloperidol. ToM was assessed at weeks 0, 8, 12, and 16 using the first-order belief, higher-order belief, faux-pas, and Reading the Mind in the Eyes tests. The primary outcome was the change in the ToM performance scores from baseline to after 16 weeks of treatment.
Results: The participants received paliperidone (n = 29) or haloperidol (n = 31). For the first-order belief task, there were no between-group differences (P > 0.05) but time differences in both groups (P < 0.05). For the higher-order belief task, there were no between-group differences (P > 0.05), but there were time differences in both groups (P < 0.05) and a time×group interaction in the paliperidone group only (P < 0.05). For the faux-pas task, there was a difference between groups at week 16 (P < 0.05), and the improvement in time was significant for the paliperidone group only (P < 0.05). For the Reading the Mind in the Eyes task, there was an improvement over time for the paliperidone group only (P < 0.05). Safety was manageable in both groups.
Conclusion: Paliperidone treatment might be more effective than haloperidol in improving ToM task performance in schizophrenia.
Trial Registration: chictr.org.cn_identifier ChiCTR-IPR-15007635.
Keywords: schizophrenia, theory of mind, longitudinal study, paliperidone, haloperidol
Introduction
Social cognition refers to the mental processes used to detect, monitor, and analyze social signals from others and subsequently choose corresponding behavioral responses.1 Patients with schizophrenia have impairments in various domains of social cognition.2,3 An essential component of social cognition is the theory of mind (ToM)4–6 which refers to our ability to infer others’ beliefs, thoughts, and intentions and understand our own, allowing everyday social interaction, social intelligence, and social competence.7,8 ToM is impaired in schizophrenia.9–13 These “mentalizing” deficits have been observed in first- and higher-order false belief tasks.14,15
Patients with schizophrenia display decreased activation in the left middle and inferior prefrontal cortex (PFC) areas during tasks involving mental state attribution,16,17 and decreased activity in the posterior orbital and middle PFC during ToM tasks compared to patients with schizophrenia.18,19 There is good evidence for an abnormal hemodynamic response in the medial prefrontal cortex (MPFC) of patients with schizophrenia during ToM tasks.20 Decreased ventromedial prefrontal cortex (VMPFC) gray matter volume (GMV) was associated with poor ToM task abilities.21 Poor faux-pas performance in people with schizophrenia is correlated with gray matter reduction in the left orbitofrontal cortex and right temporal pole;22 poor performance on the Reading Mind in the Eyes Test was found to be associated with gray matter reduction in the left ventrolateral prefrontal cortex (VLPFC).23
Several studies investigated the effect of medications specifically on ToM. Savina et al24 found that patients with schizophrenia treated with the atypical antipsychotics clozapine and olanzapine performed similarly to healthy subjects on ToM tasks, but treatment with typical antipsychotics and risperidone did not significantly improve ToM. In a small randomized controlled study, Pedersen et al25 reported that adjunctive intranasal oxytocin reduced psychotic symptoms and improved ToM and social perception in schizophrenia. Therapies that effectively improve ToM impairment might provide clinical benefits in the treatment of schizophrenia.26
The effects of some atypical antipsychotics on ToM have been studied extensively.27 Paliperidone, another atypical antipsychotic,28,29 has been investigated in a recent study that showed that social cognition and interaction training combined with paliperidone had the potential to improve cognition functions in early-onset schizophrenia.30 Previous studies demonstrated that paliperidone has a protective effect on prefrontal cortical neurons,28,29 closely related to ToM abilities.31 Therefore, it could be hypothesized that paliperidone might also demonstrate clinical benefits on ToM ability in patients with schizophrenia. Hence, this study aimed to evaluate the efficacy of paliperidone in improving ToM task performance in patients with schizophrenia compared with haloperidol.
Materials and Methods
Study Design and Participants
This study was a single-center, single-blinded (assessor), parallel-group randomized clinical trial. It was conducted in accordance with the Declaration of Helsinki and approved by Beijing Anding Hospital Ethics Committee (No. 2013 LSN No. (4)). All patients provided written informed consent. Trial Registration: chictr.org.cn_identifier ChiCTR-IPR-15007635.
Outpatients diagnosed with schizophrenia at Beijing Anding Hospital (Capital Medical University) according to the DSM-IV32 criteria were enrolled between January 2016 and March 2017. The inclusion criteria were 1) 18–45 years of age, 2) schizophrenia history of ≤10 years, 3) able to understand and comply with the requirements of the study, 4) female patients of childbearing age had to use reliable contraception and have a negative urine human chorionic gonadotropin (HCG) test at enrolment, and 5) scored no more than three of the four items of the 18-item version of the Brief Psychiatric Rating Scale BPRS (Conceptual Disorganization, Suspiciousness, Hallucinatory Behaviour, Unusual Thought Content),33 and scored no more than four on the CGI-S33,34 at the preliminary screening.
The exclusion criteria included mental retardation, drug/alcohol abuse within 4 weeks before enrolment, history of neurological disorder or organic brain disease, history of head injury with loss of consciousness, or presence of a co-morbid psychiatric disorder. Patients were excluded if they had suicidal tendencies or risk of hurting other people or were intolerant or unresponsive to paliperidone or haloperidol. Patients with catatonic schizophrenia were excluded. If any, modified electroconvulsive treatment (MECT) was discontinued within 3 months before enrolling in this study. Patients who had poor eyesight or hearing were also excluded.
The patients were admitted to the hospital due to the fluctuation of their condition. The patients did not receive antipsychotic treatment for ≥1 month before enrollment. Since the participants were able to cooperate with cognitive measurement and had no risk of suicide or impulsive injury, they were not required to be hospitalized for compulsory treatment. Moreover, a long hospitalization would have a certain impact on social cognition, so they received follow-up treatment in the outpatient department. The study was conducted in an outpatient clinic. After the patients were enrolled, they could not receive strategic and systematic psychological therapy or other rehabilitation training and could not receive physical therapy such as electroconvulsive therapy, MECT, transcranial magnetic stimulation, and transcranial direct current stimulation.
Interventions
The patients were divided into two groups according to the random number table method prepared by an independent third-party statistician. The patients were randomized to two groups: paliperidone and haloperidol for 16 weeks (oral). The cognitive assessors were blinded to the general clinical and safety assessment.
The dosage of paliperidone and haloperidol was adjusted according to the patient’s condition. After enrollment, the participants were prohibited from psychiatric medications other than those permitted by the study. Benzodiazepines were allowed for insomnia but not within 12 h of the ToM measurement. If the participants had an extrapyramidal reaction (EPR), benhyisol hydrochloride could be used, not more than once a day, not more than one tablet at a time. The participants’ medications for physical diseases were taken in accordance with medical requirements, and the type and dose were kept unchanged during the study period as far as possible.
Outcomes
The primary outcome was the change in the ToM performance scores from baseline to after 16 weeks of treatment. The secondary outcomes were treatment benefits and safety. Treatment benefits were determined by a decline in PANSS scores. Safety was assessed by adverse events (AEs), including amenorrhea and EPR. All subjects were assessed with vital signs, Positive and Negative Syndrome Scale (PANSS), hematology, clinical biochemistry, urinalysis, and concomitant medications at the initial screening and at weeks 0, 8, 12, and 16 of treatment. ECGs were conducted at weeks 0 and 16. ToM task performance was tested at weeks 0, 8, 12, and 16. Dose equivalence to chlorpromazine was estimated to compare the dose of paliperidone and haloperidol used in two groups, using the equations:35
For paliperidone: equivalent dose = actual dose×97.25 (mg/d).
For haloperidol: equivalent dose = actual dose×73.4 (mg/d).
Procedures
Informed consent was obtained, and the participants completed medical history and demographics questionnaires and PANSS. Four ToM tasks were selected, ordered in terms of developmental complexity and difficulty: a first-order false belief task, a higher-order false belief task,13 a recognition of faux-pas task,36 and the Reading the Mind in the Eyes task.12
A “first-order false belief task” requires the listener’s appreciation that a character’s belief differs from their own belief in a short story.14,39 In this study, the examiner described three stories in the first-order false belief test. For example, person A puts a basketball in a basket and then leaves the room, and then person B comes to the room and puts the basketball into a box. The participant will be asked a series of questions testing their inferences about the people in the story. The first question is a false belief question, ie, when person A returns, where does person A think the basketball is? Then the participant was asked a fact-checking question, ie, where do you think the basketball really is? The participants were asked these two questions, and if they gave the correct answer to the question of false belief or fact-checking testing questions, they were awarded a score of 1 point. Zero points were awarded for an incorrect answer.
A “higher-order false belief task” requires the participant to infer false belief of one character about the false beliefs of another character.14 These tasks measure the ability to understand embedded mental states. The examiner described three stories for the higher-order false belief test, ie, “ Ice-cream van” and “Family”40 and the Burglar story.41 The subjects were asked what one character believed about the belief of another character. One of the questions reflects ToM’s understanding; the second question serves as a control task where the participant only needs to remember and understand the story’s events to give the correct answer. Correct answers required understanding the mental states of the characters. If the participant gave the correct answer to the question of false belief or memory testing question, he or she was awarded a score of 1 point, 0 points otherwise.
The term faux-pas describes a range of social blunders, which are actions or statements that might injure or embarrass someone else or betray confidence.37 The examiner presented 20 stories containing 10 faux-pas. Each faux-pas story was followed by five questions and two control questions. Faux-pas stories and control stories were presented in mixed order. Control stories only had two control questions that focused on memory, comprehension, and attention. Scoring for all questions was 1 for a correct answer and 0 for a wrong or incomplete answer, totaling 100 possible points.
Reading the Mind in the Eyes test measures a person’s ability to infer a person’s mental state based on their facial expression.12,38 It consists of 36 black-and-white photographs of the eye region of the face, taken from different actors. Participants view these photos on a computer and are asked to choose one of four options that best express the person’s mental state on the computer monitor. The scoring is 1 for a correct answer and 0 for a wrong or incomplete answer.
The psychopathological status of the patients with schizophrenia was assessed using the PANSS42 that consisted of the Positive Symptom Scale, the Negative Symptom Scale, and the General Psychopathology Scale.
Statistical Analysis
The sample size was estimated at 30 per group based on the score of the faux-pas task, α=0.05, β=0.2, and a 30% loss to follow-up.
All analyses were carried out using SPSS 22.0 (IBM, Armonk, NY, USA). The intention to treat (ITT) population was used. The last observation carried forward (LOCF) was used for missing data. Continuous data were compared between groups using the t-test. Changes in ToM within a group were assessed using multivariable analysis of covariance (MANCOVA) with medication group and time as fixed effects and baseline scores as covariables. If the time effect was significant, tests of simple effects within treatment groups were conducted by one-way ANOVA followed by a post hoc Tukey honest significant difference test. Two-sided P-values <0.05 were considered statistically significant.
Results
Demographic Data
Sixty participants with schizophrenia were randomly assigned to paliperidone (n=29) or haloperidol (n=31) (Figure 1). All participants were right-handed. No significant differences were found between the two groups in age, education, age of onset, and duration of illness (Table 1). The gender composition also did not vary between the groups. The use of artane (2–4 mg/d) was similar between the two groups (P>0.99). The mean dose of paliperidone was 7.2±2.3 (range, 3–12) mg/d and haloperidol were 10.9±3.6 (range, 4–20) mg/d, and the dose equivalence values to chlorpromazine were 700.2±223.7 mg/d and 800.0 ±264.2 mg/d, respectively.
 |
Table 1 Characteristics of the Participants |
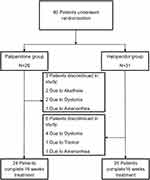 |
Figure 1 Participant flowchart. |
Mean ToM Scores in the Two Groups
For performance on the first-order false belief task (Figure 2A and Supplementary Table S1), there were no significant differences between groups (F=0.117, P>0.05), but differences were significant for time effect (F=20.989, P<0.001). There was no significant difference in the group×time interaction effect (F=2.830, P>0.05).
For the higher-order false belief task (Figure 2B and Supplementary Table S1), no significant differences were found between groups (F=3.560, P>0.05), but differences were significant for the time effect (F=10.055, P<0.001). The group×time interaction effect for this task was significant (F=3.564, P<0.001). There was a significant difference in performance from baseline to endpoint for the paliperidone group (P<0.001) but not in the haloperidol group (P>0.05).
For the faux-pas task (Figure 2C and Supplementary Table S1), there were significant differences between groups (F=6.204, Mean difference=51.4, P<0.05). The t-test showed significant differences at week 16 (P<0.05). The differences were significant for the time effect (F=5.178, P<0.05). No significant differences were found in the group×time interaction effect (F=0.681, P>0.05).
For the Reading the Mind in the Eyes task (Figure 2D and Supplementary Table S1), no significant differences were found between groups (F=3.151, P>0.05), but differences were significant for the time effect (F=4.479, P<0.05). No significant differences were found in the group×time interaction effect (F=1.874, P>0.05).
For the PANSS (Figure 3 and Supplementary Table S2), there was no difference between the paliperidone group and haloperidol group (F=0.006, P>0.05), but a significant difference in time effect in two groups (F=80.641, P<0.001), The group×time interaction effect for this task had no significant difference (F=2.335, P>0.05).
Adverse Events
The AEs between the paliperidone and haloperidol groups were compared (Table 2). The numbers of patients with at least one AE were not different between the two groups (22 vs 25, P=0.653). The numbers of patients who withdrew due to AEs were not different between the two groups (5 vs 6, P=0.833). In the paliperidone group, two, two, and one participants discontinued by weeks 8, 12, and 16, respectively, because of adverse drug reactions (amenorrhea and EPR). In the haloperidol group, three, one, and two participants discontinued by weeks 8, 12, and 16, respectively. EPRs were the main reason. The most common AEs such as akathisia, tremor, fatigue, and dystonia were not significantly different (P>0.05).
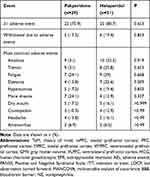 |
Table 2 Adverse Events |
Discussion
This study aimed to evaluate the efficacy of paliperidone in improving ToM task performance in patients with schizophrenia compared with haloperidol. Four ToM tasks were ordered by developmental complexity and difficulty. The results suggested that paliperidone significantly improved all four ToM tasks tested during treatment, but haloperidol only improved the first-order False Belief Task, which is the simplest task. In line with our hypothesis, the results suggest that paliperidone treatment might be superior to haloperidol in ameliorating ToM impairment in schizophrenia. At present, the treatment of schizophrenia focuses on the positive and negative symptoms. Recent research has begun to emphasize social cognitive function (which includes ToM),2–6 making this function a novel target for the treatment of schizophrenia. The present study is the first to investigate the effect of paliperidone on ToM ability in schizophrenia. The conclusions need to be validated in future trials.
The structure of paliperidone, which has a low affinity for lipid-rich environments, allows the molecule to cross the blood-brain barrier (BBB) easily.43 Animal studies28,29,44 demonstrated that paliperidone could protect the brain against neurotoxicity induced by MK-801 or acute/chronic restraint stress. Paliperidone can lead to mitochondrial protein expression change at the synaptoneurosomal level in the rats’ prefrontal cortex after chronic treatment.45 Paliperidone might have a strong neuroplastic effect and could effectively regulate the altered brain function of schizophrenia. On the other hand, animal experiments showed that haloperidol could not preferentially act on the mPFC.46,47
Although both risperidone and paliperidone act as dopamine (D2) and serotonin receptors (5-HT2A and 5-HT2C) antagonists, they have different signaling profiles.48,49 Previous research supports that different DA/5-HT levels in the PFC region directly correlate with ToM processing.31 It has been reported that paliperidone exhibits a weaker affinity for α1 and α2-adrenergic receptors than risperidone.49 Norepinephrine (NE) can promote emotion processing50–52 and emotion enhancement,53,54 which might make paliperidone improve the social-perceptual component of the ToM.55 The differences between these mechanisms could possibly contribute to some of the reasons for the different effects on ToM between paliperidone and risperidone. Furthermore, paliperidone possesses some advantages over first-generation antipsychotics. The hepatic metabolism of paliperidone is limited, and the hepatic extraction ratio is low, suggesting a lower risk of drug-drug interactions.49 Long-acting formulations (extended-release tablets and 3-monthly injections) are available, improving adherence to long-term maintenance regimens.49 Preclinical in vitro and in vivo experiments predicted that paliperidone possesses antipsychotic, antidepressant, anxiolytic, mood-stabilizing, precognitive, and anti-inflammatory effects.49
Human studies have reached inconclusive results regarding social cognition after treatment with atypical antipsychotics, including olanzapine, clozapine, and risperidone.24,27,31,56 Paliperidone is the 9-hydroxy active metabolite of risperidone.48 Some studies suggested that second-generation antipsychotics are more effective in neurocognitive function than first-generation drugs.56 Although the neurocognitive function affects social function, the latter is more complex and has many influencing factors. There is no consensus on which drugs are better for improving social functioning. Haloperidol is one of the most important and representative antipsychotic drugs,26,33,35,46,47 but a study showed that haloperidol has no protective effect on TOM.24 As a new antipsychotic drug, paliperidone has effects on a variety of receptors, which may have a protective effect on TOM. Therefore, taking haloperidol as the control group, it is meaningful to study the effects of the two drugs on TOM in SCH patients.
While the present study demonstrated significant improvement from paliperidone on ToM task performance, there were several limitations. First, this study was a single-center study and had a small sample size, which could introduce bias. Second, although the behavioral experiments study found that paliperidone can improve ToM, its mechanism is unclear. In addition, the 4-month process to measure ToM was short. Third, the patients had a relatively short course of the disease, which might lead to a better response. Further research into the physiological mechanisms of paliperidone is needed. Despite these limitations, we believe this study is a thorough investigation of the effects of paliperidone vs haloperidol on ToM ability in schizophrenia. The results showing the beneficial effects of paliperidone provide insight into pharmacologic therapies of schizophrenia and could promote future directions for research into the clinical manifestation of ToM impairment.
Conclusion
The atypical antipsychotic paliperidone might improve or protect ToM ability, but maybe not the typical antipsychotic haloperidol. Although the present study on drug treatment of ToM in patients with schizophrenia is limited, the results of this study might suggest that paliperidone can improve ToM abilities in patients with schizophrenia. These conclusions need to be validated in future trials. Future trials should be multicenter, with a large sample size, with a long period of ToM assessment, and include various types of patients.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author Jia Hongxiao at any time, upon reasonable request.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki and approved by Beijing Anding Hospital Ethics Committee (No. 2013 LSN No. (4)). All patients provided written informed consent.
Acknowledgments
We thank Dr. Baron-Cohen, who kindly provided test materials for the higher-order false belief test, the Faux-Pas test, and the Reading the Mind in the Eyes test.
Funding
This study was funded using an investigator-initiated grant from Xi’an Janssen Pharmaceutical Company. This funder had no role in study design, collection, analysis, and interpretation of the data, in the report’s writing, and in the decision to submit the paper for publication. This study was also partially supported by Beijing Hospital Authority Youth Programme (code:QML20191001), Beijing Natural Science Foundation (Grant no.7212050), Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (Grant no.ZYLX202129) Beijing Hospitals Authority's Ascent Plan (Grant no.DFL20191901), Capital’s Funds for Health Improvement and Research (Grant no.2018-1-2122, 2020-4-2126).
Disclosure
Dr Hongxiao Jia report grants from Xi’an Janssen Pharmaceutical Company, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci Biobehav Rev. 2013;37(9Pt B):2166–2180. doi:10.1016/j.neubiorev.2013.09.012
2. Chung YS, Barch DM. The effect of emotional context on facial emotion ratings in schizophrenia. Schizophr Res. 2011;131(1–3):235–241. doi:10.1016/j.schres.2011.05.028
3. Castagna F, Montemagni C, Maria Milani A, et al. Prosody recognition and audiovisual emotion matching in schizophrenia: the contribution of cognition and psychopathology. Psychiatry Res. 2013;205(3):192–198. doi:10.1016/j.psychres.2012.08.038
4. Cermolacce M, Lazerges P, Da Fonseca D, et al. [Theory of mind and schizophrenia]. Encephale. 2011;37(Suppl 2):S117–S122. French. doi:10.1016/S0013-7006(11)70037-9
5. Premack D, Woodruff G. Does the chimpanzee have a theory of mind. Behav Brain Sci. 1978;1(4):515–526. doi:10.1017/S0140525X00076512
6. Bora E, Eryavuz A, Kayahan B, Sungu G, Veznedaroglu B. Social functioning, theory of mind and neurocognition in outpatients with schizophrenia; mental state decoding may be a better predictor of social functioning than mental state reasoning. Psychiatry Res. 2006;145(2–3):95–103. doi:10.1016/j.psychres.2005.11.003
7. Yeh ZT. Role of theory of mind and executive function in explaining social intelligence: a structural equation modeling approach. Aging Ment Health. 2013;17(5):527–534. doi:10.1080/13607863.2012.758235
8. Bailey PE, Henry JD, Von Hippel W. Empathy and social functioning in late adulthood. Aging Ment Health. 2008;12(4):499–503. doi:10.1080/13607860802224243
9. Wang YY, Wang Y, Zou YM, et al. Theory of mind impairment and its clinical correlates in patients with schizophrenia, major depressive disorder and bipolar disorder. Schizophr Res. 2018;197:349–356. doi:10.1016/j.schres.2017.11.003
10. Ho KK, Lui SS, Hung KS, et al. Theory of mind impairments in patients with first-episode schizophrenia and their unaffected siblings. Schizophr Res. 2015;166(1–3):1–8. doi:10.1016/j.schres.2015.05.033
11. Bora E, Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophr Res. 2013;144(1–3):31–36. doi:10.1016/j.schres.2012.12.013
12. Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109(1–3):1–9. doi:10.1016/j.schres.2008.12.020
13. Sprong M, Schothorst P, Vos E, Hox J, van Engeland H. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007;191:5–13. doi:10.1192/bjp.bp.107.035899
14. Mazza M, De Risio A, Surian L, Roncone R, Casacchia M. Selective impairments of theory of mind in people with schizophrenia. Schizophr Res. 2001;47(2–3):299–308. doi:10.1016/S0920-9964(00)00157-2
15. Pilowsky T, Yirmiya N, Arbelle S, Mozes T. Theory of mind abilities of children with schizophrenia, children with autism, and normally developing children. Schizophr Res. 2000;42(2):145–155. doi:10.1016/S0920-9964(99)00101-2
16. Russell TA, Rubia K, Bullmore ET, et al. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. Am J Psychiatry. 2000;157(12):2040–2042. doi:10.1176/appi.ajp.157.12.2040
17. Dodell-Feder D, Tully LM, Lincoln SH, Hooker CI. The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. Neuroimage Clin. 2014;4:154–163. doi:10.1016/j.nicl.2013.11.006
18. Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41(12):1574–1582. doi:10.1016/S0028-3932(03)00119-2
19. Brunet E, Sarfati Y, Hardy-Bayle MC. Reasoning about physical causality and other’s intentions in schizophrenia. Cogn Neuropsychiatry. 2003;8(2):129–139. doi:10.1080/13546800244000256
20. Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148(2–3):75–92. doi:10.1016/j.pscychresns.2006.05.001
21. Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S. Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol Psychiatry. 2011;70(12):1169–1178. doi:10.1016/j.biopsych.2011.07.027
22. Herold R, Feldmann A, Simon M, et al. Regional gray matter reduction and theory of mind deficit in the early phase of schizophrenia: a voxel-based morphometric study. Acta Psychiatr Scand. 2009;119(3):199–208. doi:10.1111/j.1600-0447.2008.01297.x
23. Hirao K, Miyata J, Fujiwara H, et al. Theory of mind and frontal lobe pathology in schizophrenia: a voxel-based morphometry study. Schizophr Res. 2008;105(1–3):165–174. doi:10.1016/j.schres.2008.07.021
24. Savina I, Beninger RJ. Schizophrenic patients treated with clozapine or olanzapine perform better on theory of mind tasks than those treated with risperidone or typical antipsychotic medications. Schizophr Res. 2007;94(1–3):128–138. doi:10.1016/j.schres.2007.04.010
25. Pedersen CA, Gibson CM, Rau SW, et al. Intranasal oxytocin reduces psychotic symptoms and improves theory of mind and social perception in schizophrenia. Schizophr Res. 2011;132(1):50–53. doi:10.1016/j.schres.2011.07.027
26. Kucharska-Pietura K, Mortimer A, Tylec A, Czernikiewicz A. Social cognition and visual perception in schizophrenia inpatients treated with first-and second-generation antipsychotic drugs. Clin Schizophr Relat Psychoses. 2012;6(1):14–20. doi:10.3371/CSRP.6.1.2
27. Mizrahi R, Korostil M, Starkstein SE, Zipursky RB, Kapur S. The effect of antipsychotic treatment on theory of mind. Psychol Med. 2007;37(4):595–601. doi:10.1017/S0033291706009342
28. Zhu D, Zhang J, Wu J, et al. Paliperidone protects SH-SY5Y cells against MK-801-induced neuronal damage through inhibition of Ca(2+) influx and regulation of SIRT1/miR-134 signal pathway. Mol Neurobiol. 2016;53(4):2498–2509. doi:10.1007/s12035-015-9217-z
29. Peng L, Zhu D, Feng X, et al. Paliperidone protects prefrontal cortical neurons from damages caused by MK-801 via Akt1/GSK3beta signaling pathway. Schizophr Res. 2013;147(1):14–23. doi:10.1016/j.schres.2013.03.006
30. Li Y, Sun K, Liu D, et al. The effects of combined social cognition and interaction training and paliperidone on early-onset schizophrenia. Front Psychiatry. 2020;11:525492. doi:10.3389/fpsyt.2020.525492
31. Abu-Akel A, Shamay-Tsoory S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia. 2011;49(11):2971–2984. doi:10.1016/j.neuropsychologia.2011.07.012
32. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
33. Velligan DI, Newcomer J, Pultz J, et al. Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res. 2002;53(3):239–248. doi:10.1016/S0920-9964(01)00268-7
34. Good KP, Kiss I, Buiteman C, et al. Improvement in cognitive functioning in patients with first-episode psychosis during treatment with quetiapine: an interim analysis. Br J Psychiatry Suppl. 2002;43:s45–49. doi:10.1192/bjp.181.43.s45
35. Leucht S, Samara M, Heres S, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41(6):1397–1402. doi:10.1093/schbul/sbv037
36. Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10(5):640–656. doi:10.1162/089892998562942
37. Gregory C, Lough S, Stone V, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain. 2002;125(Pt 4):752–764. doi:10.1093/brain/awf079
38. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241–251. doi:10.1111/1469-7610.00715
39. Frith CD, Corcoran R. Exploring’ theory of mind’ in people with schizophrenia. Psychol Med. 1996;26(3):521–530. doi:10.1017/S0033291700035601
40. Baron-Cohen S. The autistic child’s theory of mind: a case of specific developmental delay. J Child Psychol Psychiatry. 1989;30(2):285–297. doi:10.1111/j.1469-7610.1989.tb00241.x
41. Francesca Happe UF. Theory of Mind in Autism. Learning and Cognition in Autism. Philadelphia: Springer; 1995.
42. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.261
43. Corena-McLeod M. Comparative pharmacology of risperidone and paliperidone. Drugs R D. 2015;15(2):163–174. doi:10.1007/s40268-015-0092-x
44. MacDowell KS, Caso JR, Martin-Hernandez D, et al. The atypical antipsychotic paliperidone regulates endogenous antioxidant/anti-inflammatory pathways in rat models of acute and chronic restraint stress. Neurotherapeutics. 2016;13(4):833–843. doi:10.1007/s13311-016-0438-2
45. Corena-McLeod Mdel P, Oliveros A, Charlesworth C, et al. Paliperidone as a mood stabilizer: a pre-frontal cortex synaptoneurosomal proteomics comparison with lithium and valproic acid after chronic treatment reveals similarities in protein expression. Brain Res. 2008;1233:8–19. doi:10.1016/j.brainres.2008.07.021
46. Deutch AY, Duman RS. The effects of antipsychotic drugs on Fos protein expression in the prefrontal cortex: cellular localization and pharmacological characterization. Neuroscience. 1996;70(2):377–389. doi:10.1016/0306-4522(95)00357-6
47. Heidbreder CA, Foxton R, Cilia J, et al. Increased responsiveness of dopamine to atypical, but not typical antipsychotics in the medial prefrontal cortex of rats reared in isolation. Psychopharmacology (Berl). 2001;156(2–3):338–351. doi:10.1007/s002130100760
48. Clarke WP, Chavera TA, Silva M, Sullivan LC, Berg KA. Signalling profile differences: paliperidone versus risperidone. Br J Pharmacol. 2013;170(3):532–545. doi:10.1111/bph.12295
49. Wesolowska A, Jastrzebska-Wiesek M, Cios A, Partyka A. The preclinical discovery and development of paliperidone for the treatment of schizophrenia. Expert Opin Drug Discov. 2020;15(3):279–292. doi:10.1080/17460441.2020.1682994
50. Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. The effects of reboxetine on emotional processing in healthy volunteers: an fMRI study. Mol Psychiatry. 2008;13(11):1011–1020. doi:10.1038/sj.mp.4002091
51. Hurlemann R, Hawellek B, Matusch A, et al. Noradrenergic modulation of emotion-induced forgetting and remembering. J Neurosci. 2005;25(27):6343–6349. doi:10.1523/JNEUROSCI.0228-05.2005
52. Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161(7):1256–1263. doi:10.1176/appi.ajp.161.7.1256
53. Mammarella N, Di Domenico A, Palumbo R, Fairfield B. Noradrenergic modulation of emotional memory in aging. Ageing Res Rev. 2016;27:61–66. doi:10.1016/j.arr.2016.03.004
54. Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain. 2010;3:15. doi:10.1186/1756-6606-3-15
55. Tager-Flusberg H, Sullivan K. A componential view of theory of mind: evidence from Williams syndrome. Cognition. 2000;76(1):59–90. doi:10.1016/S0010-0277(00)00069-X
56. Kucharska-Pietura K, Mortimer A. Can antipsychotics improve social cognition in patients with schizophrenia? CNS Drugs. 2013;27(5):335–343. doi:10.1007/s40263-013-0047-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


