Back to Journals » Patient Preference and Adherence » Volume 17
Palbociclib Adherence and Persistence in Patients with Hormone Receptor Positive/Human Epidermal Growth Factor Receptor 2 Negative (HR+/HER2-) Metastatic Breast Cancer
Authors Engel-Nitz NM , Johnson MG , Johnson MP , Cha-Silva AS , Kurosky SK, Liu X
Received 30 December 2022
Accepted for publication 1 April 2023
Published 18 April 2023 Volume 2023:17 Pages 1049—1062
DOI https://doi.org/10.2147/PPA.S401480
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Nicole M Engel-Nitz,1 Mary G Johnson,1 Michael P Johnson,1 Ashley S Cha-Silva,2 Samantha K Kurosky,2 Xianchen Liu2
1Optum, Eden Prairie, MN, 55344, USA; 2Pfizer Inc, New York, NY, 10017, USA
Correspondence: Nicole M Engel-Nitz, HEOR | Optum Life Sciences, MN950-1000, 11000 Optum Circle, Eden Prairie, MN, 55344, USA, Tel +1 952-205-7770, Email [email protected]
Purpose: To assess adherence and persistence with palbociclib therapy in patients with HR+/HER2- metastatic breast cancer (mBC) in a US real-world setting.
Methods: This retrospective study evaluated palbociclib dosing, adherence, and persistence using commercial and Medicare Advantage with Part D claims data from the Optum Research Database. Adult patients with mBC who had continuous enrollment 12 months prior to mBC diagnosis and initiated first-line palbociclib with aromatase inhibitor (AI) or fulvestrant between 02/03/2015 and 12/31/2019 were included. Demographic and clinical characteristics, palbociclib dosing and dose changes, adherence (medication possession ratio [MPR]), and persistence were measured. Adjusted logistic and Cox regression models were used to examine demographic and clinical factors associated with adherence and discontinuation.
Results: Patients (n = 1066) with a mean age of 66 years were included; 76.1% received first-line palbociclib+AI and 23.9% palbociclib+fulvestrant. Most patients (85.7%) initiated palbociclib at 125 mg/day. Of the 34.0% of patients with a dose reduction, 82.6% reduced from 125 to 100 mg/day. Overall, 80.0% of patients were adherent (MPR), and 38.3% discontinued palbociclib during a mean (SD) follow-up of 16.0 (11.2) and 17.4 (13.4) months, for palbociclib+fulvestrant and palbociclib+AI, respectively. Annual income below $75,000 was significantly associated with poor adherence. Older age (age 65– 74 years (hazard ratio [HR] 1.57, 95% CI, 1.06, 2.33), age ≥ 75 years (HR 1.61, 95% CI: 1.08, 2.41)) and bone-only metastatic disease (HR 1.37, 95% CI, 1.06, 1.76) were significantly associated with palbociclib discontinuation.
Conclusion: In this real-world study, > 85% of patients started palbociclib at 125 mg/day and 1 in 3 had dose reductions during the follow-up. Patients were generally adherent and persistent with palbociclib. Older age, bone-only disease, and low-income levels were associated with early discontinuation or non-adherence. Further studies are needed to understand the associations of clinical and economic outcomes with palbociclib adherence and persistence.
Keywords: breast cancer, palbociclib, adherence, persistence, metastatic
Plain Language Summary
Breast cancer is the most frequent cancer among women. The treatment goal of metastatic breast cancer (mBC) is extending patient survival time while sustaining quality of life. Endocrine therapy (aromatase inhibitor [AI] or fulvestrant) in combination with a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) is recommended as first-line treatment for patients with hormone-receptor positive, human epidermal growth factor receptor 2 negative (HR+/HER2-) mBC. Treatment adherence is crucial for meeting treatment goals. This observational study sought to evaluate adherence and persistence with palbociclib therapy in patients with HR+/HER2- mBC identified among commercial and Medicare Advantage with Part D enrollees in the Optum Research Database between 02/03/2015 and 12/31/2019. Overall, 1066 patients, with average age around 66 years were assessed; 73.6% were post-menopausal; 76.1% were treated with first-line palbociclib combined with aromatase inhibitors and 23.9% palbociclib combined with fulvestrant. Most patients (85.7%) started palbociclib at the dose of 125 mg/day. About one-third of the patients reduced their dose strength, and most of the patients who reduced strength (83%) reduced to 100 mg/day. Overall, 80.0% of patients were adherent to treatment, and just under 40% discontinued palbociclib during average follow-up periods of 16.0 and 17.4 months, for palbociclib+fulvestrant and palbociclib+AI, respectively. Patients with income below $75,000/year were one-third less likely to be adherent. Also, age ≥65 years and bone-only metastatic disease were significantly associated with discontinuing palbociclib. More studies are needed for supportive evidence.
Introduction
Breast cancer, the most common cancer in women in the United States (US), was newly diagnosed in an estimated 280,000 women in 2021.1 Six percent of new breast cancer cases present with distant metastases, for which the 5-year relative survival rate is 29%.1
The intent of metastatic breast cancer (mBC) treatment is to extend survival while maintaining quality of life.2 Molecular subtype, defined by hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status, can inform selection of appropriate treatments.3 Approximately 60% of mBCs are HR-positive, HER2-negative (HR+/HER2-),4 a subtype that is sensitive to endocrine therapies.3
Current National Comprehensive Cancer Network (NCCN) treatment guidelines recommend endocrine therapy (aromatase inhibitor [AI] or fulvestrant) in combination with a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) as first-line treatment for patients with HR+/HER2- recurrent or de novo mBC, in the absence of visceral crisis.3 The combination of first-line CDK4/6i with endocrine therapy has shown significant improvement in progression-free survival over endocrine monotherapy in Phase III trials in patients receiving initial treatment for metastatic disease or patients who progressed after prior endocrine therapy.5–10
Palbociclib, a potent and selective oral CDK4/6i, was the first in its class to receive Food and Drug Administration approval for the treatment of HR+/HER2- advanced or mBC in the US.11 Among post-menopausal patients with HR+/HER2- advanced breast cancer without prior treatment for advanced disease, the PALOMA-2 phase III trial demonstrated significantly longer progression-free survival (PFS) in patients receiving palbociclib with letrozole compared with letrozole alone (hazard ratio [HR], 0.58; 95% confidence interval [CI], 0.46, 0.72).5 Similarly, palbociclib combined with fulvestrant showed significant benefit over fulvestrant alone in PALOMA-3, a phase III study in patients with HR+/HER2- mBC who had progressed following prior endocrine therapy (HR, 0.46; 95% CI, 0.36, 0.59).6
The effectiveness of palbociclib combination therapies has been reported outside of clinical studies. A real-world study by DeMichele et al, in 1430 patients with mBC, reported that relative to letrozole alone, palbociclib combination therapy (palbociclib plus letrozole) was associated with significantly longer median real-world PFS, 20.0 vs 11.9 months; HR, 0.58; 95% CI, 0.49–0.69.12 In 1383 patients with HR+/HER2- mBC, Brufsky et al found that the real-world breast tumor response rate was 58.6% in the palbociclib plus letrozole group and 39.1% in the letrozole group, odds ratio 2.21 (95% CI, 1.503.25).13
Outside of controlled clinical trial settings, ensuring that patients take oral anti-cancer therapies, such as palbociclib, as prescribed (ie, are adherent) and for an extended period of time (ie, are persistent) is crucial for meeting treatment goals. Maintaining medication compliance for an extended duration, however, may be stymied by real-life factors including cost, adverse events, and patient perception, among others.14 Inadequate compliance with oral anti-cancer therapies has been known to lead to worsened outcomes and increased healthcare costs.14–19
Palbociclib treatment utilization has been reported in retrospective studies, but measures of adherence and persistence with palbociclib have not been characterized among patients treated in real-world clinical practice.20 A medical record review of patients who received palbociclib+AI in the US reported that 21.1% of patients discontinued treatment over a mean follow-up period of 9.9 months; among those who discontinued, the mean duration of palbociclib treatment was 8.3 months, not accounting for patients who continued and may have been doing well on treatment at the time of measurement.20 A study of 813 patients receiving palbociclib+AI in the US Flatiron health database reported a median duration of 16.3 months in the first-line setting. However, no analysis of whether patients adhered to the recommended prescribing intervals during this time or on drivers of persistence was conducted.21
Real-world assessment of palbociclib persistence and adherence and an in-depth analysis of factors driving high/low persistence and adherence are yet to be evaluated. Current data on variations in palbociclib treatment patterns are needed to aid identification of patient subgroups susceptible to early discontinuation and non-adherence, to understand possible drivers of low compliance, and to tailor programs to address specific barriers to high adherence and persistence.
The aim of this study was to describe first-line palbociclib treatment patterns among patients with HR+/HER2- mBC in the US, including initial dose and dose changes, measures of adherence and persistence, and factors associated with high adherence and persistence.
Methods
Study Design and Data Sources
This observational cohort study analyzed commercial and Medicare Advantage with Part D claims data from the Optum Research Database (ORD). The ORD is a de-identified, longitudinal administrative healthcare claims database containing over 73 million lives21 with age and gender representation similar to the US-insured population, and with geographic coverage across the US.22 The study received an exemption from Institutional Review Board review because it used de-identified secondary data. Approval through Optum’s data disclosure review for HIPAA privacy was secured prior to linking socioeconomic data to claims and enrollment data.
Sample Selection
Patients, 18 years or older, with evidence of mBC between January 01, 2010, and December 31, 2019, were identified via ≥2 non-diagnostic claims with International Classification of Disease, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM or ICD-10-CM) codes for breast cancer (ICD-9-CM, 174.x, 175.x or ICD-10-CM, C50.x1x, C50x2x) ≥30 days apart and within 1 year, and ≥2 claims on different dates with a secondary malignancy diagnosis (ICD-9-CM, 196.2, 196.5, 196.8, 197.x – 199.0 or ICD-10-CM, C772, C77.4, C77.8, C78.xx – C89.xx) on or any time after the breast cancer diagnosis date.
Eligible patients had ≥1 pharmacy claim for palbociclib between February 3, 2015, and December 31, 2019, and enrolled continuously in both medical and pharmacy benefits for ≥12 months prior to and ≥1 month after their first palbociclib claim. The date of the first palbociclib claim was considered the index treatment date, and patients were included if they had palbociclib as first-line treatment in combination with either fulvestrant or AIs (anastrozole, exemestane, or letrozole). Receipt of fulvestrant or an AI was considered a proxy for HR+ mBC. HER2 status is not well captured through diagnosis or procedure codes; therefore, patients were considered to have HER2- mBC, and thus eligible for inclusion, if there was no evidence of a prescription for an anti-HER2 therapy at any time, a method employed frequently in prior studies using administrative claims as a proxy for categorizing subtype.23,24
In addition, eligible patients had demographic and insurance information, no evidence of clinical trial participation, no evidence of pregnancy during the study period, and no evidence of another cancer (≥2 claims for same type of cancer ≥30 days apart and within 1 year) in the 24 months prior to the index treatment date. Patients were followed from the index date through the earliest of health plan disenrollment, discontinuation of palbociclib, death, or end of the study period (December 31, 2019).
Study Measures
Patient demographics, clinical, and insurance characteristics were measured at the index date, including age, gender, geographic region, insurance type, race or ethnicity, level of education, and household income. Clinical characteristics examined included: age at metastatic date, comorbidities during baseline, death during follow-up, and type and site of metastases during variable baseline of up to 24 months pre-index through 30 days post-index. Menopausal status was assigned based on a published algorithm.24 Patients were considered post-menopausal if they were female ≥60 years old, received bilateral oophorectomy prior to the index date; or had a diagnosis of postmenopausal status or menopause during the entire data period.
Patients were allocated to cohorts based on receipt of first-line palbociclib+AI or palbociclib+fulvestrant regimens, with a line of therapy algorithm adapted from similar claims analyses.12,23–25 The date of the first agent prescribed after mBC diagnosis was captured as the first-line treatment start date. Treatments given within 30 days of this first agent were grouped into the first-line regimen, which continued until the earliest of addition or change to a new agent, discontinuation (treatment gap of ≥90 days), death, disenrollment or end of the study. Biosimilars were grouped together with the original drug for identification of the regimens.
Adherence with palbociclib treatment was measured using the medication possession ratio (MPR).26,27 MPR was calculated as the sum of the number of days supply of palbociclib divided by the number of days between the first and the last fill; at least two fills of palbociclib were required to compute MPR. MPR was categorized as 0-<20%, 20-<40%, 40-<60%, 60-<80%, and 80–100%; “adherent” was defined as MPR of 80% or greater. A sensitivity analysis adjusted for inpatient stays, assuming that the facility supplied medications. MPR was assessed over the patient’s variable follow-up time.
Palbociclib persistence was measured as days to therapy discontinuation and the total number of prescription fills for palbociclib prior to discontinuation; discontinuation was defined as a gap in therapy of ≥90 days. The date of discontinuation was defined as the date when the last prescription filled prior to the gap in therapy ran out of supply. A sensitivity analysis adjusted for inpatient events.
Statistical Analyses
Demographic, clinical, socioeconomic, and treatment characteristics were analyzed descriptively and stratified by first-line regimen (palbociclib+AI or palbociclib+fulvestrant). Comorbidity burden was measured using the Charlson Comorbidity Index over the baseline period.28 Persistence with palbociclib and time to first-dose decrease were analyzed using Kaplan–Meier (KM) methods. The median time to event and the 95% confidence interval around the median were reported. Logistic regression analyses assessed baseline patient characteristics associated with palbociclib adherence (ie, MPR of 80% or greater) and a Cox proportional hazards assessed persistence (ie, time to discontinuation). Age, partner endocrine agent (fulvestrant or AI), race, income, education, site of metastases, and Charlson Comorbidity Index were included as independent variables in each model. Analyses were conducted with SAS statistical software 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
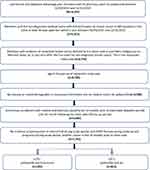
Overall, 1066 patients met the inclusion criteria (Figure 1). Mean (SD) age at the index date was 66.0 (11.6) years, and 73.6% of patients were categorized as post-menopausal. Most patients were female (98.1%), white (67.8%), and completed education beyond high school (66%, with 48.7% some college/associate degree and 17.3% college or above). During the period from 24-month pre-index to 30 days after index date, diagnosis codes for visceral metastases were present for 33.4% of the patients, and bone-only metastases were documented in 18.4% of patients. Among this population of patients who received palbociclib in the first-line of treatment, 811 (76.1%) patients received palbociclib+AI and 255 (23.9%) palbociclib+fulvestrant. Demographic characteristics were similar for patients who received palbociclib+AI and palbociclib+fulvestrant, with mean (SD) follow-up time of 17.4 (13.4) months and 16.0 (11.2) months, respectively (Table 1).
 |
Table 1 Characteristics of Patients with MBC Who Received First-Line Palbociclib with AI or Fulvestrant |
Initial Dose and Dose Adjustments
Most patients initiated palbociclib with an on-label strength of 125 mg/day (85.7%), 11.4% received 100 mg/day, and 3.0% received 75 mg/day. The initial palbociclib starting dose was similar for patients who initiated it in combination with AI or fulvestrant.
Overall, 34.0% of patients experienced a reduction in their initial dose during the follow-up period, and 1.5% experienced an increase. The remaining patients had no change during follow-up. Among patients with a reduction as their first change (n = 362), 82.6% reduced from 125 to 100 mg/day (Table 2).
 |
Table 2 First-Line Palbociclib Treatment Characteristics |
Among patients who received palbociclib+AI or palbociclib+fulvestrant, 35.0% and 30.6% experienced a reduction as their first-dose change, respectively. In both groups, the most common reduction was from 125 mg/day to 100 mg/day (82.0% and 84.6%). The Kaplan–Meier median time to the first-dose decrease was not reached. The probability of maintaining the initial dose or higher was 77.5% and 79.9% at 3 months, 71.0% and 72.4% at 6 months, and 63.7% and 67.1% at 12 months, for palbociclib+AI and palbociclib+fulvestrant, respectively (Figure 2).
 |
Figure 2 Time to Dose Decrease. Abbreviations: AI, aromatase inhibitor; F, fulvestrant; LOT, line of therapy. |
Adherence and Persistence
During follow-up, the palbociclib MPR was 0.88 (0.16), and 79.9% of patients were adherent. MPR was similar for palbociclib+AI and palbociclib+fulvestrant recipients.
Overall, 80.3% of patients receiving palbociclib+AI and 75.9% of patients receiving palbociclib+fulvestrant remained on treatment at 6 months of follow-up (Figure 3). The KM median time to discontinuation was 19.9 months (95% CI, 18.3–22.7) among patients receiving palbociclib+AI, and 15.2 months (95% CI, 12.1–18.1) among patients receiving palbociclib+fulvestrant. Persistence rates for palbociclib+AI and palbociclib+fulvestrant were 90.5% and 91.4% at 3 months, 80.3% and 75.9% at 6 months, and 68.7% and 59.1% at 12 months (Figure 3).
Factors Associated with Adherence and Persistence
Income of less than $75,000/year was significantly associated with a lower likelihood of adherence compared to those with a higher income (odds ratio 0.66, 95% CI: 0.45, 0.97); other factors were not statistically significant in the model. In the assessment of palbociclib persistence, older age (65–74 years and 75+ years) was significantly associated with an increased likelihood of discontinuation compared with younger patients (18–50 years): for age 65–74, (HR 1.57, 95% CI, 1.06, 2.33) and for age ≥75 (HR 1.61, 95% CI: 1.08, 2.41). Having bone-only metastasis was significantly associated with an increased likelihood of discontinuation relative to other types of metastases (HR 1.37, 95% CI, 1.06, 1.76) (Figures 4a and b).
Figure 4 Continued.
Discussion
Maintaining adherence and persistence to oral anti-cancer therapies is a critical step towards reaching treatment goals for patients with HR+/HER2- mBC. To date, palbociclib adherence and persistence in a real-world setting has not been well described, although multiple clinical trials have demonstrated significant efficacy and a manageable safety profile for palbociclib in combination with endocrine therapy for treatment of HR+/HER2- mBC.5,6,11
Most patients in the study initiated palbociclib at 125 mg/day,11 with similar starting doses regardless of the partner endocrine agent (AI or fulvestrant). About one-third of the patients reduced their dose, and most continued treatment with an adjustment to 100 mg/day.
Dose reductions in this study, although for larger proportions of patients, are directionally consistent with the findings of the IRIS real-world chart review.20 In IRIS, most patients initiated and maintained palbociclib at a starting dose of 125 mg/day; overall, 17.3% of patients needed dose reduction during the follow-up period. Dose adjustments in our study are closer to those in the PALOMA-2 trial, palbociclib plus letrozole, in which slightly more than one-third of the participants had reduced palbociclib dosing.5,20 In addition, in a study by Patt et al of 813 HR+/HER2- patients followed for a median of 21 months, palbociclib was initiated at 125 mg/day by 86.5% of patients; a total of 43.0% of the patients discontinued palbociclib+AI, with 11.0% discontinuing because of toxicity over a median duration of 16.3 months.21 Dose adjustments and discontinuations are typically the result of safety and disease progression considerations, which are not easily identifiable in claims data.
Overall, patients in our study were well-adherent to palbociclib based on the MPR measure. This is the first study to examine adherence with palbociclib in patients with mBC. Like other therapeutic areas, suboptimal adherence to treatment is an ongoing challenge. A 2008 retrospective cohort study by McCowan et al showed that patients with breast cancer with sub-optimal adherence to tamoxifen had poorer survival outcomes relative to more adherent patients.19 In a 2016 meta-analysis, Greer et al reported that adherence to antineoplastic cancer therapies varied broadly from 46% to 100% by patient population, type of medication, follow-up duration, and adherence definitions.16
In this study, 38.3% of patients discontinued palbociclib during the variable follow-up period (roughly one-third after KM adjustment censoring). This represents a lower rate than that reported by Patt et al and a higher discontinuation rate than reported in a real-world data study by Taylor-Stokes et al.20 Our results are more consistent with the PALOMA-2 and PALOMA-3 clinical studies that reported discontinuation rates of 39% and 37%, respectively, because of disease progression.5,6 We did not evaluate reasons for discontinuation as administrative claims do not document the reasons enrollees change treatment. In addition to progression, however, other common reasons for discontinuation include side effects such as neutropenia, other toxicities, patient choice, and economic considerations.5,6,29,30
A mixed picture emerged about the role of age, metastatic site, and income on adherence and persistence in our study population. In general, older age was associated with greater propensity to discontinue treatment as was the presence of bone only disease. It is difficult to discern in an analysis with claims data why patients with bone-only disease appeared more likely to discontinue palbociclib treatment. One plausible explanation is that bone-only metastasis in mBC patients has a relatively good prognosis versus patients with other metastasis such as visceral diseases.6,13,31 After disease progression is controlled, palbociclib may likely be switched to endocrine therapy (ET) only because palbociclib in combination with ET is costly and may cause additional adverse reactions such as neutropenia.
Income below $75,000, was significant in predicting non-adherence, although not associated with treatment discontinuation. The role of income may be of particular concern, as all the patients included in the study had both medical and pharmacy insurance coverage. This suggests potential limitations in the current health care system that may not adequately account for other social factors that can drive treatment patterns.
Our results represent one of the first efforts to provide current data on variations in palbociclib treatment patterns in the real-world practice setting and point to factors needed to aid identification of patient subgroups at risk for early discontinuation and non-adherence and to understand drivers of low adherence. An understanding of these factors is required to design programs such as the medication adherence monitoring and counseling programs32 used in the Optimizing Oral Targeted Anticancer Therapies studies33 to mitigate barriers to adequate adherence and persistence, and to improve patient outcomes.34
Limitations
These results must be viewed against key limitations, including their lack of generalizability to other populations. Some limitations are inherent to retrospective databases as claims are collected for payment purposes and rather than for research. Filled prescriptions do not mean that patients consumed the medications or took them as recommended. Claims do not capture physician-provided samples or medications provided as part of a clinical trial. While the study excluded patients with claims evidence of clinical trial participation, it is possible that not all clinical trial participants were identified. A diagnosis code on a medical claim may represent a rule-out criteria during the diagnostic process rather than actual disease. The study mitigated this issue by requiring 2 diagnosis codes >30 days apart on non-laboratory/non-imaging claims. In addition, claims data do not include information to specifically identify reasons for treatment discontinuation.
Conclusions
In this population of mBC patients who received CDK4/6i therapy, most persisted with their initial dose during a median follow-up of almost 14 months, although a notable proportion had a dose reduction. Substantial proportions of patients were adherent and persistent to palbociclib treatment, although evidence that metastatic site, age, and income levels were associated with medication adherence and discontinuation suggest the need for programs that can target specific populations for improvement.
Ethics Statement
The authors obtained Institutional Review Board waiver for this study as no identifiable protected health information was accessed in the conduct of this study in accordance with the United States Department of Health and Human Services Privacy Rule requirements for de-identification codified at 45 C.F.R. § 164.514(b). Patient privacy was preserved, and Health Insurance Portability and Accountability Act (HIPAA) data handling rules and were complied with throughout.
Acknowledgments
The authors wish to thank Bernard Tulsi and Deja Scott-Shemon for writing and editorial support, Noah Webb for the preparation of graphic elements, and Carrie Song, Malar Machi, Felix Cao, Lynn Wacha, Damon Van Voorhis and Scott Li, all of Optum, for dataset programming support. Thanks also to Cori Blauer-Peterson for additional analytic support, and to Susan Peckous and Breanna Essoi for project management.
A selection of the study’s results was presented as a poster at the 39th Annual Miami Breast Cancer Conference (March 3-6, 2022).
Funding
This study was sponsored by Pfizer Inc.
Disclosure
Ashley S. Cha-Silva, Samantha K. Kurosky and Xianchen Liu, are employees of and hold stock in Pfizer. Nicole M. Engel-Nitz, Mary G. Johnson and Michael P. Johnson are employees of Optum, which was contracted by Pfizer to conduct this study, and hold stock in UnitedHealth Group. The authors report no other conflicts of interest in this work.
References
1. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer v8.2021; 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf.
2. Smith I. Goals of treatment for patients with metastatic breast cancer. Semin Oncol. 2006;33:S2–5. doi:10.1053/j.seminoncol.2005.07.030
3. Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161–170. doi:10.1016/j.steroids.2012.11.001
4. Gong Y, Liu YR, Ji P, Hu X, Shao ZM. Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci Rep. 2017;7:45411. doi:10.1038/srep45411
5. Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925–1936. doi:10.1056/NEJMoa1607303
6. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, Phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi:10.1016/S1470-2045(15)00613-0
7. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017;35:3638–3646. doi:10.1200/JCO.2017.75.6155
8. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29:1541–1547. doi:10.1093/annonc/mdy155
9. Slamon DJ, Neven P, Chia S, et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi:10.1200/JCO.2018.78.9909
10. Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017;35:2875–2884. doi:10.1200/JCO.2017.73.7585
11. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi:10.1056/NEJMoa1810527
12. DeMichele A, Cristofanilli M, Brufsky A, et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2- metastatic breast cancer in US real-world clinical practice. Breast Cancer Res. 2021;23:37. doi:10.1186/s13058-021-01409-8
13. Brufsky A, Liu X, Li B, McRoy L, Layman RM. Real-World Tumor Response of Palbociclib Plus Letrozole Versus Letrozole for Metastatic Breast Cancer in US Clinical Practice. Target Oncol. 2021;16:601–611. doi:10.1007/s11523-021-00826-1
14. Given BA, Spoelstra SL, Grant M. The challenges of oral agents as antineoplastic treatments. Semin Oncol Nurs. 2011;27:93–103. doi:10.1016/j.soncn.2011.02.003
15. Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol. 2011;86:471–474. doi:10.1002/ajh.22019
16. Greer JA, Amoyal N, Nisotel L, et al. A Systematic Review of Adherence to Oral Antineoplastic Therapies. Oncologist. 2016;21:354–376. doi:10.1634/theoncologist.2015-0405
17. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi:10.1007/s10549-010-1132-4
18. Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer. 2013;108:1515–1524. doi:10.1038/bjc.2013.116
19. McCowan C, Wang S, Thompson AM, Makubate B, Petrie DJ. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer. 2013;109:1172–1180. doi:10.1038/bjc.2013.464
20. Taylor-Stokes G, Mitra D, Waller J, Gibson K, Milligan G, Iyer S. Treatment patterns and clinical outcomes among patients receiving palbociclib in combination with an aromatase inhibitor or fulvestrant for HR+/HER2-negative advanced/metastatic breast cancer in real-world settings in the US: results from the IRIS study. Breast. 2019;43:22–27. doi:10.1016/j.breast.2018.10.009
21. Patt DLX, Li B, McRoy L, Layman RM, Brufsky A. Real-World Treatment Patterns and Outcomes of Palbociclib Plus an Aromatase Inhibitor for Metastatic Breast Cancer: flatiron Database Analysis. Clin Breast Cancer. 2022;22:601–610. doi:10.1016/j.clbc.2022.05.002
22. Optum-Addressing the need for real-world observational research solutions. Available from: https://www.optum.com/content/dam/optum3/optum/en/resources/white-papers/heor-observational-research-sol-wp.pdf.
23. Goyal RK, Cuyun Carter G, Nagar SP, et al. Treatment patterns, adverse events, and direct and indirect economic burden in a privately insured population of patients with HR+/HER2- metastatic breast cancer in the United States. Expert Rev Pharmacoecon Outcomes Res. 2021;21:699–710. doi:10.1080/14737167.2020.1804871
24. Li N, Du EX, Chu L, et al. Real-world palbociclib dosing patterns and implications for drug costs in the treatment of HR+/HER2- metastatic breast cancer. Expert Opin Pharmacother. 2017;18:1167–1178. doi:10.1080/14656566.2017.1351947
25. Ray S, Bonthapally V, McMorrow D, Bonafede M, Landsman-Blumberg P. Patterns of treatment, healthcare utilization and costs by lines of therapy in metastatic breast cancer in a large insured US population. J Comp Eff Res. 2013;2:195–206. doi:10.2217/cer.13.1
26. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi:10.1111/j.1524-4733.2007.00213.x
27. Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi:10.1111/j.1524-4733.2006.00139.x
28. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi:10.1093/aje/kwq433
29. Kish JK, Ward MA, Garofalo D, et al. Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res. 2018;20:37. doi:10.1186/s13058-018-0958-2
30. Watson GA, Deac O, Aslam R, et al. Real-World Experience of Palbociclib-Induced Adverse Events and Compliance With Complete Blood Count Monitoring in Women With Hormone Receptor-Positive/HER2-Negative Metastatic Breast Cancer. Clin Breast Cancer. 2019;19:e186–e194. doi:10.1016/j.clbc.2018.09.002
31. Rugo HS, Brufsky A, Liu X, et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- metastatic breast cancer. NPJ Breast Cancer. 2022;8:114. doi:10.1038/s41523-022-00479-x
32. Bandiera C, Locatelli I, Courlet P, et al. Adherence to the CDK 4/6 Inhibitor Palbociclib and Omission of Dose Management Supported by Pharmacometric Modelling as Part of the OpTAT Study. Cancers. 2023;2:15.
33. Bandiera C, Cardoso E, Locatelli I, et al. Optimizing Oral Targeted Anticancer Therapies Study for Patients With Solid Cancer: protocol for a Randomized Controlled Medication Adherence Program Along With Systematic Collection and Modeling of Pharmacokinetic and Pharmacodynamic Data. JMIR Res Protoc. 2021;10:e30090. doi:10.2196/30090
34. Husinka L, Koerner PH, Miller RT, Trombatt W. Review of cyclin-dependent kinase 4/6 inhibitors in the treatment of advanced or metastatic breast cancer. J Drug Assess. 2020;10:27–34. doi:10.1080/21556660.2020.1857103
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.