Back to Journals » Local and Regional Anesthesia » Volume 8
Pain relief after transversus abdominis plane block for abdominal surgery in children: a service evaluation
Authors Bergmans E, Jacobs A, Desai R, Masters O, Thies K
Received 15 November 2014
Accepted for publication 30 December 2014
Published 7 April 2015 Volume 2015:8 Pages 1—6
DOI https://doi.org/10.2147/LRA.S77581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Stefan Wirz
Elonka Bergmans,1 Alet Jacobs,1 Rachel Desai,1 Oliver W Masters,2 Karl C Thies1
1Department of Anaesthesia and Pain Medicine, Birmingham Children's Hospital, Birmingham, 2Department of Anaesthesia, Royal Gwent Hospital, Newport, UK
Abstract: We carried out a prospective service evaluation of the quality of pain control after preoperative transverse abdominis plane (TAP) block in 100 children undergoing abdominal surgery. Data were collected on type of procedure, age, weight, level of the block, local anesthetic used, additional analgesia, and hourly pain scores. Of the 100 patients, 87 were included in the evaluation, 77% of who were less than 1 year old. Adequate pain relief was achieved in 93% of all patients. Almost half (47%) of our patients did not require intravenous (IV) opioids in the postoperative period and 27% did not need any IV opioids at all. Our results confirm the good quality of perioperative analgesia achieved with a TAP block as part of a multimodal approach in children undergoing abdominal surgery. Depending on the patient's age and the type of procedure, a TAP block may eliminate the need for IV opioids.
Keywords: intravenous opioids, pain control, TAP block, perioperative analgesia, postoperative analgesia
Introduction
Despite a rise in abdominal surgical activity at our institution (Birmingham Children’s Hospital, Birmingham, UK) the use of epidural analgesia has fallen by more than 50% over the past 5 years. Among other factors this is due to the introduction of new regional anesthesia techniques such as the ultrasound-guided transversus abdominis plane (TAP) block.1 Recent studies suggest that the TAP block is an effective regional technique for perioperative analgesia in abdominal surgery, but there is little supporting evidence of this in children.2–5 In order to assess our own practice we evaluated the quality of postoperative analgesia in children who had received a preoperative TAP block for abdominal surgery.
Methods
We carried out a prospective service evaluation on the quality of pain control after preoperative TAP block in 100 children undergoing abdominal surgery. Prospective service evaluations are well-established quality assessment instruments in the UK’s National Health Service. This study was approved and registered with Birmingham Children’s Hospital’s clinical governance department (reg. BCH AN 86/2010) and conducted as a prospective evaluation of practice and outcome in line with UK legislation. The results were anonymized and do not allow identification of individual patients. The hospital’s clinical governance department advised that publication of the results does not require patient or parental consent.
Data collection commenced in January 2011 and continued until January 2013. Data were collected on type of procedure, age, weight, type of TAP block (subcostal vs lateral, additional rectus sheath block), type and volume of local anesthetic used, intra- and postoperative analgesia given, complications, and hourly pain scores up to 48 hours post-surgery using age-appropriate and validated tools (ie, the Neonatal and Infant Pain Scale [NIPS] in children up to 3 months old, the Face, Legs, Activity, Consolability Scale [FLACC] in children up to 6 years old, and self reporting scores [Wong Baker Scale] in older children).6,7 Pain scoring in the perioperative period is mandatory at our institution and part of the hourly vital-function assessment after surgery. All patients included were followed up for at least 48 hours and visited once a day by a member of the pain service to monitor the quality of the data recorded and to ensure that opioids were not withheld if indicated.
Patients with pre-existing chronic pain conditions were excluded from the study. Patients who received their TAP blocks after surgery were also excluded because such patients often recover from anesthesia before the TAP block is fully working and require intravenous (IV) opioids in the immediate postoperative period.
Results
Patients
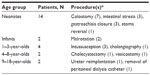
We collected data on 100 patients. Of these, 13 were excluded; eight patients because of insufficient data and five because of violation of the exclusion criteria. A total of 87 patients were included in the study. Sixty-seven patients (77%) were less than 1 year old (Table 1). In terms of weight, 25 patients weighed 3 kg or less, 22 patients weighed between 3 and 5 kg, and 40 patients weighed more than 5 kg.
Techniques applied
TAP blocks were placed after induction of general anesthesia and before the start of surgery. The transverse abdominal plane was identified using a “hockey stick” ultrasound probe (SLA 25) or a linear ultrasound probe (HFL38) in older children. A 21-gauge hypodermic needle was used to inject the local anesthetic under real-time ultrasound imaging. The level of the block was tailored to the area of analgesia required by the surgical incision:
- for upper-abdominal surgery the subcostal approach with hydro-dissection (73 patients) was followed as described by Hebbard8
- for lower abdominal surgery, the TAP block was sited more laterally (14 patients)9,10
- analgesia was supplemented with a contra-lateral rectus sheath block for incisions reaching the midline (ten patients)
- for incisions crossing the midline a bilateral TAP block was sited (15 patients).
Local anesthetic
Levobupivacaine was used in all patients. A total of 43 patients received 1 mL · kg–1 of 0.25%, 28 received 2 mL · kg–1 of 0.125%, and 16 received levobupivacaine in different concentrations and volumes never exceeding a dose of 2.5 mg·kg–1.
Surgical procedures
The surgical procedures are listed in Table 1.
Perioperative analgesia and opioid consumption
Fifty-four patients (62%) received IV opioids during surgery. The use of opioids during the procedure was at the discretion of the anesthetist. Some anesthetists administered opioids preemptively but in the majority of cases the decision was based on the cardiovascular response to stimulation; a heart rate increase of 10% from baseline was usually interpreted as insufficient analgesia and was treated with opioids. Patients were given either fentanyl in doses of 1–4 mcg · kg–1 (31 patients), remifentanil as continuous infusion 0.01–0.10 mcg · kg–1 · min–1 (four patients), morphine up to 100 mcg · kg–1 (13 patients) or a combination of these (six patients). Thirty-three patients (40%) did not require IV opioids during surgery (Figure 1).
According to the hospital’s pain protocol all patients received paracetamol 6-hourly postoperatively combined with ibuprofen and/or codeine if the pain score exceeded 3. A total of 46 patients (53%) received morphine via nurse-controlled analgesia (NCA) or patient-controlled analgesia (PCA) while the remaining 41 patients did not receive IV opioids. The prescription of postoperative NCA/PCA was also at the discretion of the anesthetist. Children under the age of 6 years old received morphine via NCA continuously at a rate of 10 mcg · kg–1 · h–1 with a bolus option of 10–20 mcg · kg–1 every 20 minutes and older children received morphine via PCA continuously at a rate of 4 mcg · kg–1 · h–1 with a bolus option of 20 mcg · kg–1 every 5 minutes. Neonates received Morphine via NCA at at a rate of 4 mcg·kg–1·h–1 and a bolus option of 10 mcg·kg–1 every 20 minutes. Four children did not receive maintenance but had a bolus option only.
Twenty-four patients did not receive any IV opioids during or after the procedure (Figure 1). Table 2 delineates the age groups and procedures for this subgroup. The hourly pain assessment assured that opioids were not withheld from our patients if they were indicated.
Postoperative IV opioid requirement was related to patient age. Of neonates, 73% did not receive postoperative IV opioids, whereas in the 9–18-year-old group only 25% did not receive IV opioids in the postoperative period (Figure 2). Morphine consumption in the first 24 hours in the NCA/PCA group ranged from 6.7 mcg · kg–1 · hr–1 in neonates to 17 mcg · kg–1 · hr–1 in the oldest age group (Figure 3). Morphine requirement declined over 48 hours in all age groups.
Quality of postoperative pain control
Pain scales (NIPS/FLACC/Wong-Baker) were from 0 to 7, 0 to 10, and 0 to 10 respectively, with 0 reflecting no pain and the maximum scores of 7 and 10 respectively, reflecting unbearable pain. We defined “adequate pain relief” as pain scores of <4 consistently (no intervention required) throughout the observation period. A review of the pain scores demonstrated adequate pain relief in over 93% of all patients. The pain scores were recorded hourly (Figure 4). Six per cent of our patients showed episodes of pain scores >3, which were successfully controlled with enterally administered ibuprofen or codeine in line with the hospital’s pain protocol. Only one patient did not show a reduction of pain score to <4 after intervention and required conversion to intravenous morphine per NCA in the ward.
Complications
No complications of the TAP block were observed during the study.
Discussion
We carried out this study to assess the quality of pain control in children who received a TAP block for abdominal surgery.
The vast majority (93%) of our patients had adequate pain control (pain scores <4) during the first 48 hours following surgery. Almost half (47%) of the patients did not require IV opioids in the postoperative period and 27% did not receive any IV opioids at all. This is a remarkable finding, as the TAP block supposedly only covers the somatic sensation to the abdominal wall and the parietal peritoneum but not visceral pain arising from the intra-abdominal organs. On the other hand, the TAP is in one continuum with the paravertebral space. We assume that the high volumes of local anesthetic that we use (1–2 mL · kg–1) facilitate spread into the paravertebral space, resulting in some visceral analgesia. This seems to be pronounced in neonates and small infants, alleviating or even eliminating the need for IV opioids.
Our results indicate that pain seems to be slightly more intense between 12 and 36 hours after surgery, than in the early postoperative period, which could be explained by the TAP block wearing off. By this time pain is usually well controlled with paracetamol and/or ibuprofen and/or codeine.
Additional IV morphine requirement seems to increase with age. Interestingly additional IV morphine consumption was higher in infants than in 1–4-year-olds. This could be due to difficulties in differentiating pain from other causes of distress in this particular age group.
The number of patients in our sample older than 1 year of age was low (23%). Therefore the results for the older children should be interpreted with caution. As mentioned before, it seems that the additional IV opioid requirement increases with age and the literature shows that the efficacy of a TAP block is less in adults than in children.11,12
Howard et al investigated postoperative morphine requirements in 10,000 patients.13 Unfortunately the results are difficult to compare with ours, as surgical procedures are not specified per age group in that study. However, the average morphine requirements that we found were less than those indicated by Howard et al. The fact that only 47% of our patients needed IV opioids postoperatively despite having undergone major abdominal surgery suggests that a preoperatively sited TAP block indeed reduces opioid requirement. The thorough pain monitoring, as described earlier, rules out under-treatment as a cause of the reduced opioid demand.
Pain relief was adequate in the vast majority of our patients. This was true even in patients receiving large midline-crossing upper-abdominal incisions, who are notoriously difficult to keep comfortable on systemic analgesia alone.
Limitations
Obviously there are several limitations to our study, which are mainly due to its descriptive nature. First the approach to the TAP block was not standardized, which is reflected in the different doses of local anesthetics used. Second, there was no control group, which makes it difficult to put our results into perspective. Third there is a certain selection bias because the decision to site a TAP block was at the discretion of the anesthetist and, due to lack of supporting data, mainly based on personal preference. However, we feel that the prospective design and the meticulous data collection contribute to the validity of our findings and compensate for these limitations.
Conclusion
Our results confirm the good quality of perioperative analgesia achieved with a TAP block as part of a multimodal approach in children undergoing abdominal surgery. Depending on the age of the patient and the type of the procedure, a TAP block may eliminate the need for IV opioids.
Disclosure
The authors declare no conflicts of interest in this work.
References
Mai C, Young M, Quraishi S. Clinical implications of the transversus abdominis plane block in pediatric anesthesia. Pediatr Anesth. 2012;22(9):831–840. | |
Hamer C, Murphy P, Diwan R. Does neonatal transverse abdominal plane block remove the need for postoperative opioid infusion? A case series of neonatal laparotomies. Pediatr Anesth. 2012;22:913–914. | |
Masters O, Thies KC. TAP block and low-dose NCA for major upper abdominal surgery. Pediatr Anesth. 2011;21(1):87–88. | |
Jacobs A, Bergmans E, Arul GS, Thies KC. The transversus abdominis plane (TAP) block in neonates and infants - results of an audit. Paediatr Anaesth. 2011;21(10):1078–1080. | |
Long JB, Birmingham PK, De Oliveira GS Jr, Schaldenbrand KM, Suresh S. Transversus abdominis plane block in children: a multicenter safety analysis of 1994 cases from the PRAN (Pediatric Regional Anesthesia Network) database. Anesth Analg. 2014;119(2):395–399. | |
Backus AL. Validation of the Neonatal Infant Pain Scale [master’s thesis]. Paper 280. Allendale, MI: Grand Valley State University; 1996. Available from: http://scholarworks.gvsu.edu/cgi/viewcontent.cgi?article=1297&context=theses. Accessed January 19, 2015. | |
Voepel-Lewis T, Zanotti J, Dammeyer JA, Merkel S. Reliability and validity of the face, legs, activity, cry, consolability behavioral tool in assessing acute pain in critically ill patients. Am J Crit Care. 2010;19(1):55–61. | |
Hebbard P. Subcostal transversus abdominis plane block under ultrasound guidance. Anesth Analg. 2008;106(2):674–675. | |
Rafi AN. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. 2001;56(10):1024–1026. | |
Suresh S, Chan VW. Ultrasound guided transversus abdominis plane block in infants, children and adolescents: a simple procedural guidance for their performance. Pediatr Anesth. 2009;19(4):296–299. | |
Sahin L, Sahin M, Gul R, Saricicek V, Isikay N. Ultrasound-guided transversus abdominis plane block in children: a randomised comparison with wound infiltration. Eur J Anaesthesiol. 2013;30(7):409–414. | |
Petersen PL, Mathiesen O, Stjernholm P et al. The effect of transversus abdominis plane block or local anaesthetic infiltration in inguinal hernia repair: a randomised clinical trial. Eur J Anaesthesiol. 2013;30(7):415–421. | |
Howard RF, Lloyd-Thomas A, Thomas M, et al. Nurse-controlled analgesia (NCA) following major surgery in 10,000 patients in a children’s hospital. Pediatr Anesth. 2010;20(2):126–134. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.