Back to Journals » Lung Cancer: Targets and Therapy » Volume 10
Pain management in patients with malignant mesothelioma: challenges and solutions
Authors Saunders J, Ashton M, Hall C , Laird B, MacLeod N
Received 29 October 2018
Accepted for publication 22 January 2019
Published 2 April 2019 Volume 2019:10 Pages 37—46
DOI https://doi.org/10.2147/LCTT.S192558
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sai-Hong Ignatius Ou
J Saunders,1 M Ashton,1,2 C Hall,3,4 B Laird,3,4 N MacLeod1
1Beatson West of Scotland Cancer Centre, Glasgow G12 0YN, UK; 2Institute of Cancer Sciences, University of Glasgow, UK; 3Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XR, UK; 4St Columba’s Hospice, Edinburgh EH5 3RW, UK
Abstract: Malignant pleural mesothelioma (MPM) is an aggressive cancer with a considerable symptom burden and poor prognosis. Focus on maintaining patients’ quality of life and pain control is therefore paramount. Pain management in MPM is complex due to its multifactorial etiology resulting from direct tumor infiltration of the surrounding soft tissue, bone, and encasement of the intercostal nerves. A variety of treatment modalities, including pharmacological and non-pharmacological options, are often required to achieve adequate pain control in this challenging disease. This review article examines the current challenges and solutions available for pain management in MPM.
Keywords: malignant, pleural, mesothelioma, pain, radiotherapy
Introduction
Malignant pleural mesothelioma (MPM) is a rare cancer affecting the pleural lining. It has a considerable symptom burden and poor prognosis, with a median life expectancy of 9–14 months from diagnosis.1 Exposure to asbestos, with its long latency period, accounts for the continued increase in incidence seen within the UK.2 Worldwide around 14,200 new cases of MPM are diagnosed each year. Incidence rates vary across the world, with 2,700 new cases diagnosed each year in the UK, an increase of around two-thirds since the 1990s.3 Patients often present with a variety of symptoms including shortness of breath, pain, and cancer cachexia.1 Diagnosis often occurs at an advanced stage of the disease which is refractory to multiple treatment modalities. Focus on quality of life and pain control is therefore paramount to the care of patients with MPM.4
Pain management in MPM is complex and challenging as a consequence of its multifactorial etiology. Patients can experience diffuse, dull, and pleuritic chest pain which characteristically increases in severity with disease progression.5 Pain is initiated not only by tumor infiltration but also as a result of investigation and management of the disease.6 To achieve optimum pain control adequate assessment and understanding of this mixed pathophysiology is key to the selection of appropriate pain management. This article will explore the pathophysiology of pain in MPM and will describe the various treatments that are available to try and help alleviate pain in this condition.
Pathophysiology
Pain is defined “as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.”7 Cancer pain is often mixed in origin being both nociceptive and neuropathic pain. Nociceptive pain includes somatic and visceral pain from innervated skin, soft tissue, bone, and organs, while somatic pain is experienced as a localized dull or ache sensation visceral pain, is deep and squeezing, with poor localization, and can involve referred pain to other sites.8 Direct stimulation or increased sensitivity from an inflammation process, such as cancer, activates nociceptive receptors resulting in pain.9 Neuropathic pain, alternatively, occurs from an injury affecting the central or peripheral nervous system, such as compression or destruction of the nerve.8 MPM’s often presents with mixed nociceptive–neuropathic pain due to direct infiltration of soft tissue and bone, as well as encasement of the intercostal nerves. Its aggressive nature can lead to major challenges in pain management and has the potential to progression to severe intractable pain.10
Pharmacological management
Since its publication in 1986, the WHO’s Analgesic Ladder for Cancer Pain Relief, detailed in Figure 1, has remained to be the cornerstone of pharmacological management of cancer pain. It outlines a stepwise approach that has been demonstrated to relieve 80% of cancer pain.11
  | Figure 1 WHO ladder for cancer pain relief. |
The first step of the ladder advices the initiation of paracetamol and non-steroidal anti-inflammatory drugs. The addition of a weak opioid, such as codeine, or tramadol, occurs at step 2 for moderate pain, with a strong opioid advised for severe pain at step 3. Adjuvant analgesics can be added at any step and are useful for the management of neuropathic pain.12 To ensure that the WHO ladder has maximum benefit, five recommendations support its implementation. First, oral forms of medication should be prescribed whenever possible. This grants patients more control and independence to manage their pain. Analgesia should be given at regular intervals, recognizing the pharmacokinetics of different medication. Slow or modified release mediations with appropriate quick acting medication for breakthrough pain are commonly prescribed in accordance to the patient’s pain intensity. While the WHO ladder provides a framework, no standard doses for the treatment of pain are advised. This is a recognition that adaption of pain medication is required for each patient. Finally, guidance highlights that pain management is an emotive and challenging topic. Careful education is beneficial to assist with successful application of analgesics.13
Although national guidelines advocate the continuation of regular paracetamol in conjunction with strong opioids for cancer pain, there is a lack of evidence for its efficacy.14 The Paracetamol vs Placebo in Conjunction With Strong Opioids for Cancer Pain trial is on-going to ascertain whether continuation of paracetamol provides symptomatic benefit.15 Codeine is often utilized first line as a weak opiate for moderate pain control. It is a prodrug, of which 5%–10% is converted to morphine by the liver. This metabolism varies between individuals with 30 mg of codeine approximately equivalent to 3 mg dose of morphine.16 Given this, debate exists to adapt and omit step 2 from the ladder due to equivocal effects of low dose strong opioids, such as morphine, and the potential for poor metabolizers of codeine. No study has yet clarified the efficiency and/or tolerability of using low dose strong opioids as an alternative in managing mild–moderate pain. Guidance is divided for the use of step 2 analgesia.17
A recent Cochrane review16 on opioids in cancer pain demonstrated that around 19 out of 20 people who experience moderate to severe pain, treated with opiates, and able to tolerate them, have a reduction in pain to mild or no pain within 14 days. This review highlighted that while opiates are commonly utilized as the mainstay of treatment for cancer pain, there is a paucity of quality evidence to support their use. Morphine is widely considered as the first choice opiate for severe pain as a result of its availability, familiarity, and low cost.18 Alternative opioids to morphine including oxycodone and hydromorphone are available. A Cochrane review demonstrated oral oxycodone provides similar pain relief and side effects as morphine and can be used as an alternative first-line oral opioid for cancer pain relief.19
Morphine can be administered via multiple routes including orally or in a liquid form. Current guidance for opioids in palliative care recommends that oral sustained release morphine should be offered first line.20 For select patients, it is recognized that alternative routes of administration may be considered. For patients with stable analgesic requirement, transdermal patches can be utilized. Alternatively, subcutaneous administration of opioids is most frequently considered in patients with unstable analgesic requirements to ease titration to achieve adequate pain control.
MPM exhibits two of the most difficult to control pains; cancer induced bone pain and neuropathic pain. Pain experience by MPM patients has a strong neuropathic component due to local effects on the neurovascular bundle. Adjuvant drugs are utilized in addition to opioids to target specific neuropathic pain mechanisms. The most frequently used are tricyclic antidepressants and antiepileptics, such as gabapentin and pregabalin.12 A systematic review by Bennett21 suggested that the addition of adjuvants do not reduce pain intensity greater than one point on a 0–10 numerical rating scale. Important to clinical practice, this review highlighted that any benefits resulting from adjuvant therapy should be observed within 4–8 days and unlikely to improve beyond this time by increasing the dosage. In MPM, continual review of oral analgesia requirement will be the key to achieve adequate control due to its rapid disease progress.
Radiotherapy
The safe delivery of tumoricidal doses of radiotherapy in MPM is challenging due to a combination of complex tumor shape, extensive disease burden, respiratory motion, and the close proximity of normal radiosensitive structures.22 The application of radiotherapy in MPM has therefore largely been limited to the palliation of symptoms, using modest doses which can be tolerated by normal tissue.
Seeding of malignant cells along instrument tracts at sites of diagnostic or therapeutic intervention affects ~40% of MPM patients and can be associated with painful subcutaneous tumor deposits.23 The use of post-procedure radiotherapy to decrease this risk is controversial and clinical practice varies widely, despite a number of negative studies.24,25 The recent publication of two, large, Phase III, randomized controlled multicentre studies has provided practice changing evidence in this field. The SMART study randomized 203 patients to either immediate (21 Gy in three fractions within 42 days of pleural intervention) or deferred radiotherapy (21 Gy in three fractions given within 35 days of procedure-tract metastases diagnosis). The Prophylactic Irradiation of Tracts trial recruited 375 patients who were randomized 1:1 to receive either 21 Gy in three fractions within 42 days of intervention or no prophylactic irradiation.26 Neither study found any significant difference in the incidence of tract site metastases between the cohorts and both concluded that there is no role for routine prophylactic radiotherapy in MPM.27
This recommendation now forms part of the current American Society of Clinical Oncology practice guidelines and it is therefore likely that prophylactic irradiation will fall out of practice.28 In contrast, radiotherapy has been widely used to palliate pain in this patient cohort for decades. Nevertheless, a systematic review published in 2014 highlighted the limited evidence supporting its role with no clear consensus on the optimal radiotherapy regime.29
In light of this paucity of evidence, the SYmptom Study of radioThErapy in MeSothelioma (SYSTEMS) study was conducted.30 This prospective, multicentre, single arm Phase II study was the first to use validated outcome measures to assess pain responses following radiotherapy in patients with MPM and remains the most robust source of evidence for the use of radiotherapy in this setting. A total of 40 patients were recruited from three centers over 18 months. Analgesia was optimized prior to embarking on a standard radiotherapy schedule of 20 Gy in five fractions, targeted at sites of pain. Treatment was delivered using parallel opposing pairs and while vulnerable organs could be shielded out, there was no “organ at risk” dosimetry data collected, reflecting the modest dose employed and the familiarity of clinicians with this palliative protocol. The results of SYSTEMS, published in 2015, demonstrated that, of the 30 patients assessable at week 5, 47% had experienced a clinically significant pain response with minimal toxicity. Consequently, the American Society of Clinical Oncology (ASCO) guidelines published in 2018 recommend that radiation therapy should be offered as an effective treatment modality for symptomatic disease.28 Importantly, the SYSTEMS study noted that there was no other palliative benefit of radiotherapy seen in this setting.30
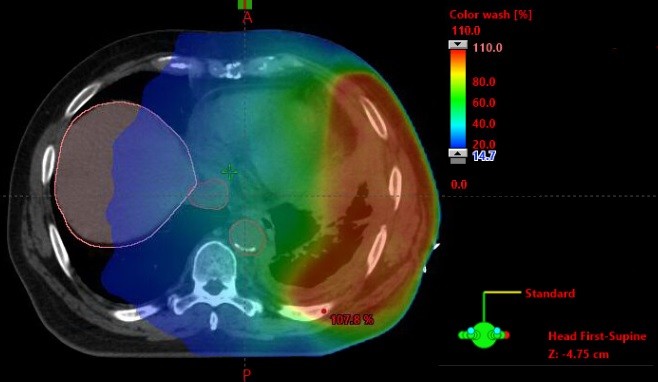
The role of dose escalated, hypofractionated radiotherapy for pain control in MPM is now being investigated in the SYSTEMS-2 study.31 This multicentre, randomized Phase II study will compare the effect of standard dose radiotherapy (20 Gy in five fractions over 1 week) with a dose-escalated regime (36 Gy in six fractions over 2 weeks). The primary outcome is pain control at week 5 compared to baseline, assessed using the Brief Pain Inventory. Secondary endpoints include radiological response, toxicity, overall survival, and quality of life. The advance of increasingly sophisticated radiotherapy techniques underpins the solution to some of the logistical and practical challenges of radiotherapy dose escalation studies. Intensity-modulated radiotherapy (IMRT) in which multiple beams of radiotherapy of different shapes and intensity are delivered continuously as the gantry moves around the patient as detailed in Figure 2. This permits dose escalation to the tumor while keeping the dose received by nearby normal tissues at a safe level (Figure 3). All patients in SYSTEMS-2 will be planned using IMRT or 3-D conformal radiotherapy techniques. Organs at risk will be outlined according to the location of the target site to ensure that dose constraints to normal tissues are not exceeded. Any patient for whom a satisfactory plan cannot be achieved for the dose escalated arm will not be eligible for randomization. It is hypothesized that a higher dose of radiation will provide an improvement in analgesic effect and duration. The outcome of this study will provide much needed clarification of the role radiotherapy can play in pain control for MPM patients, in addition to providing an important insight into the potential for further dose escalation in this disease.
Interventional pain management
Due to the wide range of structures and multi-modal pathophysiology of pain in MPM, the resultant pain syndrome may be resistant to conventional pharmacological regimens, such as costopleural syndrome. In this syndrome, pain will often present with mixed nociceptive and neuropathic pain features as the autonomic, intercostal, and occasionally brachial plexus nervous structures are involved.32 In this instance, interventional techniques may be employed. It has been suggested previously that these techniques are used as an adjuvant to common analgesic regimens at any stage rather than seen as a last resort.33 In terms of MPM, the National Mesothelioma Framework has advocated that cervical cordotomy is an option to help alleviate challenging pain syndromes.34 However, this treatment is not universally available, and there is variability in its provision in different areas.35
High cervical cordotomy involves the creation of a permanent lesion in the ascending pain pathways of the spinothalamic tract (often with heat created by radiofrequency) in the antero-lateral spinal cord. This is effective for unilateral cancer related pain below the level of the C4 dermatome, ie, below the shoulder.36 Cordotomy previously was performed as an open surgical procedure under general anesthetic with a wake-up test intra-operatively, but since the 1960s an awake percutaneous approach (percutaneous cervical cordotomy [PCC]) is most commonly employed.37
The spinothalamic tract carries pain and temperature sensation to the brain from the contralateral side of the body, thus is useful in unilateral thoracic pain syndromes often found with MPM. The tract is approached most easily at C1/2 and thermoablation at this level controls pain below C4 on the contralateral side. The extent of analgesia depends on the position of the electrode within the tract (the fibers are arranged somatotopically) and also the size of the lesion created. Because PCC is selective for pain and temperature sensation, it achieves pain relief without numbness and preserves motor power and proprioception.37
A systematic review examining the use of cordotomy for mesothelioma-related pain in 2013, found that of nine included case series involving 160 patients, all studies showed good pain relief in the majority, the greatest benefits in pain reduction were seen in the initial-post procedure phase.35 Side effects, such as headache, motor weakness, and mirror pain, occurred relatively frequently but were transient in nature and respiratory dysfunction was rare. Overall, the quality and quantity of evidence was limited and the authors concluded that further data were needed to aid decision-making on its continued provision. This review advised that a registry be set up, and in 2014 a UK National PCC registry was launched with the Invasive Neurodestructive Procedures in Cancer Pain Registry. This registry has recorded over 200 cases prospectively, including the safety and efficacy of this technique.36 Data from this registry are awaited. A prospective study of 45 patients has shown that 80% of patients reported >75% pain relief from cordotomy at 4-week follow-up.36
The most recent European Society for Medical Oncology (ESMO) guidelines on the Management of Cancer pain in adult patients states that: “Cordotomy should be offered in a MDT setting with palliative medicine, oncology and pain medicine teams to support the care pathway.36 In the case of patients who are unable to tolerate percutaneous cervical cordotomy because of the intractable nature of pain and the incapacity to lie supine in theatre, surgical cordotomy remains an option”. They recommend that “Cordotomy should be available to patients with otherwise poorly controlled cancer-related pain”.
Other interventional techniques include peripheral nerve injections. A “nerve block” describes a procedure utilizing a needle to deliver a local anesthetic or an ablative agent, such as phenol, alcohol, or glycerine, for analgesic purposes.38 Diagnostic blocks may be employed initially to ascertain the correct anatomical area or afferent pathway to subsequently target with a permanent block. Patients with thoracic chest wall pain may benefit from procedures targeting the intercostal nerve, the posterior root of the thoracic radicular nerve, and the paravertebral space. Intercostal blocks and neurolysis can be done at the patient’s bedside. Due to the potential risk of pneumothorax, it is suggested that direct needle placement is guided by ultrasonography. The benefit is seen due to the loss of sensation distal to the point of injection following the path of the nerve toward the anterior chest wall.38 One series reporting intercostal procedures for chest pain management in patients with metastatic rib lesions showed that 56% of patients described reduced analgesic use post procedure.39 When an intercostal nerve block provides temporary relief, the subsequent options are to repeat the block with a more permanent form of chemical neurolysis with agents, such as phenol,40 heat via radiofrequency,41 or freezing (cryoneuolysis).42
Surgery
Surgery as part of a tri-modality approach is the most aggressive treatment option in MPM. Its role in survival outcomes and palliation of symptoms remains under debate with several approaches described. The most radical surgery is extrapleural pneumonectomy (EPP), which consists of en-bloc resection of the visceral and parietal pleura with the lung, pericardium, and diaphragm. This surgical approach is limited to select patients, as many are unfit for such radical management due to advanced disease, frailty, and multiple comorbidities.43 A systematic review by Cao et al44 reported a significant mortality rate following EPP of 0%–11.8% and morbidity of 22%–82%, resulting in a median overall survival of 9.4–27.5 months. Debate regarding EPP has continued following the Mesothelioma and Radical Surgery (MARS) feasibility study, which randomized patients to receive chemotherapy and best supportive care only vs induction chemotherapy, EPP, and adjuvant hemi-thoracic radiotherapy. The trial concluded that, although limited, the data suggested that EPP within tri-modality therapy offered no benefit and possibly harmed patients.45 This outcome received criticism for forming such a conclusion. The MARS researchers designed a feasibility trial due to the anticipated challenge in recruiting patients comparing EPP with non-surgical management. The objective was to assess the possibility of completing a larger trial to clarify the role of EPP and not designed with the outcome to test the benefit or absence of EPP. The power of the study was low due to the small number; 50 patients who were recruited over 3 years. Investigators of the MARS trial state that 670 patients would need to be identified to gain any significant difference on overall survivial between EPP and no EPP. Thus, due to the design and power of the MARS trail, it did not allow the outcome of surgery vs no surgery to be adequately assessed.46
In recent years, interest in the less aggressive surgical approach of pleurectomy and decortication (P/D) has grown. P/D involves the resection of both parietal and visceral pleura, sparing the lung parenchyma. Advancement in techniques has led to variants of P/D procedures which are utilized in both curative and palliative management. Extended P/D, which consists of parietal and visceral pleurectomy, removal of gross tumor with resection of diaphragm, and/or pericardium, is performed in patients with potentially curative intent as an alternative to EPP.47 Flores et al48 determined that patients who underwent P/D in fact had a decrease postoperative morbidity and mortality when compared to EPP. The review, however, importantly recognized the impact of selection bias outcomes on falsely inflating survival results. Concern, therefore, remains on the interpretation of whether EPP or extended pleurectomy and decortication provides more successful results. The MARS249 trial aims to resolve this issue and investigate the survival and patient reported outcomes with extended P/D following chemotherapy vs chemotherapy only.
Palliative debulking surgery and parietal pleurectomy are performed to provide symptom control in MPM. This type of surgery is not undertaken with a curative intent but removal of the visceral pleura, and relieve of a trapped lung can, reduce chest wall pain.50 Video-Assisted Thoracic Surgery (VATS) offers a minimally invasive alternative for patients who are unfit for radical EPP or extended P/D. Recurrent pleural effusion can cause dyspnoea and discomfort for patients with MPM. The MesoVATS randomized controlled trial51 compared video-assisted thoracoscopic partial pleurectomy vs talc pleurodesis for the management of pleural effusion in MPM. While VATS has the benefit of being a less invasive approach, it demonstrated no improvement in overall survival and this approach resulted in more complications and longer hospital stay. Results assessing patients quality of life demonstrated worse function at 1 month improving at 3, 6, and 12 months with VATS compared to tacl pleurodesis; but no significant difference between the groups was identified. As discussed previously, interventions for investigation or treatment can increase the risk of seeding of malignant cells along instrument tracts.23 While most surgical research does not focus on pain management, it clearly has an impact on morbidity and quality of life of patients. The complexity of radical surgery, and on-going debate as to its role, has led to the advisement of its use only within a multi-disciplinary framework, as part of a clinical trial at specialized centers.46
Systemic Anticancer Therapy (SACT)
Chemotherapy is the first- and second-line treatment for unresectable tumors.52 While research has focused on chemotherapy’s role on patient survival, limited data focus on its effect on pain control. At present, the most widely used regimen for patients with unresectable MPM consists of cisplatin with pemetrexed. This standard treatment resulted following the publication of the Phase III EMPHACIS trial,53 a large-scale randomized controlled trial which involved 456 chemotherapy-naive patients to receive cisplatin and pemetrexed or cisplatin alone. Patients treated with the combination of pemetrexed and cisplatin had a greater survival time of 12.1 months compared to 9.3 months, as well as superior response rates and progression-free survival when compared to patients who received cisplatin alone. The addition of vitamin supplementation also demonstrated a reduction in toxicity. A modified version of the Lung Cancer Symptom Scale underwent formal validation in patients with MPM and was administered to patients of the EMPHACIS trial. Results from the 90% of patients who completed the questionnaire which was presented by Gralla et al54 at ASCO 2003 reported an improvement of overall symptom score in patients receiving combination chemotherapy, with a statistically significant improvement in pain, cough, and dyspnoea by cycle 4, week 12 in patients receiving pemetrexed plus cisplatin. The addition of bevacizumab, an anti-vascular endothelial growth factor, to current standard chemotherapy, of pemetrexed and cisplatin, has been highlighted following the MAPS study.55 This multi-center Phase III trial of 448 newly diagnosed patients with MPM randomly allocated patients to standard treatment or in combination with bevacizumab,. The addition of bevacizumab to standard chemotherapy resulted in an increase in median survival to 18.8 months in the bevacizumab arm compared to 16.1 months with standard chemotherapy. Westeel et al56 presented the MAPS trial results of its treatments impact on patients quality of life at ASCO 2018. The results of health-related quality of life were assessed in 95.5% of patients through use of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire QLQ-C30 and the Lung Cancer specific module QLQ-LC13. The addition of bevacizumab to cisplatin and pemtrexed significantly improved patients pain (HR 0.81, 95% CI 0.67–0.99; P=0.041) and peripheral neuropathy (HR 0.73, 95% CI 0.6–0.89, P=0.002). These results demonstrate that chemotherapy plays a role not only in overall survival but also provides symptomatic relief in MPM. Unfortunately, most MPM patients progress following first-line chemotherapy. No optimum regimen has yet been identified for second-line treatment.57
Careful balance between symptomatic improvement, at the risk of chemotherapy toxicities, remains a challenge as MPM patients progress. For fragile or elderly patients, carboplatin is often substituted for cisplatin. This aims to decrease toxicity, and in fact, has been reported to have similar outcomes between patients treated with cisplatin and pemetrexed.58 A focus on active symptom control in MPM patients was highlighted by the randomized control trial by Muers et al.59 This trial was undertaken before the identification that the addition of pemetrexed was demonstrated to provide superior survival outcomes. The trial reviewed the impact of first-line chemotherapy on survival compared to active symptom control alone. Four hundred nine patients were randomly assigned to receive symptomatic treatment alone, symptomatic treatment and chemotherapy, including cisplatin, vinblastine, and mitomycin, or symptomatic treatment plus single agent vinorelbine. Unfortunately, the numbers were insufficient to determine a benefit between chemotherapy regimes, although a trend toward a survival benefit for patients receiving single agent vinorelbine was noted. Ultimately they concluded that addition of chemotherapy to symptom control offered no significant benefit in terms of overall survival and quality of life. Given the current recognition of superior survival outcomes with the addition of pemetrexed, updated research would be welcome to review the impact of current first-line chemotherapy on survival compared to active symptom control alone.
As with many other tumor sites, interest in immunotherapy treatment is growing, particularly in a disease like MPM where treatment options are limited. Immunotherapy aims to stimulate the body’s natural ability to fight cancer. At present, immunotherapy is not a standard treatment for MPM.52 Agents including check point inhibitors are being investigated as potential treatments for MPM. Pembrolizumab, a programmed death-1 (PD1) inhibitor, is currently under review for its impact on MPM. The PD1 receptor is a checkpoint which when triggered by either of its ligands (PDL1 or PDL2) initiates apoptosis of effector T cells and preservation of regulatory T Cells. One percent of cells express PDL1 in up to 40% of MPM tumors. Checkpoint inhibitors interrupt the cancers ability to co-opt this pathway and silence the immune systems antitumor response.60 A Phase 1b trial of 25 patients with PDL1 expressed in 1% of tumor cells demonstrated a response to treatment with Pembrolizumab. Disease control was seen in 18 patients, who had a response duration of 12 months. Immune-related adverse effects were observed in three patients with grade 3 treatment-related toxicity observed in another five.61 This promising response has led to the PROMISE–meso Phase III randomized control trial comparing prembrolizumab with gemcitabine/vinorelbine in MPM.62 Research into combination of PD1/PDL1 agents in combination with CTLA-4 agents have been assessed for second or third-line treatments options in the MAPS2 trial.63 This randomized 125 patients to receive single-agent nivolumab or combination nivolumab and ipilimumab (a CTLA-4 agent). The results were favorable to other second or third-line therapies, which on average have a 3-month progression-free survival. Nivolumab monotherapy had a medial progression-free survival of 4 months while combination therapy was 5.6 months. Serious adverse events, however, including three patient deaths from treatment-related complications following combination therapy, was highlighted. Outcomes of such trials will advance knowledge to the promising potential immunotherapy presents in the management of MPM.
Conclusion
Pain management in MPM remains complex due to its multifactorial etiology. Despite progress in the options for treatment in MPM prognosis remains poor. Focus on symptom control is therefore paramount. While effective pain control can be gained through pharmacological treatment, many patients require multiple modalities to achieve symptomatic relief.
Current guidance reinforces the greatest evidence in support of the role of radiotherapy for pain control in MPM. Despite its use, the optimal radiotherapy dose and fraction remains unknown. The SYSTEMS study was key in demonstrating a significant pain response with minimal toxicity with radiotherapy. Now with advances in technology solutions, practical challenges of increasing radiotherapy doses have been achieved. It is hoped that IMRT will allow higher doses of radiation predicted to provide an improvement in analgesic effect and duration. The outcome of the SYSTEMS-2 trial will provide clarity as to this predicted effect of greater doses of radiotherapy on pain control.
At present, both surgical and SACT require further research to focus not only on survival outcomes but also to address the symptomatic impact of treatment. While surgery and SACT may prolong patients’ survival, the consequences of toxicities and complications to patients’ quality of life and pain control requires further investigation. Does undergoing such treatments outweigh the benefit of active symptom control alone? Research to answer this question will continue to allow open communication between patients and clinicians as to the most effective palliative treatment. While interventional procedures, such as cordotomy, demonstrate an exciting opportunity for complex pain control, its availability remains a barrier to widespread use. Guidance highlights cordotomy as playing an important role in MPM for pain management but it remains to be perceived as an option of last resort. Further experience in this field may overcome the challenges to its access and change its role to become an adjuvant at any stage to common analgesic regimens.
Pain is an emotive subject for both patients and their families due to its impact physically and emotionally. MPM is sadly often an aggressive disease with complex pain pathophysiology. Medical professionals strive to achieve the best quality of life for patients through multiple treatment modalities. While pain control in MPM remains to have challenges, it is hoped that research continues to identify solutions to alleviate pain in patients with MPM.
Disclosure
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
British Thoracic Society Standards of Care Committee. BTS statement on malignant mesothelioma in the UK, 2007. Thorax. 2007;62 (Suppl 2): ii1–ii19. | ||
McDonald JC, McDonald AD. The epidemiology of mesothelioma in historical context. Eur Respir J. 1996;9(9):1932–1942. | ||
Cancer Research UK [Internet]. Mesothelioma statistics. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/mesothelioma. Accessed October 8, 2018. | ||
Salminen EK, Silvoniemi M, Syrjänen K, Kaasa S, Kloke M, Klepstad P. Opioids in pain management of mesothelioma and lung cancer patients. Acta Oncol. 2013;52(1):30–37. | ||
Macleod N, Kelly C, Stobo J, et al. Pain in malignant pleural mesothelioma: a prospective characterization study. Pain Med. 2016;17(11):2119–2126. | ||
Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 (Suppl5):v31–v39. | ||
Merskey HBN. Classification of Chronic Pain. 2nd ed. Vol. 1994. Seattle: IASP Press; 1994. | ||
Webb JA, Leblanc TW. Evidence-based management of cancer pain. Semin Oncol Nurs. 2018;34(3):215–226. | ||
Simmons CPL, Macleod N, Laird BJA. Clinical management of pain in advanced lung cancer. Clin Med Insights Oncol. 2012;6:S8331–S8346. | ||
Jackson MB, Pounder D, Price C, Matthews AW, Neville E. Percutaneous cervical cordotomy for the control of pain in patients with pleural mesothelioma. Thorax. 1999;54(3):238–241. | ||
Ventafridda V, Tamburini M, Caraceni A, de Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer. 1987;59(4):850–856. | ||
Laird B, Colvin L, Fallon M. Management of cancer pain: basic principles and neuropathic cancer pain. Eur J Cancer. 2008;44(8):1078–1082. | ||
Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician. 2010;56(6):514–517, e202–e205. | ||
NHS Scotland Palliative Care Guidelines [webpage on the Internet]. Available from: https://www.palliativecareguidelines.scot.nhs.uk/. Accessed February 14, 2019. | ||
ClinicalTrials.gov [Internet].University of Edinburgh. Identifier: NCT02706769. A double-blind randomised parallel group trial of paracetamol versus placebo in conjunction with strong opioids for cancer related pain. Available from: https://clinicaltrials.gov/ct2/show/record/NCT02706769. Accessed October 9, 2018. | ||
Wiffen PJ, Wee B, Derry S, Bell RF, Moore RA. Opioids for cancer pain – an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;7:CD012592. | ||
Ripamonti CI. Pain management. Ann Oncol. 2012;23(Suppl 10):x294–x301. | ||
Wickham RJ. Cancer pain management: comprehensive assessment and nonopioid analgesics, part 1. J Adv Pract Oncol. 2017;8(5):475–490. | ||
Schmidt-Hansen M, Bennett MI, Arnold S, Bromham N, Hilgart JS. Oxycodone for cancer-related pain. Cochrane Database Syst Rev. 2015;2:Cd003870. | ||
Teoh PJ, Camm CF. NICE opioids in palliative care (clinical guideline 140) – a guideline summary. Ann Med Surg. 2012;1:44–48. | ||
Bennett MI. Effectiveness of antiepileptic or antidepressant drugs when added to opioids for cancer pain: systematic review. Palliat Med. 2011;25(5):553–559. | ||
Stevens CW, Forster KM, Smythe WR, Rice D. Radiotherapy for mesothelioma. Hematol Oncol Clin North Am. 2005;19(6):1099–1115, vii. | ||
Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest. 1995;108(3):754–758. | ||
Bydder S, Phillips M, Joseph DJ, et al. A randomised trial of single-dose radiotherapy to prevent procedure tract metastasis by malignant mesothelioma. Br J Cancer. 2004;91(1):9–10. | ||
O’Rourke N, Garcia JC, Paul J, Lawless C, McMenemin R, Hill J. A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother Oncol. 2007;84(1):18–22. | ||
Bayman N, Ardron D, Ashcroft L, et al. Protocol for pit: a phase III trial of prophylactic irradiation of tracts in patients with malignant pleural mesothelioma following invasive chest wall intervention. BMJ Open. 2016;6(1):e010589. | ||
Clive AO, Taylor H, Dobson L, et al. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (smart): a multicentre, open-label, phase 3, randomised controlled trial. Lancet Oncol. 2016;17(8):1094–1104. | ||
Kindler HL, Ismaila N, Armato SG, et al. Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(13):1343–1373. | ||
Macleod N, Price A, O’Rourke N, Fallon M, Laird B. Radiotherapy for the treatment of pain in malignant pleural mesothelioma: a systematic review. Lung Cancer. 2014;83(2):133–138. | ||
Macleod N, Chalmers A, O’Rourke N, et al. Is radiotherapy useful for treating pain in mesothelioma? A phase II trial. J Thorac Oncol. 2015;10(6):944–950. | ||
Ashton M, O’Rourke N, Macleod N, et al. SYSTEMS-2: a randomised phase II study of radiotherapy dose escalation for pain control in malignant pleural mesothelioma. Clin Transl Radiat Oncol. 2018;8:45–49. | ||
Parker C, Neville E. Lung cancer * 8: management of malignant mesothelioma. Thorax. 2003;58(9):809–813. | ||
Eisenberg EMF, Birkhahm J, Paladín A, Varrassi G. Time to modify the WHO analgesic leader? Pain Clin Update. 2005;13(5):1–4. | ||
Department of health. Clinical programmes: Cancer. Mesothelioma framework. Published 27.02.2007. Available from: https://webarchive.nationalarchives.gov.uk/20130124034731/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_072347.pdf. Accessed February 14, 2019. | ||
France BD, Lewis RA, Sharma ML, Poolman M. Cordotomy in mesothelioma-related pain: a systematic review. BMJ Support Palliat Care. 2014;4(1):19–29. | ||
Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(Suppl 4):iv166–iv191. | ||
Feizerfan A, Antrobus JHL. Role of percutaneous cervical cordotomy in cancer pain management. Cont Educ Anaesth Crit Care Pain. 2014;14(1):23–26. | ||
Hochberg U, Elgueta MF, Perez J. Interventional analgesic management of lung cancer pain. Front Oncol. 2017;7(12):17. | ||
Wong FC, Lee TW, Yuen KK, Lo SH, Sze WK, Tung SY. Intercostal nerve blockade for cancer pain: effectiveness and selection of patients. Hong Kong Med J. 2007;13(4):266–270. | ||
Matchett G. Intercostal nerve block and Neurolysis for intractable cancer pain. J Pain Palliat Care Pharmacother. 2016;30(2):114–117. | ||
Ahmed A, Bhatnagar S, Khurana D, Joshi S, Thulkar S. Ultrasound-guided radiofrequency treatment of intercostal nerves for the prevention of incidental pain arising due to rib metastasis. Am J Hosp Palliat Care. 2017;34(2):115–124. | ||
Ju H, Feng Y, Yang BX, Wang J. Comparison of epidural analgesia and intercostal nerve cryoanalgesia for post-thoracotomy pain control. Eur J Pain. 2008;12(3):378–384. | ||
Ambrogi V, Mineo D, Gatti A, Pompeo E, Mineo TC. Symptomatic and quality of life changes after extrapleural pneumonectomy for malignant pleural mesothelioma. J Surg Oncol. 2009;100(3):199–204. | ||
Cao C, Tian D, Park J, Allan J, Pataky KA, Yan TD. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer. 2014;83(2):240–245. | ||
Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the mesothelioma and radical surgery (MARs) randomised feasibility study. Lancet Oncol. 2011;12(8):763–772. | ||
Weder W, Stahel RA, Baas P, et al. The Mars feasibility trial: conclusions not supported by data. Lancet Oncol. 2011;12(12):1093–1094. | ||
Lang-Lazdunski L. Surgery for malignant pleural mesothelioma: why, when and what? Lung Cancer. 2014;84(2):103–109. | ||
Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135(3):620–626, 626.e1–3. | ||
ClinicalTrials.gov [Internet]. Royal brompton & harefield NHS foundation trustidentifier: NCT02040272. Mesothelioma and radical surgery 2: a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma (mars 2). Available from: https://clinicaltrials.gov/ct2/show/record/NCT02040272. Accessed October 8, 2018. | ||
Ricciardi S, Cardillo G, Zirafa CC, et al. Surgery for malignant pleural mesothelioma: an international guidelines review. J Thorac Dis. 2018;10(Suppl 2):S285–S292. | ||
Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet. 2014;384(9948):1118–1127. | ||
Woolhouse I, Bishop L, Darlison L, et al. British Thoracic Society guideline for the investigation and management of malignant pleural mesothelioma. Thorax. 2018;73(Suppl 1):i1–i30. | ||
Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–2644. | ||
Gralla RJ, Hollen PJ, Am L, Liepa AM, et al. Improving quality of life in patients with malignant pleural mesothelioma: Results of the randomized pemetrexed and cisplatin vs. cisplatin trial using the LCSS-meso instrument. Proc Am Soc Clin Oncol. 2003;22:abstr 2496. | ||
Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma Avastin cisplatin pemetrexed study (MAPs): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387(10026):1405–1414. | ||
Westeel V, Eberst G, Anota A, et al. Impact on health-related quality of life of the addition of bevacizumab to cisplatin-pemetrexed in malignant pleural mesothelioma in the maps phase III trial. J Clin Oncol. 2018;36(Suppl 15):8505–8505. | ||
Cinausero M, Rihawi K, Sperandi F, Melotti B, Ardizzoni A. Chemotherapy treatment in malignant pleural mesothelioma: a difficult history. J Thorac Dis. 2018;10(Suppl 2):S304–S310. | ||
Santoro A, O’Brien ME, Stahel RA, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaïve patients with malignant pleural mesothelioma: results of the International expanded access program. J Thorac Oncol. 2008;3(7):756–763. | ||
Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet. 2008;371(9625):1685–1694. | ||
Bibby AC, Maskell NA. Current treatments and trials in malignant pleural mesothelioma. Clin Respir J. 2018;12(7):2161–2169. | ||
Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1B trial. Lancet Oncol. 2017;18(5):623–630. | ||
ClinicalTrials.gov [Internet]. European Thoracic Oncology Platform. Identifier: NCT02991482. PembROlizuMab Immunotherapy Versus Standard Chemotherapy for Advanced prE-treated Malignant Pleural Mesothelioma (PROMISE-meso). Available from:https://clinicaltrials.gov/ct2/show/NCT02991482. Accessed October 8, 2018. | ||
Zalcman G, Mazieres J, Greillier L, et al. Second or 3rd line nivolumab versus nivolumab plus ipilimumab in malignant pleural mesothelioma patients: updated results of the IFCT-1501 MAPS2 randomized phase 2 trial. In: 2017 ESMO Congress. Abstract LBA58_PR; September 10, 2017. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.