Back to Journals » Journal of Blood Medicine » Volume 7
Pain frequency, severity and QT dispersion in adult patients with sickle cell anemia: correlation with inflammatory markers
Authors Garadah TS , Jaradat AA, AlAlawi ME, Hassan AB , Sequeira RP
Received 8 June 2016
Accepted for publication 31 August 2016
Published 31 October 2016 Volume 2016:7 Pages 255—261
DOI https://doi.org/10.2147/JBM.S114585
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Taysir S Garadah,1,2 Ahmed A Jaradat,2 Mohammed E AlAlawi,1 Adla B Hassan,1 Reginald P Sequeira2
1Salmanyia Medical Complex, Ministry of Health, 2College of Medicine and Medical Sciences, Arabian Gulf University, Manama, Kingdom of Bahrain
Background: Inflammatory markers are increased during vaso-occlusive crisis (VOC) in adult patients with sickle cell anemia (SCA), but this is not clear in clinical steady state.
Aim: The present study aims to establish the frequency and intensity of bone pain episodes in adult patients with SCA in clinical steady state and to determine the correlation between different inflammatory markers, other variables including QT dispersion (QTd) and pain frequency and intensity in SCA.
Patients and methods: Patients were classified into two groups: group 1, those with more than three hospital admissions in the last 6 months, and group 2, those with no hospital admission. Pearson correlation between variables such as body mass index (BMI), level of tumor necrosis factor (TNF-α), interleukin-1 (IL-1), C-reactive protein (CRP), hemoglobin (Hb), reticulocyte count, white blood cell count (WBC), ferritin, lactate dehydrogenase (LDH), parathormone (PTH), vitamin D3 (25-OH cholecalciferol) and bone pain frequency with severity was evaluated.
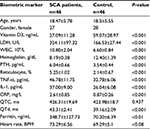
Results: Forty-six patients were enrolled in this study with a mean age of 18.47±5.78 years, with 23 patients in each group. Vitamin D3 and Hb were lower (17.04±5.77 vs 37.59±4.83 ng/L, P<0.01 and 7.96±0.3 vs 8.44±0.27 g/dL, P<0.01, respectively); the inflammatory markers showed significantly higher level of TNF-α, IL-1 and CRP (56.52±5.43 pg/ml, 44.17±4.54 pg/ml and 3.20±0.72 mg/L, respectively, P<0.05); WBC, LDH and reticulocyte count were also significantly higher and the QTd was higher (45.0±2.22 vs 41.55±0.8 ms, P<0.05) in group 1 when compared with group 2. Pearson correlation coefficient showed significant positive correlation between serum level of TNF-α and bone pain frequency (r=0.414, P<0.005) and serum level of IL-1 (r=0.39, P<0.008).
Conclusion: There is a strong positive correlation between TNF-α, IL-1 and WBC and bone pain frequency in steady state in adult patients with SCA. CRP and low hemoglobin had weak positive correlation. QTd was significantly longer in patients who had hospitalizations with VOC.
Keywords: sickle cell anemia, analog pain scale, immunological markers
Introduction
Sickle cell anemia (SCA) is a hereditary hemolytic anemia where the synthesis of hemoglobin (Hb) is abnormal and the inheritance can be heterozygous (HbS) or homozygous (HbSS).
Homozygous patients with SCA often develop vaso-occlusive crises (VOCs) and recurrent episodes of hemolysis causing multi-organ infarction and dysfunction.1,2 Complications can be of acute nature with chest syndrome, acute abdominal or bone pain syndrome, renal insufficiency and cerebral vascular accident.3
Bone pain in patients with SCA varies from acute clinical painful VOC or osteomyelitis to more chronic and debilitating disease, such as osteoporosis, osteopenia and osteonecrosis, and chronic infections with impaired growth.4 Osteopenia may be asymptomatic but can cause pain, fractures, deformity and vertebral collapse requiring chronic analgesia or surgical interventions.5
Recurrent hemolytic anemia leads to bone marrow hyperplasia and subsequent bone deformity. Bone pain and other complications are a major source of morbidity in SCA and affect patients’ quality of life.6
Saleh et al showed a better understanding of the pathophysiology of VOC. The interaction of red blood cells (RBCs) with endothelial cells, reaction with adhesion molecules, cytokines, polymorphonuclear neutrophils and platelets further clarified the understanding of the SCA manifestations.7 The clinical manifestations of SCA depend on the way of clinical assessment and patients enrolled in the study, ethnicity and geographical distribution.8 Many patients with SCA have high risks of osteopenia, osteoporosis including low body weight status, and vitamin D deficiency.9
QT dispersion (QTd) on electrocardiogram (ECG) was suggested as a marker of ventricular recovery times to distinguish between homogeneous myocardium and non-homogeneous myocardium in repolarization of myocardium.10 It was defined as maximum QT interval minus minimum QT interval. The QTd value is in the range of 10–70 ms with a mean of 29±26 ms in adolescents.11 It was shown to increase in various cardiac diseases including acute myocardial infarction, left ventricle hypertrophy of hypertension and congestive cardiac failure. Patients with dilated cardiomyopathy and the long QT syndrome indicated repolarization abnormality.12,13 One study claimed that QTd >40 ms has a sensitivity and specificity of 88% and 57%, respectively, for predicting the inducibility of sustained ventricular tachycardia during an electrophysiology study.14
The QTd has been evaluated for its prognostic role in patients with end-stage renal disease, requiring hemodialysis, and diabetes.15–17
The low vitamin D3 status and the frequency and intensity of bone pain crises in children with SCA are well documented,6–9 but such a relationship is not clear in adult patients who may live until the fourth or fifth decade. Furthermore, the best predictor of adverse outcome in such disease entity is not obvious.
The present study aims to, 1) establish the frequency and intensity of bone pain crises in adult patients with SCA in Bahrain in clinical steady state with no VOC, and 2) determine the correlation between different biomarkers and QTd in one perspective and pain frequency and intensity in SCA in another perspective.
Patients and methods
Study population
Forty-six adult patients (27 females) with SCA were studied, with a mean age of 12–40 years. The study was carried out from January 2013 to December 2013 (12 months), in Salmanyia Medical Complex. The patients were selected from the hematology clinic, and they were compared with 46 patients with bone pain but proven to be with no SCA.
Inclusion criteria
Patients were included if they were diagnosed with sickle cell disease (SCD) with homozygous hemoglobin (HbSS) using Hb electrophoresis and solubility screening test for sickling. Patient written informed consent to participate in this study was obtained. The study and protocols were approved by the Salmanyia Medical Complex Review Board.
Exclusion criteria
Patients with a history of acute chest pain in the last 3 weeks and blood disorders other than SCA were excluded. Patients with bone fracture, severe renal failure or hepatic failure or who had acute VOCs within 10 days prior to the enrollment were excluded.
Methods
Each patient gave a full medical history about the daily frequency of chest or bone pain, and previous hospitalization within the last 12 months prior to the enrollment, and medications. The weight and height were measured, and the body mass index (BMI) was calculated. Patients were defined to be in a steady state if they had no history of blood transfusion during the last 4 months, no history of intercurrent illness such as infection and inflammation during the last 4 weeks and no treatment with medications such as antibiotics that may affect the blood counts during the last 3 weeks.
Blood samples were obtained at base line, 1 month and 3 months after enrollment to determine the level of tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), C-reactive protein (CRP), Hb, reticulocyte count, lactate dehydrogenase (LDH), vitamin D3, parathormone (PTH) and serum ferritin. Patients were examined clinically for bone pain or tenderness in the peripheral joints, chest or vertebrae within the last 2 days prior to assessment.
Every patient was provided with a booklet for recording the frequency of bone or muscular pain on a daily basis and marking the intensity of pain with the visual analog scale (VAS) with the following color coding of five grades: grade 1: white color, 1–2/10 points, grade 2: green color, 3–4/10 points, grade 3: yellow color, 5–6/10 points, grade 4: orange color, 7–8/10 points and grade 5: red color, 9–10/10 points.16 The frequency and severity of bone pain were tabulated on a daily basis, then weekly basis and on a monthly basis for 3 months. The frequency and severity of pain were calculated as the mean per month ± SD.
The severity of pain was defined as follows: 1: mild, if the color code noted is white or green, 2: severe, if the color code is red or orange, and 3: moderate severity, if the color code is yellow.
Serum level of vitamin D3 was measured using commercially available vitamin D kits (Chromsystems Instruments & Chemicals GmbH, Gräfelfing, Germany). Quantification of PTH in serum was performed by enzyme-linked immunosorbent assay (Creative Diagnostics, Shirley, NY, USA). Serum TNF-α and IL-1 levels were measured using the enzyme-linked immunosorbent assay technique (enzyme-amplified sensitivity immunoassay kits; BioSource Europe SA, 8 B-1400, Nivelles, Belgium).
The 46 patients enrolled in the study were classified into two groups: group 1, those with more than three hospital admissions in the last 6 months, and group 2, those with no hospital admission. All patients had a 12-lead ECG.
QT interval was corrected for heart rate (QTC), and the K value was 0.415 for women and 0.397 for men, as modified by Shipley and Hallaran.18,19 The ECG was recorded at a speed of 25 mm/s. The value of QTd was a mean of three calculated values on V1 to V6 chest leads. The methods of QTd calculation were as previously reported.20
Statistical analysis
The statistical analysis software package (IBM SPSS Statistics for Windows, version 21.0; IBM Corp., Armonk, NY, USA) was used for data analysis. Clinical characteristics and biometric data of patients with SCA and the normal population are presented as mean ± SD. Student’s t-test was used to compare the mean difference between two groups with SCA and the control. QTd interval was adjusted for body surface area in all the patients.
The correlation between different variables and the frequency and severity of bone or muscular pain was performed using Pearson correlation coefficient. P<0.05 was considered as statistically significant.
Results
This study included 46 patients with SCA, with a mean age of 18.47±5.78 years (range 12–40) of whom 27 (60%) were females.
Table 1 summarizes the clinical and biometric characteristics of patients with SCA compared with age- and gender-matched normal population. Patients with SCA had significantly lower Hb (8.73 vs 9.98 g/dL, P<0.001) and vitamin D3 (21.11 vs 47.02 ng/mL, P<0.001) levels. Furthermore, there were higher levels of ferritin (317.35 vs 80.09 ng/mL, P<0.001), white blood cell count (WBC; 10.80±2.04 vs 6.60±0.84 109/L, P<0.01), reticulocyte (6.2 vs 2.08%, P<0.001), LDH (485.36 vs 195.95 U/L, P<0.001) and PTH (68.96 vs 41.56 pg/mL, P<0.001).
The inflammatory markers in SCA were significantly higher for TNF-α: 46.78±11.75 vs 32.78±6.06 pg/mL, P<0.01, IL-1: 37.00±9.0 vs 26.04±6.08 pg/mL, P<0.01 and CRP: 2.61±0.85 vs 0.87±0.26 mg/L, P<0.01 compared with the control; likewise, QTd was higher (43.31±2.41 vs 39.16±2.09 ms), and QTC was higher but with no significant statistics.
Table 2 summarizes the clinical and laboratory findings data of patients with SCA in group 1 who had more than three hospitalizations and group 2 with no hospitalization.
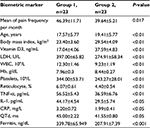
The patients in group 1, compared with group 2, had significantly higher pain frequency per month (46±11.71 vs 39±5, P<0.017), with lower vitamin D3 in blood (17.04±4.06 vs 37.59±4.83 ng/mL).
Furthermore, the WBC, LDH and reticulocyte count were significantly higher in group 1 compared with group 2. The inflammatory markers showed significantly higher levels of TNF-α, IL-1 and CRP (56.52±5.43 vs 36.59±6.76 pg/ml, P<0.0144; 17±4.54 vs 29.5±5.74 pg/m, P<0.05; and 3.20±0.72 vs 1.99±0.41 mg/L, P<0.05, respectively) in group 1 compared with group 2.
Patients in group 1 compared with group 2 had a significantly higher QTd on ECG with a QTd interval of 45.00±2.22 vs 41.55±0.80 ms, P<0.05.
The frequency of bone pain per month over 6 months was 46.39±11.71 vs 39.64±5.21, P<0.017, in group 1 compared with group 2.
The intensity of pain recorded in a daily diary using color code of VAS is depicted in Figure 1 for both groups. The pain frequency per month in group 1 was observed to be severe in 27, moderate in ten and mild intensity in nine patients, while in group 2, the intensity was severe in 12, moderate in eight and mild in 19 patients.
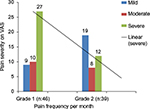  | Figure 1 The mean of pain frequency per month for the two groups (1 and 2) in patients with SCA and pain intensity using VAS. Abbreviations: SCA, sickle cell anemia; VAS, visual analog scale. |
The Pearson correlation coefficient showed significant positive correlation between the high serum level of TNF-α and bone pain frequency (r=0.414, P<0.005). Also, the serum level of IL-1 was positively correlated with pain frequency (r=0.39, P<0.008).
The Pearson correlation coefficient showed no significant correlation between a high level of WBC and pain frequency (r=0.258, P=0.107), and also PTH (r=0.216, P=0.153), ferritin (r=0.289, P<0.054) and CRP (r=0.049, P=0.747). Furthermore, both low level of Hb as well as BMI had negative correlations (r=0.244, P=0.107; and r=0.196, P=0.263), respectively.
Discussion
Acute pain is a common clinical manifestation of SCA and patients seek medical attention; however, some of these episodes are short-lived and are managed at home.21,22 Frequency of pain peaks between the age of 19 and 39 years; the higher frequency of pain is associated with a higher mortality rate in patients over age 19 years.23 In one report, pain in SCA was assessed using the data recorded in a daily diary by adults with sickle cell disease.24
In this study, bone pain frequency and intensity were significantly higher among those with lower Hb. A painful event without seeking hospital care was the norm rather than the exception. A similar discrepancy between home-managed pain and hospital treatment has been observed in pediatric patients.21 The episodes of pain can affect any area of the body, with the back, chest and extremities, but the abdomen being most commonly affected; the pain severity can range from trivial to excruciating.
The intensity of bone pain in adult patients with SCA was assessed and analyzed using VAS with color coding. Patients in group 1 in steady state had significantly more pain frequency per month compared with patients in group 2, and the intensity of severe pain episodes was higher in group 1 (27/56; 58.7%) compared with (12/39; 30.7%) in group 2.
Clinical characteristics of adult patients with SCA compared with those without the evidence of lower Hb and higher reticulocyte and LDH indicate a state of long-standing increment of hemolysis. These findings are consistent with other previous reports where the mean Hb and hematocrit concentrations were 7.9 g/dL and 22.9%, respectively, with an absolute reticulocyte count of five times the normal.25
When the Hb level was <7.9 g/dL, there was a weak positive correlation with bone pain frequency on VAS, and with severe intensity in the absence of VOC, or in the steady state.
It seems that adult patients with SCA in steady state had a continued low grade of RBC hemolysis that contributes significantly to higher serum LDH, as evidenced in the previous report of definitive chromium radiolabeling red cell survival studies,26 and by several other authors where LDH level increases as Hb level decreases,27–29 accompanied by increased LDH due to lysis of cells from other organs as well.
WBC was significantly higher in steady state in adult patients with SCA in group 1 compared with group 2, which supports the theory of the baseline inflammatory status in this group of patients. In one report, patients with SCA had WBCs higher than normal, particularly under the age of 18 years. The elevated WBC is linked to the pathophysiology of sickle cell disease and increases the morbidity in this group of patients.30
Vitamin D3 was significantly lower in patients in group 1 compared with group 2. This may explain partially the increase in pain frequency and intensity in group 1. One recent report showed association of increased inflammatory markers and vitamin D3 deficiency in SCA.31 Ferritin level was significantly higher among patients in group 1 versus group 2, which highlights the long-term outcome in patients, due to multiple blood transfusions in group 1. In a previous report, high serum ferritin level had poor prognostic adverse outcome in such a group of patients.32,33
The QTd was significantly longer in group 1 indicating the more heterogeneity of the QT interval, which leads to more arrhythmia and adverse outcomes. This is consistent with a recent report where both QTC and QTd were significantly higher in SCA in addition to abnormal cardiac autonomics.34
In a study by Fu et al,35 QTd of repolarization was determined to be the main electrophysiological cause for sudden death in patients with ischemic heart disease and dilated cardiomyopathy.
The three inflammatory markers, namely, CRP, IL-1 and TNF-α were significantly higher in group 1 compared with group 2. This was in agreement with other trials, as patients with SCA were observed to have higher TNF-α, IL-1 and CRP levels that were related to increased frequency of pain in the steady state.36–38
In one report, there was an inverse relation between vitamin D3 level and the TNF-α level that may be associated with endothelial dysfunction in steady state with activation of monocyte that plays a role in the pathophysiology of VOC in SCA.39 So, what seems a steady state is actually a misnomer of the interval between severe VOC, where patients had biochemical and microvascular occlusion that is insufficient to cause the full overt tissue infarction of severe painful crisis,40 but it is responsible for a low sub-acute state of low pain status that is below the threshold of precipitating crises.
In one study, the inflammatory marker, CRP, was established as the most significant correlate of hospitalizations for painful episodes, whereas markers of increased hemolytic status, such as endothelial activation and coagulation activation, correlated positively with VOC events by univariate analysis.41
Over recent years, the role of CRP as a plasma biomarker for low-grade systemic inflammation has been intensely investigated for its predictive associations with adverse outcomes in vascular diseases, such as cardiovascular42 and peripheral arterial diseases.43
Many studies have shown altered proinflammatory cytokine levels in the plasma of patients with SCA during both steady state and acute VOC,44,45 but no consistent pattern of cytokines involvement in SCA has emerged that correlates with specific clinical outcomes. However, these cytokines, in turn, could be responsible for driving the low-grade or chronic inflammatory response, evidenced by the presence of mild–moderate, baseline elevations of acute phase reactants, such as CRP.46
These results highlight the clinical relevance of inflammation in microvessel occlusive complications and provide a basis for further evaluation of TNF-α and IL-1 as potential biomarkers for predictive clinical outcomes in adult patients with SCA, and for the future trials of anti-inflammatory therapies as primary treatment of patients with SCA.
The findings presented in this study should be interpreted cautiously as there are a wide variety of major contributions to the pathogenesis of SCD.
The results confirm previous reports in the field but they do not represent a step forward in the field of SCD. They support the view that the chronic inflammatory response is an ongoing process not only during crises but also during steady-state conditions.
Conclusion
There is a strong positive correlation between the inflammatory biomarkers, TNF-α, IL-1 and WBC, and bone pain frequency in steady state in adult patients with SCA. QTd is significantly longer among patients with SCA who had more than three hospitalizations over a 6-month interval.
Acknowledgment
This study was funded by College of Medicine & Medical Sciences, grant number 81.
Disclosure
The authors report no conflicts of interest in this work.
References
Alhamdan NA, Almazrou YY, Alswaidi FM, Choudhry AJ. Premarital screening for thalassemia and sickle cell disease in Saudi Arabia. Genet Med. 2007;9(6):372–377. | ||
Nasserullah Z, Alshammari A, Abbas MA, et al. Regional experience with newborn screening for sickle cell disease, other hemoglobinopathies and G6PD deficiency. Ann Saudi Med. 2003;23(6):354–357. | ||
Omojola MF, Annobil S, Adzaku F, Addae SK, Mohammed S. Bone changes in sickle cell anaemia. East Afr Med J. 1993;70(3):154–158. | ||
Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–773. | ||
Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. | ||
Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol. 2005;129(4):482–490. | ||
Saleh AW, Hillen HF, Duits AJ. Levels of endothelial, neutrophil and platelet-specific factors in sickle cell anemia patients during hydroxyurea therapy. Acta Haematol. 1999;102(1):31–37. | ||
Pearson HA. Reply: sickle cell disease in the Kingdom of Saudi Arabia: east and west. Ann Saudi Med. 1999;19(3):281–282. | ||
Goodman BM 3rd, Artz N, Radford B, Chen IA. Prevalence of vitamin D deficiency in adults with sickle cell disease. J Natl Med Assoc. 2010;102(4):332–335. | ||
Kautzner J, Malik M. QT interval dispersion and its clinical utility. Pacing Clin Electrophysiol. 1997;20(10 pt 2):2625–2640. | ||
Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol. 2000;36(6):1749–1766. | ||
Ashikaga T, Nishizaki M, Arita M, et al. Effect of dipyridamole on QT dispersion in vasospastic angina pectoris. Am J Cardiol. 1999;84(7):807–810. | ||
Galinier M, Vialette JC, Fourcade J, et al. QT interval dispersion as a predictor of arrhythmic events in congestive heart failure. Importance of aetiology. Eur Heart J. 1998;19(7):1054–1062. | ||
Goldner B, Brandspiegel HZ, Horwitz L, Jadonath R, Cohen TJ. Utility of QT dispersion combined with the signal-averaged electrocardiogram in detecting patients susceptible to ventricular tachyarrhythmia. Am J Cardiol. 1995;76(16):1192–1194. | ||
Langen KJ, Ziegler D, Weise F, et al. Evaluation of QT interval length, QT dispersion and myocardial m-iodobenzylguanidine uptake in insulin-dependent diabetic patients with and without autonomic neuropathy. Clin Sci (Lond). 1997;93(4):325–333. | ||
Wu VC, Lin LY, Wu KD. QT interval dispersion in dialysis patients. Nephrology (Carlton). 2005;10(2):109–112. | ||
McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007–1019. | ||
Bazett HC. An analysis of the time-relations of electrocardiograms. Ann Noninvasive Electrocardiol. 1997;2(2):177–194. | ||
Shipley RA, Hallaran WR. The four-lead electrocardiogram in two hundred normal men and women. Am Heart J. 1936;11(3):325–345. | ||
Pye M, Quinn AC, Cobbe SM. QT interval dispersion: a non-invasive marker of susceptibility to arrhythmia in patients with sustained ventricular arrhythmias? Br Heart J. 1994;71(6):511–514. | ||
Dampier C, Setty BN, Eggleston B, Brodecki D, O’neal P, Stuart M. Vaso-occlusion in children with sickle cell disease: clinical characteristics and biologic correlates. J Pediatr Hematol Oncol. 2004;26(12):785–790. | ||
Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94–101. | ||
Baum KF, Dunn DT, Maude GH, Serjeant GR. The painful crisis of homozygous sickle cell disease. A study of the risk factors. Arch Intern Med. 1987;147(7):1231–1234. | ||
Aisiku IP, Smith WR, McClish DK, et al. Comparisons of high versus low emergency department utilizers in sickle cell disease. Ann Emerg Med. 2009;53(5):587–593. | ||
West MS, Wethers D, Smith J, Steinberg M. Laboratory profile of sickle cell disease: a cross-sectional analysis. The Cooperative Study of Sickle Cell Disease. J Clin Epidemiol. 1992;45(8):893–909. | ||
Ballas SK, Smith ED. Red blood cell changes during the evolution of the sickle cell painful crisis. Blood. 1992;79(8):2154–2163. | ||
Atkinson AJ, Colburn WA, DeGruttola VG, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. | ||
Bargoma EM, Mitsuyoshi JK, Larkin SK, et al. Serum C-reactive protein parallels secretory phospholipase A2 in sickle cell disease patients with vasoocclusive crisis or acute chest syndrome. Blood. 2005;105(8):3384–3385. | ||
Brittain JE, Parise LV. Cytokines and plasma factors in sickle cell disease. Curr Opin Hematol. 2007;14(5):438–443. | ||
Tassel C, Arnaud C, Kulpa M, et al. Leukocytosis is a risk factor for lung function deterioration in children with sickle cell disease. Respir Med. 2011;105(5):788–795. | ||
Hassan AB, Garadah T, Diab DE, et al. Increased serum levels of tumour necrosis factor-alpha and interleukin-6 in adult Bahraini sickle cell patients with hypovitaminosis D and low bone mineral density. Int J Curr Res. 2015;7(9):20702–20707. | ||
Brownell A, Lowson S, Brozovic M. Serum ferritin concentration in sickle cell crisis. J Clin Pathol. 1986;39(3):253–255. | ||
Hussain MA, Davis LR, Laulicht M, Hoffbrand AV. Value of serum ferritin estimation in sickle cell anaemia. Arch Dis Child. 1978;53(4):319–321. | ||
Kolo PM, Sanya EO, Olanrewaju TO, Fawibe AE, Soladoye A. Cardiac autonomic dysfunction in sickle cell anaemia and its correlation with QT parameters. Niger Med J. 2013;54(6):382–385. | ||
Fu GS, Meissner A, Simon R. Repolarization dispersion and sudden cardiac death in patients with impaired left ventricular function. Eur Heart J. 1997;18(2):281–289. | ||
Francis RB Jr, Haywood LJ. Elevated immunoreactive tumor necrosis factor and interleukin-1 in sickle cell disease. J Natl Med Assoc. 1992;84(7):611–615. | ||
Kuvibidila S, Gardner R, Ode D, Yu L, Lane G, Warrier RP. Tumor necrosis factor alpha in children with sickle cell disease in stable condition. J Natl Med Assoc. 1997;89(9):609–615. | ||
Pathare A, Al Kindi S, Alnaqdy AA, Daar S, Knox-Macaulay H, Dennison D. Cytokine profile of sickle cell disease in Oman. Am J Hematol. 2004;77(4):323–328. | ||
Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–2135. | ||
Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. | ||
Krishnan S, Setty Y, Betal SG, et al. Increased levels of the inflammatory biomarker C-reactive protein at baseline are associated with childhood sickle cell vasocclusive crises. Br J Haematol. 2010;48(5):797–804. | ||
Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145(1):21–29. | ||
Vainas T, Stassen FR, de Graaf R, et al. C-reactive protein in peripheral arterial disease: relation to severity of the disease and to future cardiovascular events. J Vasc Surg. 2005;42(2):243–251. | ||
Bourantas KL, Dalekos GN, Makis A, Chaidos A, Tsiara S, Mavridis A. Acute phase proteins and interleukins in steady state sickle cell disease. Eur J Haematol. 1998;61(1):49–54. | ||
Duits AJ, Schnog JB, Lard LR, Saleh AW, Rojer RA. Elevated IL-8 levels during sickle cell crisis. Eur J Haematol. 1998;61(5):302–305. | ||
Singhal A, Doherty JF, Raynes JG, et al. Is there an acute-phase response in steady-state sickle cell disease? Lancet. 1993;341(8846):651–653. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.