Back to Journals » Journal of Pain Research » Volume 16
Pain Fluctuations of Women with Subacute Herpetic Neuralgia During Local Methylcobalamin in Combination with Lidocaine Treatment: A Single-Blinded Randomized Controlled Trial
Authors Xu G , Tang W, Zhou C, Xu J, Cheng C, Gong W, Dong S, Zhang Y
Received 24 January 2023
Accepted for publication 12 April 2023
Published 15 April 2023 Volume 2023:16 Pages 1267—1284
DOI https://doi.org/10.2147/JPR.S404713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
Gang Xu,1 Weizhen Tang,1 Chaosheng Zhou,1 Jie Xu,1 Chao Cheng,1 Weiwei Gong,2,3 Shihong Dong,2 Yu Zhang1
1Department of Rehabilitation Medicine, Affiliated Tenth People’s Hospital of Tongji University, Shanghai Tenth People’s Hospital, Shanghai, 200072, People’s Republic of China; 2Department of Rehabilitation Medicine, Tongji University School of Medicine, Shanghai, 200092, People’s Republic of China; 3Department of Rehabilitation Medicine, Shanghai First Rehabilitation Hospital, Shanghai, 200090, People’s Republic of China
Correspondence: Gang Xu, Department of Rehabilitation Medicine, Affiliated Tenth People’s Hospital of Tongji University, Shanghai Tenth People’s Hospital, 301 Middle Yanchang Road, Shanghai, 200072, People’s Republic of China, Tel +8621-66306496, Fax +8621-66301051, Email [email protected]
Purpose: To evaluate the efficacy and pain fluctuations of methylcobalamin in combination with lidocaine local injection treatment for subacute herpetic neuralgia (SHN).
Methods: Seventy-nine women (60.4 ± 2.7 years) with thoracic SHN were enrolled and randomized to receive a combination of methylcobalamin and lidocaine local injection (MI, N=40), or a combination of lidocaine patch 5% and oral methylcobalamin (PO, N=39) for four weeks. Repeated-measures analyses of variance were used to evaluate the effect on pain levels. Generalized estimation equations were used to analyze the cause-effect relationship between pain fluctuations and influencing factors.
Results: At the treatment endpoint, the group, treatment time, and group interacted with treatment time effects of the pain scores and area were statistically significant (P< 0.001), The pain scores were 2.9 ± 0.9 (MI) and 4.3 ± 1.5 (PO). 80.00% (MI) or 28.21% (PO) of patients had pain scores ≤ 3, the odds ratio was 2.84 (95% CI: 1.68 to 4.79). The incidence of postherpetic neuralgia was 5.0% (2/40) at 3 months. Pain fluctuated repeatedly during treatment. The pain fluctuation increased from 8.75 log folds in the afternoon, to 79.85 log folds at night. With the ADLs level increasing from 1 to 3, the pain fluctuated from 4.28 to 17.70 log folds. Allodynia, itching, sleep quality, and ADLs were the significant influencing factors (P< 0.05).
Conclusion: This study validated the efficacy of methylcobalamin combined with lidocaine for SHN, and confirmed that pain levels in patients with SHN had an obvious circadian rhythm. ADLs were an important cause of pain fluctuations.
Keywords: subacute herpetic neuralgia, methylcobalamin, lidocaine, pain fluctuation, activities of daily living
Introduction
Subjects with herpes zoster (HZ) that are > 50 years old are more likely to have severe zoster-associated pain (ZAP) and develop postherpetic neuralgia (PHN),1 which may severely impact the quality of life (QoL), decreasing physical functionality and activities of daily living (ADL), and creates severe difficulties in both professional and personal life.2,3 Clinical studies have shown that the pain intensity caused by neuropathic pain exhibits a circadian pattern clinically.4–8 Peripheral neuropathic pain is often worse at night and significantly impairs sleep.6 Odrcich`s data suggested that the pain intensity of PHN progressively increased throughout the day.9 In patients with thoracic PHN with allodynia, touch-evoked pain appears to play an important role in progressively higher pain levels towards the end of the day.9 The difference in pain intensity from 8:00 AM to 8:00 PM was more pronounced in females than in males.10 Previous studies have reported that acute HZ interfered in all health domains, especially sleep (64% of participants), enjoyment of life (58%), and general activities (53%).11 Coplan et al investigations demonstrated that PHN often restricts walking and mobility.12 This would imply that physical activity either evokes or exacerbates neuropathic pain, and cumulative activity throughout the day may lead to progressive increases in pain.9 Pain-related disability includes body actions, activity limitations, and participation restrictions induced by pain. In patients with PHN, pain-related disability is one of the most important outcomes and key to treatment.13 Therefore, appropriate assessments of factors for pain-related disability are required for pain management.
Acute ZAP occurs in >95% of patients older than 50 years of age; 60–70% of patients continue to experience persistent pain one month after disease onset.14,15 It is estimated that 5–20% of those with HZ go on to develop PHN.16 The median age of patients with HZ and PHN is 56.3 and 65.9 years, respectively.17 Women are at higher risk of HZ and PHN.18,19 The main pathological mechanism of PHN is the reactivated varicella-zoster virus (VZV)-induced injury of nerve fibers from the affected dorsal root ganglion.20,21 The persistence of local pain after the onset of vesicles implies that the damaged neural tissues, such as afferent fibers, epidermal nerve fibers, and endings, play an important role in the pathogenesis of PHN.22 Subacute herpetic neuralgia (SHN), which is defined as pain beyond the acute phase that persists for approximately 30 to 120 days after the onset of vesicles.23,24 Prompt antiviral therapy may reduce the incidence and severity of PHN. However, these therapies do not completely alleviate ZAP.25–28 Treatment for ZAP is focused on symptom control.29–31 However, the analgesic effect is often not satisfactory.18 Given the continued high incidence of HZ, it remains important to determine better ways to treat ZAP.18 SHN is the early stage of nerve injury, and timely treatment at this critical stage can effectively reduce the incidence of PHN.32,33 A specific mechanism-based intervention, such as a promoting nerve repair strategy based on neurotrophic agents, methylcobalamin (MeB), has shown a more persistent analgesic effect.34
The present study aimed to evaluate the long-term effect of local MeB12 injection in combination with lidocaine on SHN, and the acute within-day mutual reactivity of pain fluctuations, pain intensity, phenotypes, activities of daily living (ADLs), and sleep quality throughout the circadian cycle in patients with thoracic SHN during treatment.
Methods
Study Design
This study was a prospectively registered randomized controlled trial (ChiCTR2000034972; 26 July 2020 to 15 August 2021) conducted at the Affiliated Tenth People’s Hospital of Tongji University, following the Ethics Review Board of Affiliated Tenth People’s Hospital of Tongji University approval (SHSY-IEC-4.0/20-32). All procedures involving human participants were performed by the ethical standards of the institutional and national research committee and the 1964 Helsinki Declaration, its later amendments, or comparable ethical standards. Eligible participants gave written informed consent after the provision of the trial information sheet and counseling by staff not involved in the trial. Data management was performed at the Affiliated Tenth People’s Hospital of Tongji University. To reflect the living state characteristics of women, the age of the enrolled women was controlled at 55–65 years old. We report in compliance with the CONSORT statement.35
Inclusion Criteria
Eligibility criteria included: a diagnosis of thoracic SHN for at least 30 days and no more than 4 months; ≥55 years of age women; cutaneous and/or subcutaneous pain and/or other discomforts at the dermatomal T4-6 levels; or adjacent cutaneous dermatomes temporally and spatially associated with the HZ rash; and a pain score ≥ 4 on the 11 point numerical rating scale (NRS) in the past 24 h; and pain area greater than or equal to one palm area.
Exclusion Criteria
Subjects who met the following criteria were excluded from this study: presentation or history of a serious or unstable medical or psychological condition, and insomnia that would compromise participation in the study, or confound the assessment of SHN.
Rationale Behind Sample Size Determination
The use of topical lidocaine is supported by clinical practice guidelines, including first-line treatment by the European Federation of Neurological Societies36 and second-line by the Canadian Pain Society.37 Lidocaine patch 5% (LP) is one of the currently available treatment options for PHN patients.38,39
We propose a strategy based on neurotrophic drugs to promote nerve repair to treat pain. The expected proportion of pain intensity NRS of 3 or lower for the 28-day treatment was 85% compared with a 46.2% response rate of LP at the endpoint,40 with a one-sided 5% significance level. With an assumption of error of 0.05 and an anticipated dropout rate of 15%, a minimum of 34 patients per group was required for 80% statistical power. Considering the subsequent analysis of the impact factors of pain fluctuations, the number of patients in each group increased by another 15%. Thus, a total of 78 patients were needed for this study.
Patient Recruitment
Subjects with SHN were recruited at the department of rehabilitation, Affiliated tenth people’s hospital of Tongji university from August 2020 to August 2021. The participant belonged to the Asian/Chinese race women and ranged between 55 and 65 years old.
Randomization
An independent research physician evaluated the eligible subjects who agreed to enroll for the trial and provided written informed consent. All the enrolled subjects` demographic and baseline characteristics were recorded at the baseline visit. Another independent physician performed the randomization procedure. Assignment to local injection or oral medication was in a 1:1 ratio with each assignment secured inside a sealed opaque envelope holding a unique identifier. The randomization list was created by a random number generator. On treatment Day 1, the eligible patient was assigned their unique identifier corresponding with the sealed envelope sequence.
Intervention
The randomized subjects were categorized into two groups: MeB combined with lidocaine local injection (MI) group, and LP 5% combined with oral MeB (PO) group.
The subjects in the MI group received MeB (1000--1500 μg, Eisai Co. Ltd, Tokyo, Japan) plus 2.0% lidocaine (20–30 mg, Fuda Co. Ltd, Shanghai, China) local injection. All drugs were mixed to get a 4.0–6.0 mL injection, which was administered with a 25-gauge needle and a sterile hypodermic syringe. The drugs were injected into the most painful area reported by the patients, with 0.3–0.5 mL of liquid injected per region. The patients were observed for 1 hour after the first administration and were subsequently discharged without any symptoms of discomfort. The treatment programs were performed once daily every morning, between 8 and 11 a.m, five times a week for 4 wks.
Because patients were prone to external use of LP, we used LP in combination with oral MeB as a control group. Subjects in the PO group received topical LP, 5% (10 ×14 cm, lidocaine 700 mg, 5% w/w, Tide Pharmaceutical Co., Ltd, Beijing, China) once daily by a therapist, in addition, to oral a 500-μg MeB tablet (Eisai Co. Ltd, Tokyo, Japan) three times daily. Up to three LP, 5% were simultaneously applied as needed pain area for 12 hours daily. To ensure the compliance of patients, the dosage and method of LP were controlled by doctors.
Single Blind
Given the nature of the injection, it was impossible to blind the physician and subjects. The independent physicians who did not participate in clinical treatment conducted the pain assessment.
Adverse Events
Adverse events were monitored and recorded during the treatment period by a treating physician who immediately reported to a researcher. The researcher then recorded the data on standardized clinical notes for each participant.
Participant Compliance
The number of treatment sessions attended and the number of missed or canceled appointments were recorded by the treating physician.
Primary Outcome
All patients underwent a structured interview to assess the clinical and phenomenological characteristics of their pain sensations, such as the location, descriptive scope characteristics, intensity, data on pain duration, and QoL were obtained by Zoster Brief Pain Inventory (ZBPI) and health-related QoL,12 and Euro-Qol VAS.41
Pain intensity was measured using the ZBPI, to assess the worst pain level and period of the previous day. ZAP is generally described as continuous spontaneous pain, paroxysmal pain,42 tactile allodynia,22 itchings,43,44 and numbness. Further measures consisted of five phenotypes of pain.
Because the pain associated with HZ was located in the partial regions innervated by distal nerve branches instead of the entire nerve distribution,45 one unit of the pain scope in this study was defined as the whole palm area of the patient-self`s right hand. Subjects’ perceived pain regions were mapped in a sagittal fashion from the lateral to the midline, and the unit proportions were then calculated.
At the endpoint, patients were asked whether they felt a reduction of 4 points (approximately 50%) in pain score in the preceding 28 days compared with how they felt before receiving treatment in this study, the proportion of patients who had a pain score ≤ 3, and the numbers of patients using analgesics after treatment.
Health-Related QoL
The secondary measures were to assess interference with health-related QoL using the ZBPI during the four-week treatment period. The ZBPI QoL scores were used to measure selected ADL and health,12 which included the interference of HZ-related pain with seven QoL components: general activity, mood, walking ability, normal work, relationship with others, sleep, and enjoyment of life. The EuroQoL VAS is a validated measure of health-related QoL that can assist with subjective measurements of the current health state using a scale of 0 to 100.41
Pain Fluctuations and Influencing Factors
Given the aim of the current study, while the efficacy was evaluated, and acute within-day mutual reactivity of circadian rhythm of pain fluctuations, ADLs, and sleep quality were particularly concerning.
To assess the circadian rhythm of pain fluctuations, one day and night were divided into 4 periods following living habits, starting from the morning (5:00 to 12:00); the noon and afternoon (12:00 to 17:00); the evening (17:00 to 22:00); and the night (22:00 to 5:00).
As no standard definition for pain fluctuation exists,46 it is difficult to accurately recall the pain intensity.47–49 Pain fluctuation was defined as a change of greater than 2 scores on NPS in comparison to the pain rating at every treatment visit in this study.
The pain fluctuation extent was divided into 4 levels. The patients were inquired about the worst pain level and the period of the previous day, and the answer options were “much worse pain (greater than 4 scores on NPS)”, “worse pain (greater than 2 scores on NPS)”, “as usual”, and “pain relief (greater than 2 scores on NPS)” to compare with current pain levels at the time of visit (coded as 3, 2, 1, and 0, respectively). During each treatment, the treating doctor asked the patient about the changes in pain intensity and treatment response. As long as the patient reported a significant deterioration in pain, they were required to recollect their ADL over the past 24 hours. We analyzed the within-day factors for significant fluctuations in pain and the average change in pain intensity per week.
To be more in line with the limb movement characteristics of women in this age group, the walking ability and normal work of ZBPI-QoL were replaced with a variety of unconstrained body activities associated with daily living and housework (higher ADLs). Referring to the 8 items of instrumental activities of daily living (IADL),50 we divide usually 10 weight-bearing movements into three levels, namely, light level (including self-bath; defecation difficulty; taking a bus for more than 30 minutes; intentional chest-expanding or upper extremity stretch exercises), medium level (such as cutting and cooking; washing coats; hanging and sunning coats or quilts) and high-intensity level (walking more than 500 meters with heavy objects [≥2.5 kg]; cleaning the house and mopping the floor; driving a car or riding a battery motorcycle for more than 30 minutes). The patients were inquired if they did any of these ADLs, then these ADLs were coded as 3 (high-intensity level), 2, 1, and 0, respectively.
We also recorded the patient’s sleep time and quality by self-reported to replace the sleep component of ZBPI-QoL scores. The participants were asked how many hours per night and day they slept. Total diurnal sleeping time was categorized into <1h, 1–2h, 2–3h, and >4h of sleep, respectively. “How does pain interfere with your sleep in the past day?” The answer options are “difficulty falling asleep due to pain”, “unable to fall asleep after waking up due to pain”, “continue to fall asleep after waking up due to pain”, and “able to sleep without being woken up” (Combine with sleep time and quality, coded as 3, 2, 1, and 0, respectively). Participants answered the question to address the perceived acute effect of pain on sleep.
Statistical Analysis
Data are expressed as the mean ± SD for continuous variables, or number (%) for categorical variables.
ANOVA of repeated measures was performed to assess pain intensity and area changes. Mauchly’s tests the hypothesis of sphericity and when if this violated the hypotheses, the analyses will be based on the Greenhouse-Geisser test. For comparisons of the proportions of patients who achieved 30% (equal to two units of pain reduction from their baseline pain scores) and 50% (equal to four units of pain reduction from their baseline pain scores) perceived overall change, Chi-square tests were performed. Student’s t-test was used to analyze the ZBPI-QoL and EQ-VAS scores variance for group comparisons.
Second, the generalized estimating equation (GEE) models with log links having robust variances were used to analyze the influencing factors of pain fluctuations by ordinal logistics regression, and a working correlation matrix with independence was selected for the minimum quasi-likelihood under the independence model criterion (QIC) value. In the Repeated module, select the Patient ID and Periods in the Subject variables box, and the Treatment time points in the Within subject variables box. Selecting the Independent structure of the same patient’s contribution data from the Working Correlation Matrix. Others remain default. Set the ordinal response model type in the Type of Model module, and select ordinal logistics to fit the logistics regression model that corrects data correlation. Specify the outcome variable in the Response module. Select the Pain fluctuations variable into the Dependent Variable, and select the minimum value as the reference value in the Reference Category. In the Predictors module, select the Periods, Groups, Treatment time points, Pain subtypes, Pain intensity, Pain area, Whether doing ADLs, and Sleep quality variables to be included in the model into Covariables. In the Model module, select Periods, Groups, Treatment time points, Pain subtypes, Pain intensity, Pain area, Whether doing ADLs, and Sleep quality variables into the Model box. In the Statistics module, in addition to the default, check to Include empirical parameter estimates to obtain the OR value. The GEE parameter estimates were expressed as the partial regression coefficients (β), odds ratio (OR), and 95% confidence intervals (95% CIs). A p-value <0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS version 22.0 (IBM, Armonk, NY, USA).
Results
Participants and Complications
A total number of 85 women with T4-6 SHN met the inclusion criteria and were thus eligible for the 4-week treatment. Six subjects were excluded for various reasons, leaving 79 women who met the inclusion criteria and thus eligible for the 4-week treatment. Most women (94.94%) had completed primary or further education. The subjects in the MI group (N=40) received a MeB and lidocaine local injection combination. One was discontinued due to changing to another analgesic drug after receiving 5 days of treatment. Subjects in the PO group (N=39) received LP combined with an oral MeB tablet. Two subjects did not complete the study due to a lack of improvement after 1 week of treatment in the PO group, whereas another one was discontinued due to increasing the dose of an analgesic drug after receiving 7 days of treatment. The therapeutic responses of 79 participants were analyzed on an intention-to-treat basis. Figure 1 and Table 1 presented the baseline treatment allocation, sociodemographic, and clinical characteristics.
 |
Table 1 Demographic, Clinical Characteristics, and Therapeutic Response of Patients with Subacute Herpetic Neuralgia |
 |
Figure 1 Consort flow chart. Notes: Adapted from Schulz KF, Altman DG, Moher D for the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. Copyright: © 2010 Schulz et al. Creative Commons Attribution License35. |
Pain Intensity and Area
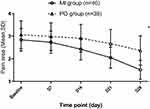
The differences groups in mean pain intensity, area, and QoL after treatment are shown in Table 1, Figures 2, and 3. The Greenhouse-Geisser’s estimate of deviation from sphericity ω=0.58 (pain scores) or 0.51 (pain area). The significant TIME differences showed that treatment time had an impact on the reduction of pain intensity (F=105.47, p<0.001) and area (F=489.88, p<0.001). The significant GROUP differences showed that the pain intensity (F=8.32, p=0.005) and area (F=15.93, p<0.001) reduction in the MI group were more significant than that in the PO group. As the treatment time elapse, the pain intensity and area were gradually decreasing and reached the minimum at the end of the treatment on the 28th day. Significant interactions between GROUP and TIME demonstrated that the extent of reduction of pain intensity (F=9.46, p<0.001) and area (F=65.71, p<0.001) between the two groups was different with the prolongation of the treatment time. A significant reduction in the pain scores and area following local injection treatment compared with those before treatment (p<0.001), or compared with those following oral treatment (p<0.001). The extent of pain intensity and area reduction in the MI group was greater (Figures 2 and 3).
Consistent with the overall results, significant differences in other efficacy measures were noted in the 2 groups, such as the proportion of patients who achieved ≥30% and ≥ 50% pain reduction, the proportion of patients who had a pain score of ≤ 3, and the numbers of patients using analgesics after treatment (Table 1).
Other Different Categorized Pain Intensities
Some participants complained of continuous, paroxysmal, allodynia, numbness, and itching. These scores as assessed at the treatment endpoint revealed that subjects who received local MeB therapy had statistically significant effects on most subtypes of pain (Table 1, p<0.05) compared with those who received oral treatment except tightness (tight feeling), allodynia and itching. The proportion of patients with tightness, allodynia, or paresthesia increased slightly at the end of treatment.
Health-Related QoL
The differences among the groups in the mean scores for ADLs and QoL based on the ZBPI and the EQ-VAS at the 28-day assessment were shown in Figure 4 and Table 1. After 28 days of treatment, the patients in the two groups had significantly lower ADL and QoL scores in the ZBPI and higher EQ-VAS scores, but these items were significantly different among the 2 groups.
Pain Fluctuation
To establish a cause-effect relationship between pain fluctuations and the influencing factors, GEE was used to examine the significance of the factors with the inclusion of other variables. From the analysis of the GEE in Table 2, it could be seen that the critical value of the difference in pain fluctuation was statistically significant (P<=0.001), so it was necessary to deduct the central effects. After controlling the central effects, the β of circadian rhythm and doing DALs exceeded 2.0. Taking the pain fluctuation in the morning as the basic point, the log value of the cumulative probability OR (log OR) of pain level did show a progressive fluctuation, which increased additionally from 8.75 (95% CI [6.73, 11.38]) of the log OR folds in the afternoon, to an additional 79.85 (95% CI [47.69, 133.67]) folds at night, with morning-evening differences. Taking the pain fluctuation of the inactive patients as the basic point, with ADL level increased from 1 to 3, the pain fluctuation was also raised from 4.28 (95% CI [2.37, 7.72]) to 17.70 (95% CI [7.60, 41.23]) folds. This result suggested that doing ADLs was an important cause of pain fluctuations.
 |
Table 2 Parametric Estimated Value Based on the Collected Data for Generalized Estimating Equation |
Safety
Thirty-nine subjects completed injection treatment. The injections were well tolerated by these patients. In MI groups, mild adverse events were reported for subjects who had a subcutaneous hemorrhage, but the bleeding stopped after 1 minute. No serious side effects were reported in any of the groups, such as acute lidocaine intoxication, excessive sensory loss, dysesthesias, or other serious adverse events.
Discussion
Treatment Response
A significant response was observed up to 28 days relative to the baseline in the PO groups. The pain scores were 4.3 ±1.5. This result demonstrated a benefit to LP plus oral MeB treatment in reducing pain for SHN. But only 21 of the 39 (53.85%) subjects achieved a pain reduction of 30% at the end of the 28-day treatment. It is difficult to distinguish between the significant benefits of our LP plus oral MeB and LP used alone.40
Reactivated and subsequently replicated VZVs in neural cells exhibit anterograde axonal transport to skin.51 Epidermal nerve fibers and endings, and skin were the major damaged regions by VZV.52,53 According to guidelines, topical treatment should be considered in the management of localized neuropathic pain.54 Our neural repairing strategies should focus particularly on the most painful area of the local subcutaneous neural fibers. The findings of this study indicated there was a significant difference in the mean pain scores and the area between the two treatment methods (p <0.001, Figures 2, and 3), representing an additional decrease per time point using local injection therapies. The pain severity in the MI group decreased significantly, and the mean pain score at the 28-day endpoint was 2.9 ±0.9. Thirty-six of the 40 (90%) subjects achieved a pain reduction of 30% or greater. Thirty-two of the 40 (80%) subjects perceived worst pain <3, and the odds ratio was 2.84 (95% CI: 1.68 to 4.79). As the patient’s pain intensity decreased, the number of patients using analgesics was gradually reduced. Thirty-four subjects with received local injections had stopped using analgesics at the endpoint. The mean pain score with local injection treatment significantly decreased relative to the oral treatment, with continued efficacy over 6 months, the incidence of PHN was 5.0% (2/40) at 3 months. Pain relief levels from local MeB injection were superior to those from systemic administration as demonstrated by odds ratios. HZ is a neurocutaneous viral disease, so PHN is a typical neuropathic pain, in which the underlying mechanisms involve the affected nerve axonal damage and myelin disruption by the VZV.55–57 Our rationale is based on previous studies that proposed that approximately 83.3% of ZAP or PHN is localized neuropathic pain.58,59 Therefore, the mechanism-based intervention strategy may be a neural repairing strategy based on local neurotrophic technique. On the one hand, the elder is at risk for cobalamin deficiency.60,61 In many patients, the causes of cobalamin deficiency cannot be removed, and lifelong cobalamin replacement therapy is required.60 In some circumstances, it may be warranted to correct cobalamin deficiency rapidly, as in the case of neurologic symptoms, due to the risk of irreversible sequelae.62,63 On the other hand, MeB can attenuate neuropathic pain, which is accompanied by inhibition of intraepidermal nerve fiber loss and mitochondria impairment.64 Clinical experience has shown that high-dose MeB can relieve symptoms of neuropathy.65–67 The results of this study indicated that local injections can directly deliver MeB to VZV-damaged tissues, obtain neurological responses, and relieve pain, which is consistent with results from previous studies.34,68 Widely used nerve blocks or regional anesthesia suggests that local administration is superior to systemic administration for peripheral nerve injury.69,70 Local translation of axon enables spatiotemporal specificity in the neuronal response to axon injury71,72 or regrowth of injured axons.73–75 For the most representative disease of localized neuropathic pain, PHN,54 the local pain regions may be the region of interest, some neurotrophic agents may be the suitable drug, and a local higher dose of MeB may be the appropriate dose. Local injections could deliver directly MeB to the VZV-damaged regions, and reduce the pain intensity, consistent with previous studies results.65,76 Cobalamin supplements are generally well-tolerated even when prescribed at high doses.60 Kaji`s result indicated that ultra-high-dose MeB did not show major side effects on amyotrophic lateral sclerosis in a long-term Phase II/III randomized controlled study.77 A meta-analysis of randomized controlled trials denied any effects of cobalamin supplementation on cancer incidence or mortality, rather showing a lower risk of melanoma.78 Following the latest S2k guidelines, lidocaine patches are recommended as second-line treatment for PHN.31 Some evidence has indicated that lidocaine, a voltage-gated Na+ channel blocking agent, could regulate the release of pro-inflammatory and anti-inflammatory cytokines, and inhibit peripheral and central sensitization,79 resulting in analgesic effects.80 Lidocaine likely added relevant effects when given in the context of a multimodal pain management approach.81 Adverse events occur no more frequently than when using a placebo, provided that contra-indications and some caveats are considered.81 In this study, lidocaine was injected in combination with MeB to avoid local pain stimulation during injection at the lesion.
PHN can induce heterogeneously painful symptoms and negative sensory signs because different mechanisms can act simultaneously in the same patient, which might be responsible for different manifestations.82,83 In addition, both clinical manifestations and pathophysiologic mechanisms usually change with time.45 A subset of patients complained of continuous pain, paroxysmal pain, allodynia, itching, and numbness. With local therapies, the number of patients with various subtypes of pain and pain intensity changed. These scores at the local treatment endpoint revealed that local therapy had statistically significant effects on most pain subtypes (p<0.05). This finding suggested that local MeB injection could significantly reduce different characteristics of pain and discomfort (Table 1). The conversion of pain subtypes implied that our strategies did not focus on a particular type of pain but rather on repairing damaged-nerve fibers. Symptoms in some subjects sustained or transformed into the tightness, allodynia, or itching, which implied that tightness, allodynia, or itching did not respond well to MeB. Further studies are needed to confirm these findings in a larger number of patients.
The clinical effectiveness of the 28-day treatment concerning pain relief was verified as the perceived overall change data and the QoL based on the ZBPI and EQ-VAS data for 4 weeks, which showed significant benefits (P < 0.05, Table 1 and Figure 4).
The results of this study demonstrated that local MeB in combination with lidocaine injection had a significant analgesic effect on pain in patients with SHN (P < 0.001), which is consistent with the results from the previous studies.34 However, throughout the treatment process, it was found that the patient’s pain scores gradually decreased, but the pain level waved from time to time. Therefore, we pay special attention to the influencing factors of patients’ pain fluctuation.
Pain Fluctuations and Influencing Factors
After considering different periods of a day, pain level and phenotypes, group effect, time effect, ADL, and sleep quality, the GEE was used to analyze the influencing factors. Table 2 showed the parametric estimated value based on the collected data by ordinal logistic regression.
The pain of PHN could fluctuate to follow a circadian rhythm.4,10 During treatment in this study, the pain level also showed a progressive fluctuation with an obvious morning-evening difference. One possible explanation for this could involve the dysregulation of inhibitory signals in spinal pain processing circuits, whereby a switch from presynaptic inhibition to excitation in the dorsal horn could be responsible for the development of pain sensation in response to non-noxious stimuli, plays an important role in neuropathic pain conditions.84,85
At the same time, the results of this study found that changes in the circadian rhythm of pain were related to some factors, such as pain levels, pain types, and ADL. Taking the pain fluctuation at the time of enrollment as the basic point, the pain fluctuation increased by 1.39 (95% CI [1.04, 1.87]) folds for an additional 14-day treatment. Three weeks of treatment had less effect on pain fluctuations than two weeks of treatment, suggesting that the pain fluctuation ranges decreased as the pain reduced.
As pain levels increased, the pain volatility surged considerably, suggesting that moderate and severe pain had a significant pain oscillation. Especially pain intensity at the 5 and 7 scores, the partial regression coefficient was higher than that at the 6 and 8 scores, indicating other factors might interfere with the pain fluctuation.
The allodynia and pruritic phenotype had a significant causal relationship with pain fluctuations, the WALD χ2 were 7.61 (allodynia) or 12.74 (itching), respectively. Taking the pain fluctuation of continuous pain as the basic point, the allodynia increased by 1.42 (95% CI [1.11, 1.82]) folds of pain fluctuation. Bumgarner et al observed that male mice exposed to dim light at night could induce cold hyperalgesia and mechanical allodynia.86 Allodynia is strongly related to sleep quality.87 Clinically, some patients with zoster-related trunk allodynia often complained that local skin could not touch clothes and quilts. Gently touching can often induce tenderness and affect sleep. In contrast, the itching increased by 1.79 (95% CI [1.30, 2.46]) folds of pain fluctuation. Some patients with zoster-related pruritus often complained that their itching worsened at night and could not help scratching. After scratching, the pain attack occurred Immediately. Scratching is almost always worse at night when it can undermine sleep.88 The results in this study were consistent with the descriptions of others.86,89
This study found that when the pain was severe, the patients could not move their limbs because of pain, and most of the patient’s ADL intensities were only 0–1 level, so the pain fluctuation was small. The nature of housewives at this age is always wanting to do some housework within their ability.90,91 When the pain was gradually relieved, most of the women wanted and dare to engage in activities such as household chores. The pain intensity did not change significantly during limb movement and even felt a little relaxed. However, when they finished their work, they would feel the pain worsen during rest, which continues until night, affecting sleep. The effect of limb movement on pain lasted longer and was more volatile than the circadian rhythm of pain. Maintenance of IADL is crucial to keeping independent life. Higher level functional capacity such as IADL depends on muscle activities, limb movement, and dynamic postural adjustments. The dynamic stretching of skeletal muscle is multi-component, which includes the neurodynamic changes, that would result from an interaction between stretching, contraction, and movement.92 The neurodynamic effects of limb movements on axon strain, stress, and tension have not been fully studied during the more terminal ranges of movement.93 ZAP is an immediate and delayed neuropathy caused by neurotropic VZV reactivation.94–96 VZV is mainly latent in the nucleus and cytoplasm of neurons in the dorsal root ganglia.96 Reactivated and subsequently replicated VZVs in neural cells exhibit anterograde axonal transport to the skin.51,97 Contrary to common infectious diseases, the destruction of axons in the subcutaneous plexus by VZV occurs from the inside out.53 During physiological limb movement, complex biomechanical changes occur in peripheral nerves, consisting of longitudinal and transverse nerve excursions and changes in diameter rather than anatomical elongation.93 Normal strain tolerances for peripheral nerve tissue are between 6% and 8%, with damage occurring at 11% strain, due to demyelination or axonal tears.98 We had reasons to speculate, in SHN patients with moderate to severe pain, the VZV-induced damaged axons also had a change of strain tolerances. The results of this study implied that limb weight-bearing movement of ADLs aggravated axonal stretch, strain, and tension, exacerbated the fluctuation of pain, and worsen pain levels.
A previous study concluded that sleep shortage was associated with an increased risk for PHN, and hyperesthesia and acute pain intensity appeared to mediate this association.99 Evidence supports a reciprocal relationship between pain and sleep in which pain disturbs sleep, and poor sleep enhances pain.100,101 A reciprocal relationship has been noted between sleep and chronic pain, especially for those with a status of widespread pain.102,103 The WALD χ2 of trouble sleeping had statistically significant, suggesting that there was an interactive relationship between sleep quality and pain volatility. We analyzed the impact of pain fluctuation on sleep from the perspective of interaction. From Wald’s test, the pain fluctuations were the significant causes of trouble sleeping. Taking the “able to fall asleep without being woken up” as the basic point, pain fluctuations increased by 2.43 (95% CI [1.21, 4.88]) folds of “unable to fall asleep after waking up”, and increased by 2.80 (95% CI [1.30, 6.04]) folds of “difficulty falling asleep due to pain”. The interaction between allodynia and itch pain with trouble sleeping was more intense.87,88 The results of this study indicated that interactions between difficulty falling asleep with pain fluctuations were pronounced, which was consistent with the descriptions of others.100,101
The pain fluctuations had no statistically significant cause-effect relationship with the groups and pain area.
Combining these results, we could conclude that local MeB in combination with lidocaine injection produced significant pain relief from SHN. Secondly, the pain levels in patients with SHN had an obvious circadian rhythm; the patient`s pain often fluctuated repeatedly during treatment, which was consistent with the tendency of neuropathic pain to be worst during the night.9 In an analysis of the influencing factors, we found pain intensity and subtypes, treatment time, doing ADLs, or sleep quality were complex interrelationships with pain volatility: the pain of SHN disturbed sleep, and in turn, the sleep interference exacerbated the pain. On the other hand, limb movement by ADLs was the external influencing factor for pain fluctuations. The circadian rhythm of pain manifested as aggravation from 8.75 folds in the afternoon to 79.85 folds in the night, which ultimately worsen pain, interfered with their sleep and undulated the continuation and stability of the treatment responses.
A growing body of research shows exercise has an impact on many biological systems, including the nervous system, leading to a focus on exercise as a means of pain reduction.104 Activities associated with daily living such as walking, housework, and gardening can be supplemented by activities typically considered to be exercise. However, exercise is a double-edged sword,105 not all measures of pain improve following exercise training.104 When the pain score was 6–8, patients were not in the mood to do anything and were unable to participate in any housework, so the pain fluctuations mostly reflected the circadian rhythm of SHN itself. On the contrary, when the mean pain was a 5 score, some women did do something. After doing ADLs, the pain fluctuated (4.36 log OR folds) higher than that at the 6 scores of pain (3.86 log OR folds). This result implied that ADLs were a significant influencing factor for neurodynamic changes in damaged axons. Although only minor changes in nerve strain during ADLs or physical activities were reported in previous studies,106,107 increasing axonal tension by limb weight-bearing movement may further hurt the VZV-destructed axons, resulting in pain worsening after ADLs. With the prolongation of treatment, especially in the middle and late treatment stages, the pain scores gradually decreased, then these women wanted and dared to do something. With the relief of pain, the effect of ADLs on pain fluctuation was decreasing, which has no statistical significance. However, ADLs were a controllable influencing factor. Taking into account the comprehensive benefits of the treatment, patients should engage in household chores as little as possible during treatment, especially strenuous limb movement, to avoid stretching damaged axons and hindering treatment.
Follow-Up
In the PO group, subjects who completed the 28-day treatment but had a poor intensity score (≥5) were considered inadequate responders and were assigned to another local treatment sequence, assessed alone, and did not include in the MI group. Therefore, the patients in the PO group did not follow up. Thirty-six subjects (90.00%) and 34 subjects (84.81%) completed 3-month and 6-month followed up, respectively. The intention-to-treat data of participants’ pain scores showed that the therapeutic effects continued beyond the treatment endpoint to the 6-month follow-up; however, a small number of subjects reported some HZ-related discomforts, such as allodynia, itching, and paresthesia. The incidence of PHN was 5.0% (2/40) at 3 months (Table 1).
Limitations
Single-blind was a limitation in this study. The patients were informed that these treatment approaches were valid interventions that had a realistic chance of being beneficial. Two physicians performed the local injections by a standard protocol, to ensure less variance in injection technique. The physicians were also instructed to treat subjects in the two groups with the same degree of rigor, enthusiasm, and optimism. The subjects were advised not to inform the observer about the treatment they received. One independent physician who did not participate in clinical management and was blinded to the subjects’ treatment allocation, carried out the assessments of the pain intensity and area on days 7, 14, 21, and 28 of the treatment, and conducted the visits follow-up. One independent physician recorded the fluctuations in pain level every period of the previous day, sleep state, and ADLs during everyday treatment.
Repeated injections every day inevitably left pinholes and pain in the treated region. In practice, our therapists strictly disinfected and avoided the pinhole sign of the previous day. Compared with the pain of SHN, the pain of these pinholes is only transient and subsides within 1 to 2 days.
The main outcome measures in this study were patient self-evaluation, especially the evaluation of pain fluctuation in the form of recall. Due to the lack of a precise definition of pain fluctuation, recalling the pain state at that time was inevitable to be affected by patients’ emotions, and the accuracy might not be high. However, when the patient’s pain became worse, she would actively report the pain and sleep situation of the last night to the doctor during treatment, which could truly reflect the patient’s pain fluctuation.
The psychological state not included in the evaluation index in this study, such as depression, anxiety, and irritability, also interacted with the pain fluctuation. According to research reports, high body fat percentage and using proton-pump inhibitors were significantly positively associated with poor health-related quality of life and depression in older adults.108,109 These associations appeared to be stronger in women than in men.108 The number of older adults with multimorbidity is surging around the world, so adverse drug events and potentially severe drug-drug interactions may increase.110–112 These are underlying risks that need to be considered in future research.
This study only evaluated aged 55–65 years women with SHN. The results in this study do not represent all types of patients equally.
Conclusion
This study validated the efficacy of local MeB combined with lidocaine injection for SHN. A significant reduction in the pain scores and area following local injection plus lidocaine treatment compared with those before treatment or compared with those following lidocaine plus oral treatment.
In addition, we confirmed that pain levels in patients with SHN had an obvious circadian rhythm, and pain levels often fluctuated repeatedly during treatment. Its influencing factors included limb movement by ADLs, treatment time, and some pain subtypes. Trouble sleeping had a reciprocal relationship with pain fluctuations. The groups and pain areas had no cause-effect relationships with pain fluctuations. ADLs were a stronger but controllable influencing factor.
Patient Consent Statement
Participation in this study was voluntary and proceeded only after informed, written consent was obtained from the patient’s parent or legal guardian.
Data Sharing Statement
Data and other materials are available from the corresponding author.
Ethical Approval and Clinical Trial Registration
This study was approved by the Institutional Review Board of Affiliated Tenth People’s Hospital of Tongji University (SHSY-IEC-4.0/20-32; 26 July 2020) and registered at chictr.org.cn (ChiCTR2000034972; 26 July 2020).
Acknowledgments
We thank all the patients and researchers who participated in this study.
Funding
This study was supported by the National Natural Science Foundation of China (81771209). The funders had no role in study design, data collection, data analysis, manuscript preparation, and publication decisions.
Disclosure
The author(s) declared no potential conflicts of interest concerning this article’s research, authorship, and/or publication.
References
1. Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: a prospective study with long term follows up. BMJ. 2000;321:794–796. doi:10.1136/bmj.321.7264.794
2. Werner RN, Nikkels AF, Marinovic B, et al. European consensus-based (S2k) guideline on the management of Herpes Zoster - guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), part 2: treatment. J Eur Acad Dermatol Venereol. 2017;31:20–29. doi:10.1111/jdv.13957
3. Kramer S, Baeumler P, Geber C, et al. Somatosensory profiles in acute herpes zoster and predictors of postherpetic neuralgia. Pain. 2019;160:882–894. doi:10.1097/j.pain.0000000000001467
4. Bumgarner JR, Walker WH
5. Warfield AE, Prather JF, Todd WD. Systems and circuits linking chronic pain and circadian rhythms. Front Neurosci. 2021;15:705173. doi:10.3389/fnins.2021.705173
6. Gilron I. Impact of chronobiology on neuropathic pain treatment. Pain Manag. 2016;6:241–247. doi:10.2217/pmt-2015-0007
7. Burish MJ, Chen Z, Yoo SH. Emerging relevance of circadian rhythms in headaches and neuropathic pain. Acta Physiol. 2019;225:e13161. doi:10.1111/apha.13161
8. Hu S, Gilron I, Singh M, Bhatia A, Scoping A. Review of the diurnal variation in the intensity of neuropathic pain. Pain Med. 2022;23:991–1005. doi:10.1093/pm/pnab336
9. Odrcich M, Bailey JM, Cahill CM, Gilron I. Chronobiological characteristics of painful diabetic neuropathy and postherpetic neuralgia: diurnal pain variation and effects of analgesic therapy. Pain. 2006;120:207–212. doi:10.1016/j.pain.2005.10.007
10. Gilron I, Bailey JM, Vandenkerkhof EG. Chronobiological characteristics of neuropathic pain: clinical predictors of diurnal pain rhythmicity. Clin J Pain. 2013;29:755–759. doi:10.1097/AJP.0b013e318275f287
11. Drolet M, Brisson M, Schmader KE, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182:1731–1736. doi:10.1503/cmaj.091711
12. Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–356. doi:10.1016/j.jpain.2004.06.001
13. Dworkin RH, Perkins FM, Nagasako EM. Prospects for the prevention of postherpetic neuralgia in herpes zoster patients. Clin J Pain. 2000;16:S90–100. doi:10.1097/00002508-200006001-00016
14. Whitley RJ, Volpi A, McKendrick M, Wijck A, Oaklander AL. Management of herpes zoster and post-herpetic neuralgia now and in the future. J Clin Virol. 2010;48(Suppl 1):S20–28. doi:10.1016/S1386-6532(10)70005-6
15. Whitley RJ. A 70-year-old woman with shingles: review of herpes zoster. JAMA. 2009;302:73–80. doi:10.1001/jama.2009.822
16. Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011;86:1146–1153. doi:10.4065/mcp.2011.0305
17. Thompson RR, Kong CL, Porco TC, Kim E, Ebert CD, Acharya NR. Herpes Zoster and postherpetic neuralgia: changing incidence rates from 1994 to 2018 in the United States. Clin Infect Dis. 2021;73:e3210–e3217. doi:10.1093/cid/ciaa1185
18. Cohen EJ. Commentary on Herpes Zoster and postherpetic neuralgia. Clin Infect Dis. 2021;73:e3218–e3219. doi:10.1093/cid/ciaa1192
19. Meister W, Neiss A, Gross G, et al. A prognostic score for postherpetic neuralgia in ambulatory patients. Infection. 1998;26:359–363. doi:10.1007/BF02770836
20. Wu S, Yang S, Ou M, et al. Transcriptome analysis reveals the role of cellular calcium disorder in Varicella Zoster Virus-induced post-herpetic Neuralgia. Front Mol Neurosci. 2021;14:665931. doi:10.3389/fnmol.2021.665931
21. Devor M. Rethinking the causes of pain in herpes zoster and postherpetic neuralgia: the ectopic pacemaker hypothesis. Pain Rep. 2018;3:e702. doi:10.1097/PR9.0000000000000702
22. Petersen KL, Rice FL, Farhadi M, Reda H, Rowbotham MC. Natural history of cutaneous innervation following herpes zoster. Pain. 2010;150:75–82. doi:10.1016/j.pain.2010.04.002
23. Arani RB, Soong SJ, Weiss HL, et al. Phase specific analysis of herpes zoster associated pain data: a new statistical approach. Stat Med. 2001;20:2429–2439. doi:10.1002/sim.851
24. Desmond RA, Weiss HL, Arani RB, et al. Clinical applications for change-point analysis of herpes zoster pain. J Pain Symptom Manage. 2002;23:510–516. doi:10.1016/S0885-3924(02)00393-7
25. Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014;2014:CD006866.
26. Jang YH, Lee JS, Kim SL, et al. Do interventional pain management procedures during the acute phase of Herpes Zoster prevent postherpetic neuralgia in the elderly? A meta-analysis of randomized controlled trials. Ann Dermatol. 2015;27:771–774. doi:10.5021/ad.2015.27.6.771
27. Rabaud C, Rogeaux O, Launay O, et al. Early antiviral treatment fails to completely prevent herpes-related pain. Med Mal Infect. 2013;43:461–466. doi:10.1016/j.medmal.2013.07.012
28. Fashner J, Bell AL. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2011;83:1432–1437.
29. Mallick-Searle T, Snodgrass B, Brant JM. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J Multidiscip Healthc. 2016;9:447–454. doi:10.2147/JMDH.S106340
30. Saguil A, Kane S, Mercado M, Lauters R. Herpes Zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2017;96:656–663.
31. Gross GE, Eisert L, Doerr HW, et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J Dtsch Dermatol Ges. 2020;18:55–78.
32. Liang L, Li X, Zhang G, Sun Y, Yu H, Jiao J. Pregabalin in the treatment of herpetic neuralgia: results of a multicenter Chinese study. Pain Med. 2015;16:160–167. doi:10.1111/pme.12564
33. Liu DY, Chen JS, Lin CY, Gong QJ, Zhao Q, Wan L. Subcutaneous peripheral nerve stimulation for treatment of acute/subacute Herpes zoster-related trigeminal neuralgia: a retrospective research. Clin J Pain. 2021;37:867–871. doi:10.1097/AJP.0000000000000981
34. Xu G, Zhou CS, Tang WZ, et al. Local administration of methylcobalamin for subacute ophthalmic herpetic neuralgia: a randomized, phase III clinical trial. Pain Pract. 2020;20(8):838–849. doi:10.1111/papr.12909
35. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi:10.1136/bmj.c332
36. Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e1188. doi:10.1111/j.1468-1331.2010.02999.x
37. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19:328–335. doi:10.1155/2014/754693
38. Fornasari D, Magni A, Pais P, Palao T, Polati E, Sansone P. Changing the paradigm in postherpetic neuralgia treatment: lidocaine 700 mg medicated plaster. Eur Rev Med Pharmacol Sci. 2022;26:3664–3676. doi:10.26355/eurrev_202205_28862
39. Navez ML, Monella C, Bosl I, Sommer D, Delorme C. 5% Lidocaine medicated plaster for the treatment of postherpetic neuralgia: a review of the clinical safety and tolerability. Pain Ther. 2015;4:1–15. doi:10.1007/s40122-015-0034-x
40. Bianchi L, Piergiovanni C, Marietti R, et al. Effectiveness and safety of lidocaine patch 5% to treat herpes zoster acute neuralgia and to prevent postherpetic neuralgia. Dermatol Ther. 2021;34:e14590. doi:10.1111/dth.14590
41. EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
42. Niv D, Maltsman-Tseikhin A. Postherpetic neuralgia: the never-ending challenge. Pain Pract. 2005;5:327–340. doi:10.1111/j.1533-2500.2005.00035.x
43. Semionov V, Shvartzman P. Post herpetic itching--a treatment dilemma. Clin J Pain. 2008;24:366–368. doi:10.1097/AJP.0b013e3181633fb1
44. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87–92. doi:10.1016/j.sder.2011.04.006
45. Dworkin RH, Gnann JW
46. Rahman QA, Janmohamed T, Pirbaglou M, et al. Defining and predicting pain volatility in users of the manage my pain app: analysis using data mining and machine learning methods. J Med Internet Res. 2018;20:e12001. doi:10.2196/12001
47. Ruben MA, Jodoin AN, Hall JA, Blanch-Hartigan D. The impact of acute pain self-efficacy on pain intensity and the accurate recall of pain. Health Psychol Rep. 2017;6:136–145. doi:10.5114/hpr.2018.72068
48. Norvell KT, Gaston-Johansson F, Fridh G. Remembrance of labor pain: how valid are retrospective pain measurements? Pain. 1987;31:77–86. doi:10.1016/0304-3959(87)90008-X
49. Eich E. On the accuracy of memory for pain. Aps J. 1993;2:192–194. doi:10.1016/S1058-9139(05)80088-9
50. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi:10.1093/geront/9.3_Part_1.179
51. Zerboni L, Sung P, Lee G, Arvin A. Age-associated differences in infection of human skin in the SCID mouse model of Varicella-Zoster virus pathogenesis. J Virol. 2018;92:5. doi:10.1128/JVI.00002-18
52. Worrell JT, Cockerell CJ. Histopathology of peripheral nerves in cutaneous herpesvirus infection. Am J Dermatopathol. 1997;19:133–137. doi:10.1097/00000372-199704000-00006
53. Markus A, Grigoryan S, Sloutskin A, et al. Varicella-zoster virus (VZV) infection of neurons derived from human embryonic stem cells: direct demonstration of axonal infection, transport of VZV, and productive neuronal infection. J Virol. 2011;85:6220–6233. doi:10.1128/JVI.02396-10
54. Casale R, Mattia C. Building a diagnostic algorithm on localized neuropathic pain (LNP) and targeted topical treatment: focus on 5% lidocaine-medicated plaster. Ther Clin Risk Manag. 2014;10:259–268. doi:10.2147/TCRM.S58844
55. Mondelli M, Romano C, Rossi S, Cioni R. Herpes zoster of the head and limbs: electroneuromyographic and clinical findings in 158 consecutive cases. Arch Phys Med Rehabil. 2002;83:1215–1221. doi:10.1053/apmr.2002.33989
56. Mondelli M, Romano C, Passero S, Porta PD, Rossi A. Effects of Acyclovir on sensory axonal neuropathy, segmental motor paresis and postherpetic neuralgia in herpes zoster patients. Eur Neurol. 1996;36:288–292. doi:10.1159/000117274
57. Esiri MM, Tomlinson AH. Herpes Zoster. Demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi:10.1016/0022-510X(72)90120-7
58. Coderre TJ. Topical drug therapeutics for neuropathic pain. Expert Opin Pharmacother. 2018;19:1211–1220. doi:10.1080/14656566.2018.1501026
59. Mick G, Baron R, Finnerup NB, et al. What is localized neuropathic pain? A first proposal to characterize and define a widely used term. Pain Manag. 2012;2:71–77. doi:10.2217/pmt.11.77
60. Marchi G, Busti F, Zidanes AL, Vianello A, Girelli D. Cobalamin Deficiency in the Elderly. Mediterr J Hematol Infect Dis. 2020;12:e2020043. doi:10.4084/mjhid.2020.043
61. Clarke R, Grimley Evans J, Schneede J, et al. Vitamin B12 and folate deficiency in later life. Age Ageing. 2004;33:34–41. doi:10.1093/ageing/afg109
62. Andres E, Zulfiqar AA, Vogel T. State of the art review: oral and nasal vitamin B12 therapy in the elderly. QJM. 2020;113:5–15. doi:10.1093/qjmed/hcz046
63. Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood. 2008;112:2214–2221. doi:10.1182/blood-2008-03-040253
64. Xu J, Wang W, Zhong XX, Feng YW, Wei XH, Liu XG. Methylcobalamin ameliorates neuropathic pain induced by vincristine in rats: effect on loss of peripheral nerve fibers and imbalance of cytokines in the spinal dorsal horn. Mol Pain. 2016;12:13. doi:10.1177/1744806916657089
65. Shibuya K, Misawa S, Nasu S, et al. Safety and efficacy of intravenous ultra-high dose methylcobalamin treatment for peripheral neuropathy: a phase I/II open label clinical trial. Intern Med. 2014;53:1927–1931. doi:10.2169/internalmedicine.53.1951
66. Sil A, Kumar H, Mondal RD, et al. A randomized, open labeled study comparing the serum levels of cobalamin after three doses of 500 mcg vs. a single dose methylcobalamin of 1500 mcg in patients with peripheral neuropathy. Korean J Pain. 2018;31:183–190. doi:10.3344/kjp.2018.31.3.183
67. Han Y, Wang M, Shen J, et al. Differential efficacy of methylcobalamin and alpha-lipoic acid treatment on symptoms of diabetic peripheral neuropathy. Minerva Endocrinol. 2018;43:11–18. doi:10.23736/S0391-1977.16.02505-0
68. Okada K, Tanaka H, Temporin K, et al. Methylcobalamin increases Erk1/2 and Akt activities through the methylation cycle and promotes nerve regeneration in a rat sciatic nerve injury model. Exp Neurol. 2010;222:191–203. doi:10.1016/j.expneurol.2009.12.017
69. Helen L, O’Donnell BD, Moore E. Nerve localization techniques for peripheral nerve block and possible future directions. Acta Anaesthesiol Scand. 2015;59:962–974. doi:10.1111/aas.12544
70. Koizuka S, Nakajima K, Mieda R. CT-guided nerve block: a review of the features of CT fluoroscopic guidance for nerve blocks. J Anesth. 2014;28:94–101. doi:10.1007/s00540-013-1675-8
71. Hanz S, Perlson E, Willis D, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi:10.1016/S0896-6273(03)00770-0
72. Perry RB, Doron-Mandel E, Iavnilovitch E, et al. Subcellular knockout of importin beta1 perturbs axonal retrograde signaling. Neuron. 2012;75:294–305. doi:10.1016/j.neuron.2012.05.033
73. Li L, Yu J, Ji SJ. Axonal mRNA localization and translation: local events with broad roles. Cell Mol Life Sci. 2021;78:7379–7395. doi:10.1007/s00018-021-03995-4
74. Terenzio M, Koley S, Samra N, et al. Locally translated mTOR controls axonal local translation in nerve injury. Science. 2018;359:1416–1421. doi:10.1126/science.aan1053
75. Zheng JQ, Kelly TK, Chang B, et al. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21:9291–9303. doi:10.1523/JNEUROSCI.21-23-09291.2001
76. Xu G, Lv ZW, Feng Y, Tang WZ, Xu GX. A single-center randomized controlled trial of local methylcobalamin injection for subacute herpetic neuralgia. Pain Med. 2013;14:884–894. doi:10.1111/pme.12081
77. Kaji R, Imai T, Iwasaki Y, et al. Ultra-high-dose methylcobalamin in amyotrophic lateral sclerosis: a long-term phase II/III randomised controlled study. J Neurol Neurosurg Psychiatry. 2019;90(4):451–457. doi:10.1136/jnnp-2018-319294
78. Zhang SL, Chen TS, Ma CY, et al. Effect of vitamin B supplementation on cancer incidence, death due to cancer, and total mortality: a PRISMA-compliant cumulative meta-analysis of randomized controlled trials. Medicine. 2016;95:e3485. doi:10.1097/MD.0000000000003485
79. Knezevic NN, Tverdohleb T, Nikibin F, Knezevic I, Candido KD. Management of chronic neuropathic pain with single and compounded topical analgesics. Pain Manag. 2017;7:537–558. doi:10.2217/pmt-2017-0020
80. Ma L, Li J, Zhou J, et al. Intravenous lidocaine alleviates postherpetic neuralgia in rats via regulation of neuroinflammation of microglia and astrocytes. iScience. 2021;24:102108. doi:10.1016/j.isci.2021.102108
81. Hollmann MW, Hermanns H, Kranke P, Durieux ME. Intravenous lidocaine: it’s all about a risk-benefit analysis. Anaesthesia. 2021;76:717–718. doi:10.1111/anae.15436
82. von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi:10.1016/j.neuron.2012.02.008
83. Siqueira SR, Alves B, Malpartida HM, Teixeira MJ, Siqueira JT. Abnormal expression of voltage-gated sodium channels Nav1.7, Nav1.3 and Nav1.8 in trigeminal neuralgia. Neuroscience. 2009;164:573–577. doi:10.1016/j.neuroscience.2009.08.037
84. Guo D, Hu J. Spinal presynaptic inhibition in pain control. Neuroscience. 2014;283:95–106. doi:10.1016/j.neuroscience.2014.09.032
85. Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101:259–301. doi:10.1152/physrev.00045.2019
86. Bumgarner JR, Walker WH
87. Lovati C, D’Amico D, Bertora P, et al. Correlation between presence of allodynia and sleep quality in migraineurs. Neurol Sci. 2010;31(Suppl 1):S155–158. doi:10.1007/s10072-010-0317-2
88. Sack R, Hanifin J. Scratching below the surface of sleep and itch. Sleep Med Rev. 2010;14:349–350. doi:10.1016/j.smrv.2010.03.003
89. Patel T, Ishiuji Y, Yosipovitch G. Nocturnal itch: why do we itch at night? Acta Derm Venereol. 2007;87:295–298. doi:10.2340/00015555-0280
90. Craig L, Powell A. Shares of housework between mothers, fathers and young people: routine and non-routine housework, doing housework for oneself and others. Soc Indic Res. 2016;136:269–281. doi:10.1007/s11205-016-1539-3
91. Milkie MA, Wray D, Boeckmann I. Gendered pressures: divergent experiences linked to housework time among partnered men and women. J Comp Fam Stud. 2021;52:147–179. doi:10.3138/jcfs-52-2-002
92. Vieira DCL, Opplert J, Babault N. Acute effects of dynamic stretching on neuromechanical properties: an interaction between stretching, contraction, and movement. Eur J Appl Physiol. 2021;121:957–967. doi:10.1007/s00421-020-04583-3
93. Szikszay T, Hall T, von Piekartz H. In vivo effects of limb movement on nerve stretch, strain, and tension: a systematic review. J Back Musculoskelet Rehabil. 2017;30:1171–1186. doi:10.3233/BMR-169720
94. Jiang HF, Wang W, Jiang X, et al. ORF7 of Varicella-Zoster virus is required for viral cytoplasmic envelopment in differentiated neuronal cells. J Virol. 2017;91:1. doi:10.1128/JVI.00127-17
95. Ludlow M, Kortekaas J, Herden C, et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016;131:159–184. doi:10.1007/s00401-015-1511-3
96. Kennedy PG, Rovnak J, Badani H, Cohrs RJ. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96:1581–1602. doi:10.1099/vir.0.000128
97. Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12:197–210. doi:10.1038/nrmicro3215
98. Topp KS, Boyd BS. Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice. Phys Ther. 2006;86:92–109. doi:10.1093/ptj/86.1.92
99. Yamada K, Kubota Y, Shimizu Y, et al. Sleep shortage is associated with postherpetic neuralgia development through hyperesthesia and acute pain intensity: a community-based prospective cohort study. Pain Pract. 2019;19:476–483. doi:10.1111/papr.12766
100. Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. doi:10.1016/j.smrv.2005.08.001
101. Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Semin Neurol. 2005;25:106–116. doi:10.1055/s-2005-867079
102. Jansson-Frojmark M, Boersma K. Bidirectionality between pain and insomnia symptoms: a prospective study. Br J Health Psychol. 2012;17:420–431. doi:10.1111/j.2044-8287.2011.02045.x
103. Tang NKY, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35(5):675–687. doi:10.5665/sleep.1830
104. Leitzelar BN, Koltyn KF. Exercise and neuropathic pain: a general overview of preclinical and clinical research. Sports Med Open. 2021;7:21. doi:10.1186/s40798-021-00307-9
105. Cooper MA, Kluding PM, Wright DE. Emerging relationships between exercise, sensory nerves, and neuropathic pain. Front Neurosci. 2016;10:372. doi:10.3389/fnins.2016.00372
106. Dilley A, Lynn B, Greening J, DeLeon N. Quantitative in vivo studies of median nerve sliding in response to wrist, elbow, shoulder and neck movements. Clin Biomech. 2003;18:899–907. doi:10.1016/S0268-0033(03)00176-1
107. Boyd BS, Dilley A. Altered tibial nerve biomechanics in patients with diabetes mellitus. Muscle Nerve. 2014;50:216–223. doi:10.1002/mus.24155
108. Giovannini S, Macchi C, Liperoti R, et al. Association of body fat with health-related quality of life and depression in nonagenarians: the Mugello study. J Am Med Dir Assoc. 2019;20:564–568. doi:10.1016/j.jamda.2019.01.128
109. Laudisio A, Antonelli IR, Gemma A, et al. Use of proton-pump inhibitors is associated with depression: a population-based study. Int Psychogeriatr. 2017;30:153–159. doi:10.1017/S1041610217001715
110. Novella A, Elli C, Tettamanti M, et al. Relation between drug therapy-based comorbidity indices, Charlson’s comorbidity index, polypharmacy and mortality in three samples of older adults. Arch Gerontol Geriatr. 2022;100:34. doi:10.1016/j.archger.2022.104649
111. Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2015;7:11–22. doi:10.1177/2042098615615472
112. Taylor AW, Price K, Gill TK, et al. Multimorbidity - not just an older person’s issue. Results from an Australian biomedical study. BMC Public Health. 2010;10:45. doi:10.1186/1471-2458-10-718
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.