Back to Journals » Drug Design, Development and Therapy » Volume 14
Paeoniflorin Alleviates Abnormalities in Rats with Functional Dyspepsia by Stimulating the Release of Acetylcholine
Authors Zou X, Wang Y, Wang Y, Yang J, Guo H, Cai Z
Received 30 April 2020
Accepted for publication 11 November 2020
Published 22 December 2020 Volume 2020:14 Pages 5623—5632
DOI https://doi.org/10.2147/DDDT.S260703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Xuan Zou,1,2,* Yang Wang,1,2,* Yuheng Wang,3 Junting Yang,3 Huishu Guo,1,2 Zhengxu Cai3
1Central Laboratory, The First Affiliated Hospital of Dalian Medical University, Dalian 116001, People’s Republic of China; 2Institute (College) Integrative Medicine, Dalian Medical University, Dalian 116044, People’s Republic of China; 3Department of Neurology, The First Affiliated Hospital of Dalian Medical University, Dalian 116001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhengxu Cai
Department of Neurology, The First Affiliated Hospital of Dalian Medical University, 222 Zhongshan Road, Dalian 116001, People’s Republic of China
Email [email protected]
Huishu Guo
Central Laboratory, The First Affiliated Hospital of Dalian Medical University, 222 Zhongshan Road, Dalian 116001, People’s Republic of China
Tel +86-411-83635963 ext 7255
Email [email protected]
Introduction: Paeoniflorin is a main active component in traditional Chinese medicine. Paeoniae alba radix is widely used as a spasmolytic and pain-relieving agent for abdominal spasmodic pain. Functional dyspepsia (FD) is characterized by pain or burning in the epigastrium, fullness, bloating and nausea. However, limited information is available about the effect of paeoniflorin on FD.
Materials and Methods: In this study, iodoacetamide or clonidine-induced FD rat models were established to investigate the impacts of paeoniflorin on FD induced by different pathophysiologic disturbances.
Results: We found the therapeutic effect of paeoniflorin through assessing the gastric emptying, gastric accommodation and visceral hypersensitivity. This function of paeoniflorin was related to the release of acetylcholine (ACh), which was accompanied by reduced acetylcholinesterase (AchE) activity in stomach and hypothalamus. Paeoniflorin administration inhibited the cyclo-oxygenase-2 (COX-2) expression and increased the level of ghrelin in the stomach. Besides, the levels of occludin and ZO-1 were elevated in the duodenum from paeoniflorin-treated rats, suggesting the impaired duodenal barrier was ameliorated.
Discussion: These results indicate that paeoniflorin possesses the ability to alleviate functional dyspepsia.
Keywords: paeoniflorin, functional dyspepsia, acetylcholine, gastric function
Introduction
Functional dyspepsia (FD) is a highly prevalent gastrointestinal disorder characterized by a sensation of pain or burning in the epigastrium, fullness during or after meal, bloating, and nausea.1 The global prevalence of FD is about 20%.2 At present, therapies for FD, including pharmacotherapy, are not yet been fully effective.3 This condition adversely affects patients’ quality of life and working efficiency.4 Consequently, the identification of efficacious treatments (such as natural products) that do not involve prescription drugs may be beneficial. According to Rome Consensus definition, FD is categorized into postprandial distress syndrome (PDS, characterized by postprandial fullness and early satiation) and epigastric pain syndrome (EPS, characterized by epigastric pain and burning).5 The above adverse symptoms can be caused by delayed gastric emptying (about 20–50% of patients with FD), impaired gastric accommodation to a meal (about 40% of patients with FD) and visceral hypersensitivity to gastric distension (34% of patients with FD).6
Acetylcholine (ACh), an excitatory neurotransmitter, promotes contraction and peristalsis of gastrointestinal smooth muscle. Dopamine D2 receptors and 5-hydroxytryptamine (5-HT4) receptors affect the release of ACh and thereby resulting in gastrointestinal motility disorder. Dopamine D2 receptor antagonists and 5-HT4 receptor agonists as prokinetic drugs are extensively used in the therapy of FD, because they can increase gastric contractility.7,8 Metoclopramide, domperidone, and itopride hydrochloride (itopride) are dopamine D2 receptor antagonists, while mosapride citrate (mosapride), cisapride monohydrate (cisapride), and tegaserod are 5-HT4 receptor agonists. However, the therapeutic effects of these drugs are unsatisfactory in FD patients.9 Previous researches have confirmed that metoclopramide causes severe neurological adverse effects,10 and domperidone facilitate occurrence of arrhythmias.11 Cisapride contributed to the release of ACh from myenteric neurons via 5-HT4 receptor agonism, but was abandoned for QT interval prolongation and fatal arrhythmias.12 In the past few years, tegaserod was used to treat the irritable bowel syndrome with constipation, but it had been withdrawn due to an increased incidence of cardiovascular ischemic events.12 Itopride and mosapride were efficacious in the treatment of FD, but the use of these drugs was clinically limited because of underlying adverse effects.2,13 Thus, safer therapies for FD, such as employing natural products, should be developed.
Paeoniae alba radix (also named White Paeony Root or Baishao in Chinese) is one of the Chinese traditional tonic crude drugs, which is the dried peeled root of Paeonia lactiflora Pall (family Paeoniaceae). It is widely used as a spasmolytic and pain-relieving agent to treat menstrual disorders and to relieve abdominal spasmodic pain.14 Paeoniflorin is a monoterpene glycoside isolated from the aqueous extract of the dry root of P. lactiflora Pall and is identified as a main active component in Paeoniae alba radix. Paeoniflorin had been found to exert many pharmacological effects, including an increase of cognitive ability,15 a reduction of MPTP-induced toxicity,16 and anti-depressant effect.17 Recent studies suggested that paeoniflorin down-regulated the activity of acetylcholinesterase (AchE) in the hippocampus of rats,18 and up-regulated the expression level of 5-HT.19 Thus, we postulate that paeoniflorin may be a novel natural drug for the treatment of FD. However, the gastroprokinetic and gastric emptying activities of paeoniflorin, including its underlying mechanisms, have yet to be fully elusive.
Gastric irritation with iodoacetamide (IA) in the neonatal period induces gastric hypersensitivity, motor dysfunction and damaged accommodation in adults.20 Neonatal gastric irritation has been regarded as a typical approach to establish a FD animal model. It has been known that clonidine (CD) as a α2-adrenoceptor agonist can inhibit the release of ACh from cholinergic neurons and thereby lead to gastric motility dysfunction.21 In this study, IA or CD-induced FD rat models were established to assess the impacts of paeoniflorin on FD induced by different pathophysiologic disturbances. The effects of paeoniflorin on ACh, ghrelin, COX-2 and adhesion proteins were further investigated.
Materials and Methods
Medicinal Materials
Paeoniflorin, the aqueous extract of the dry root of P. lactiflora Pall, was obtained from Meilun Biotechnology Co., Ltd. (MB2113, Dalian, China).
Animals
Male Sprague-Dawley (SD) rats aged at 10-day old and 8-week old were purchased from Liaoning HFK BIOSCIENCE Co., Ltd. (Beijing, China). All rats were housed under the following environmental conditions: 22±1°C temperature, 45–55% humidity, 12 h light/dark cycle and freely access to food and water. All the procedures in animal experiments followed the guide for the care and use of laboratory animals and this study was approved by The First Affiliated Hospital of Dalian Medical University.
Neonatal Gastric Irritation Animal Model
Gastric hypersensitivity and gastric motor dysfunction in FD can be modeled in rat pups by gastric irritation as previously reported.22 Ten-day-old rat pups were randomly divided into the following group:
- Sham group (sucrose + normal saline; n=6);
- Control group (IA + normal saline; n=6);
- IA+paeoniflorin (15) group (IA + 15 mg/kg paeoniflorin; n=6);
- IA+paeoniflorin (30) group (IA + 30 mg/kg paeoniflorin; n=6).
Ten-day-old rat pups were given orally 0.2 mL of 0.1% IA (144–48-9, Aladdin, Shanghai, China; dissolved in 2% sucrose) for 6 consecutive days; sham pups received 0.2 mL of 2% sucrose alone. Subsequently, rat pups were returned to their cage. After 6 weeks, rats were intraperitoneally injected 15 or 30 mg/kg paeoniflorin for 2 consecutive weeks. Gastric function was detected 1 h after the last injection of paeoniflorin.
CD-Induced Motility Dysfunction Model
CD-induced FD rat model was established using the method of Kawachi et al.23 Eight-week old rats were randomly divided into the following group:
- Sham group (normal saline + normal saline; n=6);
- Control group (CD + normal saline; n=6);
- CD+paeoniflorin (15) group (CD + 15 mg/kg paeoniflorin; n=6);
- CD+paeoniflorin (30) group (CD + 30 mg/kg paeoniflorin; n=6).
Eight-week-old rats were intraperitoneally injected 15 or 30 mg/kg paeoniflorin for 2 consecutive weeks. Sham rats received normal saline alone. At 30 min after the last injection of paeoniflorin, CD at a dose of 100 μg/kg (20,283–92-5, Sigma, St. Louis, Missouri, USA) or normal saline was subcutaneously administered. Gastric function was detected 30 min after CD treatment.
Measurement of Gastric Emptying
Gastric emptying in rats was detected with the phenol red method as previously described.21 Rats were fasted for 18 h and free access to water. Rats were then administered intragastrically 1.5% carboxymethylcellulose sodium salt supplemented with 0.05% phenol red (0.5 mL/rat). Twenty minutes later, rats were euthanized and stomach was collected. After treatment with 10 mL of 0.1 M NaHCO3, gastric content harvested and centrifuged at 8000 g for 15 min (H1650-W, Xiangyi, Changsha, China). The amount of phenol red in the supernatant was measured at the absorbance at 558 nm using a microplate reader (ELX-800, BioTek, Vermont, USA). For the IA-FD model, phenol red was intragastrically administered to rats 30 min after the last injection of paeoniflorin. For the CD-FD model, rats were intragastrically administered phenol red at 5 min before the administration of clonidine.
Gastric Accommodation and Gastric Sensitivity Tests
Gastric accommodation and gastric sensitivity were detected using barostat and electrophysiological recorder as described previously.24,25 Rats were anesthetized and then a balloon with a maximum volume of 20mL was connected to a polyethylene tube. The tube was introduced from the mouth to the stomach. A pair of electrodes was fixed into the trapezius muscle. After rats regained consciousness, the tubes were connected to a barostat (C&J Technology, Toronto, Canada) and electrophysiological recorder (AD instrument, New South Wales, Australia). Rats receipted a series of 30 s balloon distensions: 20, 40, 60 and 80 mmHg at an interval of 3 min between distensions. The volume of the balloon and electromyographic (EMG) was recorded. Gastric accommodation was calculated based on the changes in balloon volume (mL) per mmHg during the distension, while gastric sensitivity is defined as the rate of change root mean square of EMG.
AchE Activity Assay
AchE activity assay was performed as previously reported.23 The stomach was harvested from fasted rats and mixed with a 10-fold volume of 0.1 mM sodium phosphate buffer (pH 6.9) containing 10 mM EDTA. The mixture was centrifuged for 1 h and sediment was washed twice with buffer. After treatment with buffer containing 1% Triton X-100 at 4°C for 1 h, supernatant was collected by centrifugation (8000 g, 4°C, 10 min) and was regarded as a stomach-derived fraction with AChE. Protein concentration was detected using a BCA Assay Kit (PC0020, Solarbio, Beijing, China) according to the manufacturer’s instructions. AChE activity was determined using an Assay Kit (BC2020, Solarbio) according to the manufacturer’s protocol. The absorbance at 412 nm was detected using a spectrophotometer (UV752N, Youke, Shanghai, China). Inhibition rate (%) was calculated as the ratio of ACh activity in the presence of a test compound and sham activity in the absence of the test compound.
Western Blot
The duodenum was lysed on ice for 5 min in cell lysis buffer (P0013, Beyotime, Shanghai, China) containing 1 mM PMSF (ST506, Beyotime). Supernatants were centrifugation at 10,000 g for 5 min at 4°C and collected. The protein concentration was detected by the BCA Assay Kit (P0011, Solarbio). Equal number of supernatants (20 μL/lane) was separated on sodium dodecyl sulfate-polyacrylamide gel and transferred into PVDF membrane (IPVH00010, Millipore, Billerica, USA). After blocking in 5% (m/v) skim milk (Q/NYLB 0039S, Yili, Huhehaote, China), membrane was incubated overnight at 4°C with the following primary antibodies: E-cadherin (1:500, 20,874-1-AP, Proteintech, Beijing, China), occludin (1:1000, 27,260-1-AP, Proteintech), zona occludens-1 (ZO-1, 1:1000, 21,773-1-AP, Proteintech), and β-actin (1:1000, sc-47,778, Santa Cruz, California, USA). After four 5-min washing with TBST buffer, membrane was incubated with HRP-conjugated goat anti-rabbit or anti-mouse (both 1:5000, A0208/A0216, Beyotime) secondary antibody for 45 min at 37°C. The protein bands on the membrane were visualized using ECL chemiluminescence substrate (PE0010, Solarbio) and the protein levels were quantified with Gel-Pro-Analyzer software.
Immunofluorescence
Stomach and duodenum tissues were cut into 6 μm thickness. Sections were incubated at 4°C overnight with the following primary antibodies: ghrelin (1:200, #31,865, CST, Boston, USA), occludin (1:200, 66,378-1-Ig, Proteintech), and cyclo-oxygenase-2 (COX-2, 1:200, A1253, ABclonal, Wuhan, China). After three 5-min washing, the sections were cultivated with Cy3-conjugated goat anti-rabbit or anti-mouse antibody (1:400, A0516/A0521, Beyotime) at room temperature for 1 h. The slides were stained with 4ʹ,6-diamidino-2-phenylindole (DAPI, C1002, Beyotime) and immunofluorescence was observed using light microscopy (BX53, Olympus, Tokyo, Japan) at original magnification × 600.
Statistical Analysis
All data were shown as mean ± SD. Student’s t-test (two tailed) and one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test were used to assess the difference among the groups. P < 0.05 was defined as statistically significant. GraphPad Prism 8.0 was used for statistical analysis of all data.
Results
Paeoniflorin Promotes Gastric Emptying, Gastric Accommodation and Gastric Sensitivity in FD Rats
The effect of paeoniflorin on IA-induced or CD-induced delayed gastric emptying was investigated by the phenol red method. It could be seen from Figure 1A, the gastric emptying of the control group administered IA or CD revealed an obvious decrease compared with the sham group (p < 0.5). Paeoniflorin at a dose of 30 mg/kg markedly enhanced gastric emptying compared with the control group (p < 0.5). We also assessed the effect of paeoniflorin on gastric accommodation and gastric sensitivity. It could be seen from Figure 1B that enhanced balloon pressure of the sham group led to an increase in balloon volume. Compared with the sham group, balloon volume of the control rats induced by IA or CD was significantly decreased under distention pressures of 40 mmHg, 60 mmHg and 80 mmHg (p < 0.5), but not at 20 mmHg. Paeoniflorin treatment markedly improved gastric accommodation in FD rats (p < 0.5). On the other hand, EMS testing results displayed that FD rats of the control group extremely increased in EMG at distension pressures of 40 mmHg, 60 mmHg and 80 mmHg compared with the sham rats (p < 0.5, Figure 1C). Administration of paeoniflorin (15 and 30 mg/kg/daily) greatly repressed the EMG activity of FD rats to gastric distension (p < 0.5). Our results suggested that paeoniflorin greatly ameliorated delayed gastric emptying, impaired gastric accommodation and gastric sensitivity in FD rats.
Paeoniflorin Stimulates the Release of ACh in FD Rats
To evaluate whether paeoniflorin stimulated the release of the neurotransmitter ACh, we examined the AChE activity in paeoniflorin-treated FD rats. Figure 2A and B showed that stomach-derived and hypothalamus-derived AChE activities of the control group were significantly increased by CD administration in comparison to the sham group (p < 0.5), but the effect induced by IA did not reach statistically significant. Moreover, paeoniflorin at doses of 15 mg/kg and 30 mg/kg efficiently suppressed AChE activity in stomach and hypothalamus of CD-induced rats (p < 0.5). The serum level of ACh in the control group was greatly down-regulated by IA or CD administration compared with the sham group, but the use of paeoniflorin restored ACh level (p < 0.5, Figure 2C). Our results suggested that paeoniflorin treatment stimulated the release of ACh in FD rats.
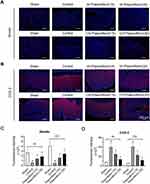
Paeoniflorin Regulates the Levels of Ghrelin and COX-2 in the Stomach of FD Rats
Ghrelin was expressed as granular neuropeptides in the stratum basale of the stomach, as revealed by immunofluorescence (Figure 3A). Compared with the sham group, the expression of ghrelin was decreased in the stomach of FD rats (p < 0.5, Figure 3A and C). Paeoniflorin treatment enhanced the ghrelin expression of FD rats. Furthermore, the expression of COX-2 was observed in the stratum basale of the stomach in comparison to the sham group (Figure 3B). However, the administration of paeoniflorin exhibited a reduction of COX-2 level in stomach of FD rats and thereby alleviating micro-inflammation (p < 0.5, Figure 3B and D).
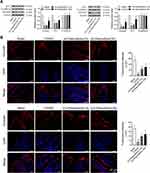
Paeoniflorin Ameliorates the Impaired Duodenal Barrier in FD Rats
A previous study has shown that duodenal barrier function is compromised in FD.26 Thus, we assessed whether adhesion proteins were altered in IA or CD-induced FD rats, and determined the effect of paeoniflorin on the expression of cell-to-cell adhesion proteins. The tight junction proteins (occludin and ZO-1) and the adherens junction proteins (E-cadherin) were detected by Western blotting and immunofluorescence. It could be seen from Figure 4A that the expression of occludin and ZO-1 at the tight junction in the control group was greatly decreased in FD rats than those in the sham group (p < 0.5). Paeoniflorin at a dose of 30 mg/kg obviously up-regulated occludin and ZO-1 levels in the duodenum of FD rats (p < 0.5, Figure 4A). In addition, no difference in protein expression of E-cadherin was observed in FD rats compared with the sham group. Paeoniflorin treatment had no effect on the adherens junction protein E-cadherin in the duodenum of FD rats (Figure 4A). The results of immunofluorescence revealed the reduced staining of occludin at the tight junction in control rats, and treatment with paeoniflorin effectively increased the occludin level (Figure 4B). Our results suggested that paeoniflorin ameliorated the impaired duodenal barrier in FD rats.
Discussion
Paeoniflorin, a glucoside, is one of the principal bioactive components of the P. lactiflora Pall and has many pharmacological functions, such as anti-inflammatory, antiallergic and antioxidant effects.15 Previous studies have found that paeoniflorin inhibits the AChE activity and promotes the 5-HT expression.19,21 AChE antagonist and 5-HT4 receptor agonists are commonly used in the treatment of FD due to the improvement of gastrointestinal motility. Therefore, this study examined the role and mechanism of paeoniflorin in rats with FD-like phenotype. Our results showed that paeoniflorin treatment accelerated gastric emptying and enhanced gastric accommodation as well as gastric sensitivity in IA or CD-induced FD rat models. Paeoniflorin stimulated the release of ACh in FD rats, accompanied with increased ghrelin and reduced COX-2. Besides, paeoniflorin administration ameliorated the impaired duodenal barrier in FD rats. These findings indicate the therapeutic effect of paeoniflorin in FD rats.
Adverse events in early life are one of risk factors for developing FD in adulthood.27 Using a previously described model of FD rats where 10-day-old rat pups got gastric irritation within a few days, we find that IA treatment results in the delayed gastric emptying, impaired gastric accommodation and visceral hypersensitivity in adult rats, which is in conformity to a previous study.22 The administration of paeoniflorin restores gastric motility in IA-induced adult rats. Besides, a model of adult rats with CD treatment is established to induce FD-like phenotype. CD, a α2-adrenoceptor agonist, induces gastrointestinal dysfunction through suppressing the release of ACh from cholinergic nerve terminals. Gastrointestinal dysfunction models induced by α2-adrenoceptor agonists have been widely applied to assess the influence of gastroprokinetic drugs in animals.28 Sninsky et al have confirmed that gastrointestinal dysfunction is induced by an α2-adrenoceptor agonist in humans.29 We find that CD treatment leads to delayed gastric emptying, impaired gastric accommodation and visceral hypersensitivity in adult rats. These results with CD treatment are in line with a previous study.30 Similarly, administration of paeoniflorin alleviates delayed gastric emptying, impaired gastric accommodation and sensitivity in a concentration-dependent manner. Therefore, we determine that paeoniflorin can efficiently ameliorate the gastrointestinal dysfunction induced by a different cause.
As we all know, the central nervous system affects gastrointestinal function. In the central nervous system, vagal efferent nerve fibers project to the stomach, which play a vital role in gastric motility, and thereby stimulating ACh release from cholinergic nerve terminals in the myenteric plexus of the stomach.31 A study has reported that itopride promotes ACh release from cholinergic nerves through antagonizing dopamine D2 receptors with anti-AChE activity.32 Another study has also found that mosapride induces ACh release from cholinergic nerves and facilitates gastric motility via the stimulation of 5-HT4 receptors.33 Consequently, the release of ACh at the cholinergic receptor sites plays an important in gastric motility. In the present study, we find that treatment with CD significantly increases the rat stomach and hypothalamus-derived AChE activities, but a weak effect is observed on AChE activity in IA-induced rats. Administration of paeoniflorin stimulates the release of Ach in IA or CD-induced FD rats. Therefore, we determine that paeoniflorin contributes to gastric motility and the release of ACh by inhibiting AChE activity in Cd-induced FD rats. Gastric irritation with IA in the neonatal period induces superficial sloughing of the gastric epithelium and mild gastritis, consequently leading to impaired gastric sensory and motor function.20 The therapeutic effect of paeoniflorin in IA-induced FD rats may be due to paeoniflorin up-regulates the cAMP and 5-HT expression, thereby promoting the release of ACh.19,34,35 Our researches determine that paeoniflorin contributes to the release of ACh, and further relieves abnormalities in rats with FD.
Treatment with IA leads to the production of ghrelin in the stomach, and then ghrelin is released into the circulation.36 Ghrelin can directly affect the activity of the arcuate nucleus neurons. The hypothalamus modulates energy expenditure after receiving both hormonal signals and peripheral neurons.37 Therefore, the release of circulating hormones, such as ghrelin, modulates the brain-gut axis through hormonal signals.16 Ghrelin has been reported to efficiently increase gastric peristalsis.25 Ghrelin levels are markedly lower in patients with FD compared to that in healthy volunteers.38 A previous study has shown the reduced expression of ghrelin in the brain and stomach of FD rats induced by IA.39 Consistent with this finding, our study finds that gastric irritation in the neonatal rat can lead to a reduction of ghrelin compared with the sham rats. Moreover, we are the first to confirm that the expression of ghrelin is reduced in the stomach of FD rats induced by CD. The administration of paeoniflorin markedly enhanced ghrelin expression, especially at a dose of 30 mg/kg. In addition, it has been shown that micro-inflammation exists in gastrointestine of FD patients.40 COX-2 is the inducible enzyme that is less expressed in normal tissues but highly expressed when stimulated by inflammation.41 A previous study found that COX-2 was highly expressed in the stomach of FD rats.42 Besides, COX-2 inhibitors significantly reduced the risk of gastrointestinal adverse effects in patients.43 Another study has shown the protective effects of paeoniflorin on rheumatoid arthritis by reducing the COX-2 protein expression level.44 In agreement with this report, our study determines that paeoniflorin treatment efficiently down-regulates the level of COX-2 and thereby alleviates abnormalities in rats with FD.
The intestinal barrier plays an important in preventing the translocation of harmful substances from gut lumen to submucosa. Previous study has confirmed that duodenal barrier is implicated in the pathomechanism of FD.45 Adhesion proteins are significant components of the intestinal barrier, including the tight junction proteins (occludin and ZO-1) and the adherens junction proteins (E-cadherin).46 The dysregulation of adhesion proteins associates with impaired duodenal integrity and micro-inflammation. Downregulation of occludin leads to an increase of permeability, thereby triggering intestinal diseases.47 A study finds that tail clamping-associated stress leads to a decrease in occludin expression in the duodenum, thereby damaging duodenal integrity of FD rats.48 Another study has also reported the impaired duodenal mucosal barrier function in patients with FD, exhibited by a reduction of ZO-1 and E-cadherin levels.26 In the present study, the decrease in occludin and ZO-1 expression in the duodenum of FD rats is induced by IA or CD-associated stress. Administration of paeoniflorin causes the upregulation of tight junction proteins (occludin and ZO-1) in the duodenum. Therefore, we postulate that paeoniflorin up-regulates the expression of occludin and ZO-1 expression in the duodenum by changes of neurotransmitter ACh in FD rats.
Conclusion
In summary, paeoniflorin possesses the ability to relieve the symptoms of FD, with attenuated inflammatory reaction. Paeoniflorin inhibits the AChE activity and increases the levels of ACh and ghrelin. Furthermore, paeoniflorin ameliorates the impaired duodenal barrier by up-regulating the expression of adhesion proteins (occludin and ZO-1). These findings suggest that paeoniflorin is a potential therapeutic compound for FD.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China [Nos. 81673727 and 81273919].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Talley NJ, Locke GR
2. Curran MP, Robinson DM. Mosapride in gastrointestinal disorders. Drugs. 2008;68(7):981–991. doi:10.2165/00003495-200868070-00007
3. El-Serag HB, Talley NJ. Systemic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19(6):643–654. doi:10.1111/j.1365-2036.2004.01897.x
4. Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther. 2013;38(2):170–177. doi:10.1111/apt.12355
5. Enck P, Azpiroz F, Boeckxstaens G, et al. Functional dyspepsia. Nat Rev Dis Primers. 2017;3:17081.
6. Asano H, Tomita T, Nakamura K, et al. Prevalence of gastric motility disorders in patients with functional dyspepsia. J Neurogastroenterol Motil. 2017;23(3):392–399. doi:10.5056/jnm16173
7. Tonini M, Cipollina L, Poluzzi E, Crema F, Corazza GR, De Ponti F. Review article: clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther. 2004;19(4):379–390. doi:10.1111/j.1365-2036.2004.01867.x
8. O’Mahony S, Dinan TG, Keeling PW, Chua AS. Central serotonergic and noradrenergic receptors in functional dyspepsia. World J Gastroenterol. 2006;12(17):2681–2687. doi:10.3748/wjg.v12.i17.2681
9. Holtmann G, Gapasin J. Failed therapy and directions for the future in dyspepsia. Dig Dis. 2008;26(3):218–224. doi:10.1159/000121350
10. Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther. 2010;31(1):11–19. doi:10.1111/j.1365-2036.2009.04189.x
11. Doggrell SA, Hancox JC. Cardiac safety concerns for domperidone, an antiemetic and prokinetic, and galactagogue medicine. Expert Opin Drug Saf. 2014;13(1):131–138. doi:10.1517/14740338.2014.851193
12. Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466–1479. doi:10.1053/j.gastro.2005.11.059
13. Monkemuller K, Malfertheiner P. Drug treatment of functional dyspepsia. World J Gastroenterol. 2006;12(17):2694–2700. doi:10.3748/wjg.v12.i17.2694
14. Chen LC, Lee MH, Chou MH, Lin MF, Yang LL. Pharmacokinetic study of paeoniflorin in mice after oral administration of Paeoniae radix extract. J Chromatogr B Biomed Sci Appl. 1999;735(1):33–40. doi:10.1016/S0378-4347(99)00408-9
15. Zhong SZ, Ge QH, Li Q, Qu R, Ma SP. Peoniflorin attentuates Abeta ((1-42))-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J Neurol Sci. 2009;280(1–2):71–78. doi:10.1016/j.jns.2009.01.027
16. Liu HQ, Zhang WY, Luo XT, Ye Y, Zhu XZ. Paeoniflorin attenuates neuroinflammation and dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease by activation of adenosine A1 receptor. Br J Pharmacol. 2006;148(3):314–325. doi:10.1038/sj.bjp.0706732
17. Mao QQ, Zhong XM, Qiu FM, Li ZY, Huang Z. Protective effects of paeoniflorin against corticosterone-induced neurotoxicity in PC12 cells. Phytother Res. 2012;26(7):969–973. doi:10.1002/ptr.3673
18. Lan Z, Chen L, Fu Q, et al. Paeoniflorin attenuates amyloid-beta peptide-induced neurotoxicity by ameliorating oxidative stress and regulating the NGF-mediated signaling in rats. Brain Res. 2013;1498:9–19. doi:10.1016/j.brainres.2012.12.040
19. Qiu ZK, He JL, Liu X, et al. Anxiolytic-like effects of paeoniflorin in an animal model of post traumatic stress disorder. Metab Brain Dis. 2018;33(4):1175–1185. doi:10.1007/s11011-018-0216-4
20. Liu LS, Winston JH, Shenoy MM, Song GQ, Chen JD, Pasricha PJ. A rat model of chronic gastric sensorimotor dysfunction resulting from transient neonatal gastric irritation. Gastroenterology. 2008;134(7):2070–2079. doi:10.1053/j.gastro.2008.02.093
21. Asano T, Aida S, Suemasu S, Tahara K, Tanaka K, Mizushima T. Aldioxa improves delayed gastric emptying and impaired gastric compliance, pathophysiologic mechanisms of functional dyspepsia. Sci Rep. 2015;5:17519. doi:10.1038/srep17519
22. Hagiwara SI, Kaushal E, Paruthiyil S, Pasricha PJ, Hasdemir B, Bhargava A. Gastric corticotropin-releasing factor influences mast cell infiltration in a rat model of functional dyspepsia. PLoS One. 2018;13(9):e0203704. doi:10.1371/journal.pone.0203704
23. Kawachi M, Matsunaga Y, Tanaka T, et al. Acotiamide hydrochloride (Z-338) enhances gastric motility and emptying by inhibiting acetylcholinesterase activity in rats. Eur J Pharmacol. 2011;666(1–3):218–225. doi:10.1016/j.ejphar.2011.05.049
24. Uchida M, Kobayashi O, Iwamoto C. Effects of L-tryptophan on gastric emptying evaluated by breath test in relation to gastric accommodation evaluated by Barostat in rats. J Pharmacol Sci. 2015;127(2):229–231. doi:10.1016/j.jphs.2014.12.008
25. Liu J, Li F, Tang XD, et al. XiangshaLiujunzi decoction alleviates the symptoms of functional dyspepsia by regulating brain-gut axis and production of neuropeptides. BMC Complement Altern Med. 2015;15:387. doi:10.1186/s12906-015-0913-z
26. Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2014;63(2):262–271. doi:10.1136/gutjnl-2012-303857
27. Liu S, Hagiwara SI, Bhargava A. Early-life adversity, epigenetics, and visceral hypersensitivity. Neurogastroenterol Motil. 2017;29:9. doi:10.1111/nmo.13170
28. Ueki S, Matsunaga Y, Yoneta T, Tamaki H, Itoh Z. Gastroprokinetic activity of nizatidine during the digestive state in the dog and rat. Arzneimittelforschung. 1999;49(7):618–625.
29. Sninsky CA, Davis RH, Clench MH, Thomas KD, Mathias JR. Effect of lidamidine hydrochloride and loperamide on gastric emptying and transit of the small intestine. A double-blind study. Gastroenterology. 1986;90(1):68–73. doi:10.1016/0016-5085(86)90076-4
30. Fulop K, Zadori Z, Ronai AZ, Gyires K. Characterisation of alpha2-adrenoceptor subtypes involved in gastric emptying, gastric motility and gastric mucosal defence. Eur J Pharmacol. 2005;528(1–3):150–157. doi:10.1016/j.ejphar.2005.10.025
31. Kawachi M, Hori N, Takei M, Kurimoto T, Akaike N, Ito Y. Gastric relaxation induced by electrical and chemical stimulation of the area postrema in the rat. Gen Physiol Biophys. 2008;27(4):243–252.
32. Iwanaga Y, Miyashita N, Morikawa K, Mizumoto A, Kondo Y, Itoh Z. A novel water-soluble dopamine-2 antagonist with anticholinesterase activity in gastrointestinal motor activity. Comparison with domperidone and neostigmine. Gastroenterology. 1990;99(2):401–408. doi:10.1016/0016-5085(90)91022-X
33. Mine Y, Yoshikawa T, Oku S, Nagai R, Yoshida N, Hosoki K. Comparison of effect of mosapride citrate and existing 5-HT4 receptor agonists on gastrointestinal motility in vivo and in vitro. J Pharmacol Exp Ther. 1997;283(3):1000–1008.
34. Chen JY, Wu HX, Chen Y, et al. Paeoniflorin inhibits proliferation of fibroblast-like synoviocytes through suppressing G-protein-coupled receptor kinase 2. Planta Med. 2012;78(7):665–671. doi:10.1055/s-0031-1298327
35. Hatat B, Yahiaoui S, Lecoutey C, et al. A novel in vivo anti-amnesic agent, specially designed to express both acetylcholinesterase (AChE) inhibitory, serotonergic subtype 4 receptor (5-HT4R) agonist and serotonergic subtype 6 receptor (5-HT6R) inverse agonist activities, with a potential interest against alzheimer’s disease. Front Aging Neurosci. 2019;11:148.
36. Tomita R. Regulation of vasoactive intestinal peptide and substance P in the human pyloric sphincter. Hepatogastroenterology. 2009;56(94–95):1403–1406.
37. Murphy KG, Dhillo WS, Bloom SR. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev. 2006;27(7):719–727. doi:10.1210/er.2006-0028
38. Yagi T, Asakawa A, Ueda H, Miyawaki S, Inui A. The role of ghrelin in patients with functional dyspepsia and its potential clinical relevance (Review). Int J Mol Med. 2013;32(3):523–531. doi:10.3892/ijmm.2013.1418
39. Ganko M, Calka J. Prolonged acetylsalicylic-acid-supplementation-induced gastritis affects the chemical coding of the stomach innervating vagal efferent neurons in the porcine dorsal motor vagal nucleus (DMX). J Mol Neurosci. 2014;54(2):188–198. doi:10.1007/s12031-014-0274-y
40. Du L, Chen B, Kim JJ, Chen X, Dai N. Micro-inflammation in functional dyspepsia: A systematic review and meta-analysis. Neurogastroenterol Motil. 2018;30(4):e13304. doi:10.1111/nmo.13304
41. Zhang S, Bian H, Li X, et al. Hydrogen sulfide promotes cell proliferation of oral cancer through activation of the COX2/AKT/ERK1/2 axis. Oncol Rep. 2016;35(5):2825–2832. doi:10.3892/or.2016.4691
42. He Y, Yang C, Wang P, et al. Child compound Endothelium corneum attenuates gastrointestinal dysmotility through regulating the homeostasis of brain-gut-microbiota axis in functional dyspepsia rats. J Ethnopharmacol. 2019;240:111953. doi:10.1016/j.jep.2019.111953
43. Jarupongprapa S, Ussavasodhi P, Katchamart W. Comparison of gastrointestinal adverse effects between cyclooxygenase-2 inhibitors and non-selective, non-steroidal anti-inflammatory drugs plus proton pump inhibitors: a systematic review and meta-analysis. J Gastroenterol. 2013;48(7):830–838. doi:10.1007/s00535-012-0717-6
44. Jia Z, He J. Paeoniflorin ameliorates rheumatoid arthritis in rat models through oxidative stress, inflammation and cyclooxygenase 2. Exp Ther Med. 2016;11(2):655–659. doi:10.3892/etm.2015.2908
45. Tanaka F, Tominaga K, Fujikawa Y, et al. Concentration of glial cell line-derived neurotrophic factor positively correlates with symptoms in functional dyspepsia. Dig Dis Sci. 2016;61(12):3478–3485. doi:10.1007/s10620-016-4329-5
46. Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014;36:157–165. doi:10.1016/j.semcdb.2014.08.011
47. Xiao Y, Liu YY, Yu KQ, Ouyang MZ, Luo R, Zhao XS. Chinese herbal medicine liu jun zi tang and xiang sha liu jun zi tang for functional dyspepsia: meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:936459. doi:10.1155/2012/936459
48. Chang X, Zhao L, Wang J, Lu X, Zhang S. Sini-san improves duodenal tight junction integrity in a rat model of functional dyspepsia. BMC Complement Altern Med. 2017;17(1):432. doi:10.1186/s12906-017-1938-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.