Back to Journals » Drug Design, Development and Therapy » Volume 13
Paclitaxel alleviated sepsis-induced acute lung injury by activating MUC1 and suppressing TLR-4/NF-κB pathway
Authors Wang YM, Ji R, Chen WW, Huang SW, Zheng YJ, Yang ZT , Qu HP, Chen H, Mao EQ, Chen Y, Chen EZ
Received 7 July 2019
Accepted for publication 1 September 2019
Published 24 September 2019 Volume 2019:13 Pages 3391—3404
DOI https://doi.org/10.2147/DDDT.S222296
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Yu-Ming Wang,1,* Ran Ji,1,* Wei-Wei Chen,1 Shun-Wei Huang,1 Yan-Jun Zheng,1 Zhi-Tao Yang,1 Hong-Ping Qu,2 Hao Chen,3 En-Qiang Mao,1 Ying Chen,1 Er-Zhen Chen1
1Department of Emergency, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Critical Care Medicine, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Department of General Surgery, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ying Chen; Er-Zhen Chen
Department of Emergency, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, No. 197 Ruijin 2nd Road, Shanghai 200025, China
Tel +86 216 437 0045
Fax +86 216 433 3548
Email [email protected]; [email protected]
Purpose: It has been reported that approximately 40% of ALI (acute lung injury) incidence resulted from sepsis. Paclitaxel, as a classic anti-cancer drug, plays an important role in the regulation of inflammation. However, we do not know whether it has a protective effect against CLP (cecal ligation and puncture)-induced septic ALI. Our study aims to illuminate the mitigative effects of paclitaxel on sepsis-induced ALI and its relevant mechanisms.
Materials and methods: The survival rates and organ injuries were used to evaluate the effects of paclitaxel on CLP mice. The levels of inflammatory cytokines were tested by ELISA. MUC1 siRNA pre-treatment was used to knockdown MUC1 expression in vitro. GO203 was used to inhibit the homodimerization of MUC1-C in vivo. The expression levels of MUC1, TLR 4 and p-NF-κB/p65 were detected by Western blot.
Results: Our results showed that paclitaxel improved the survival rates and ameliorated organ injuries especially lung injury in CLP-induced septic mice. These were accompanied by reduced inflammatory cytokines in sera and BALF (bronchoalveolar lavage fluid). We also found paclitaxel could attenuate TLR 4-NF-κB/p65 activation both in lung tissues of septic mice and LPS-stimulated lung type II epithelial cell line A549. At the upstream level, paclitaxel-upregulated expression levels of MUC1 in both in vivo and in vitro experiments. The inhibitory effects of paclitaxel on TLR 4-NF-κB/p65 activation were reversed in lung tissues of septic mice pre-treated with MUC1 inhibitor and in MUC1-knockdown A549 cells. Protection of paclitaxel on sepsis-induced ALI and decrease of inflammatory cytokines were also abolished by inhibition of MUC1.
Conclusion: Collectively, these results indicated paclitaxel could significantly alleviate acute lung injury in CLP-induced septic mice and LPS-stimulated lung type II epithelial cell line A549 by activating MUC1 and suppressing TLR-4/NF-κB pathway.
Keywords: sepsis, acute lung injury, MUC1, TLR 4, NF-κB
Introduction
Sepsis, which is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, is a major health care problem with high mortality rates worldwide.1,2 Nearly 30% of sepsis patients can develop into multi-organ dysfunction syndrome (MODS).3 It has been reported that lung might be one of the most vulnerable organs and approximately 40% ALI (acute lung injury) incidence results from sepsis.4–7 At present, there is no specific treatment for sepsis-induced ALI. The overall treatment strategies include high-flow nasal cannula and noninvasive ventilation, prone positioning, neuromuscular blockade, ECMO, supportive care and anti-inflammatory therapy.8 Recent studies suggest early short-term use of neuromuscular blocking agent (NMBA), if indicated, may be helpful in patients with sepsis-induced ALI.9,10 Anti-inflammatory therapy is also an important and effective treatment means. The role of corticosteroid in sepsis-induced ALI remains inconclusive.11,12 Statins have been reported to reduce inflammation, however, a recent multicenter trial showed no mortality benefit in sepsis-induced ALI.13
Paclitaxel, which is isolated from the bark of Taxus brevifolia tree, is one of the most widely used and traditional anticancer drugs and could arrest cell cycle in the G0/G1 and G2/M phases to induce cell death by stabilizing microtubule polymer and preventing microtubules from disassembly.14–16 Recent studies have shown that paclitaxel also played important roles in non-neoplastic diseases especially the regulation of inflammation and might exert anti-inflammatory effects by regulating the TLR-4 pathway.17 Paclitaxel was reported to inhibit chemotaxis induced by endotoxin-activated serum.18 In a mouse model of kidney injury, paclitaxel could block NF-κB activation and cytokine production.19 Paclitaxel could also attenuate the vascular leak and inflammation in LPS-induced acute lung injury in a mouse model. However, we still did not know the role of paclitaxel in mouse model of cecal ligation and puncture (CLP)-induced sepsis and the molecular mechanism of paclitaxel in sepsis-induced ALI also needed to be explored.
Numerous studies have reported that NF-κB played a crucial part in the pathogenesis of organ injury induced by sepsis. Toll-like receptor 4 (TLR 4)-NF-κB pathway was a classic signaling pathway triggered by pathogen-associated molecular pattern (PAMP) or danger-associated molecular pattern (DAMP) during sepsis.20,21 MUC1, as a kind of membrane-bound glycoprotein expressed in epithelial cells, played an essential anti-inflammatory role in response to diverse infections and served as a negative regulator of toll-like receptor signaling.22–26 However, it remained unclear whether paclitaxel inhibited TLR 4-NF-κB pathway in sepsis-induced ALI. We also did not know whether MUC1 was involved in the protective functions by paclitaxel during sepsis-induced ALI. We tried to investigate these questions.
Materials and methods
Animals
C57BL/6J mice (Male, 24–30 g, 8–10 weeks old) were purchased from Slac Laboratory Animal Corporation (Shanghai, China). All animals were kept in a humidity-controlled (30–70%) and temperature-controlled (21–23°C) room with a 12 hrs light/dark cycle. Mice did not have limited access to water and food (LabDiet, St. Louis, MO, USA). All experiments involving animals were demonstrated to be ethically acceptable by the Animal Ethics Committee of Ruijin Hospital affiliated with Shanghai Jiao Tong University, School of Medicine (No. 092), and were also in accordance with the international guidelines for care and use of laboratory animals (National Institutes of Health Publication No. 85-23, revised 1996).
CLP model
Mice were anesthetized by ketamine (75 mg/kg) and xylazine (15 mg/kg).27 Mice of sham group only received cecum exposure without ligation or puncture. All mice were resuscitated by sterile 0.9% saline at the end of the surgery and had free access to water and food. In order to evaluate the effects of paclitaxel, antibiotics should be avoided administration.
Mice were euthanized at 6 and 24 hrs after surgery. Tissues of lungs, heart, liver and kidney were harvested and fixed in 4% paraformaldehyde at 4°C or stored at −80°C immediately. Whole blood was centrifuged at 3000 rpm for 15 mins and sera were collected and stored at −80°C. Bronchoalveolar lavage fluid (BALF) was performed three times through a tracheal cannula with sterile PBS of a total volume of 1.5 mL and centrifuged at 1500 rpm for 5 mins at 4°C.
Animals and group
For survival analysis, 130 mice were divided into seven groups, i.e., sham (n=10), CLP group (n=20), CLP + paclitaxel (Taiji, China) (75, 150, 225, 300 μg/kg, n=20 for each group, paclitaxel was given 2 hrs after CLP by intraperitoneal injection), CLP + paclitaxel (150 μg/kg, n=20, paclitaxel was given immediately after CLP by intraperitoneal injection). Mice of sham and CLP group were only injected with same amount of sterile 0.9% saline intraperitoneally. In the subsequent trials involving mechanisms, only 150 μg/kg paclitaxel was given to mice 2 hrs after CLP.
MUC1 inhibitor GO203 (Selleck Chemicals, Houston, TX, USA) was dissolved in PBS for in vivo experiments and injected intraperitoneally (10, 20, 30 mg/kg, n=6 for each group). We used Western blot to analyze optimum doses. CP2 (Selleck Chemicals, Houston, TX, USA) was used as the negative control.
Cell culture and group
The human lung type II epithelial cell line A549 (Stem Cell Bank, Chinese Academy of Sciences, Shanghai, China) was cultured in DMEM (Sigma, St. Louis, MO, USA) culture medium with 10% FBS (FBS, Gibco, Grand Island, NY, USA), 100 U/mL streptomycin and 100 U/mL penicillin (Millipore, TMS-AB2-C) at 37°C in a humidified atmosphere containing 5% CO2.28 Cells were grown to confluence before LPS and paclitaxel treatment. Cells were incubated with different doses of LPS (Sigma, St. Louis, MO, USA) (1, 2.5, 5, 10 μg/mL) and different time-points (6, 12, 24, 48 hrs). Cells were then treated with different doses of paclitaxel (1, 2.5, 5, 10, 15, 20 nM) (Sigma, St. Louis, MO, USA). Cells and cell culture medium were collected and stored at −80°C for various measurements.
MUC1 siRNA transfection in vitro
A549 cells were transfected with human MUC1-siRNAs or NC siRNA (200 nM, GenePharma, Shanghai, China) with the transfection reagent lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) for 24 hrs. Human MUC1 siRNA sequences were as follows: MUC1-siRNA-1 5ʹ-CACAG UUCAA UCAGU AUAATT-3ʹ (sense), 5ʹ-UUAUA CUGAU UGAAC UGUGTT-3ʹ (antisense); MUC1-siRNA-2 5ʹ-GGGAU ACCUA CCAUC CUAUTT-3ʹ (sense), 5ʹ-AUAGG AUGGU AGGUA UCCCTT-3ʹ (antisense); MUC1-siRNA-3 5ʹ-CUCUU ACACA AACCC AGCATT-3ʹ (sense), 5ʹ-UGCUG GGUUU GUGUA AGAGTT-3ʹ (antisense).
Histological and biochemical analysis
The lungs, heart, kidney and liver tissues were fixed in the 4% paraformaldehyde over 24 hrs and stained with hematoxylin and eosin (H&E). Then, we evaluated the severity of organ injury and calculated scores according to scoring criteria reported before.29,30 Two pathologists performed the double-blind evaluation of all of the six fields of view at 100× magnification.
The lungs were weighed immediately after separation and dried in an oven at 80°C for 24 hrs to achieve a constant weight and then wet/dry weight ratio was calculated. Hepatocyte injury enzyme alanine aminotransferase (ALT), myocardial injury biomarker creatine kinase (CK) and renal injury biomarker creatinine were calculated using kits obtained from Jiancheng Bioengineering Institute (Nanjing, China).
Enzyme-linked immunosorbent assay (ELISA)
IL-1β, IL-6, TNF-α and IL-10 concentrations from mouse sera and bronchoalveolar lavage fluid (BALF) as well as culture supernatant of A549 cells were assessed by enzyme-linked immunosorbent assay (ELISA) kits (MultiSciences Biotech, Co., Ltd., Hangzhou, China) and all procedures were conformed to the manufacturer’s protocols.
Western blot assay
Protein samples (25 μg per sample) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Temecula, CA, USA). Then, membranes were blocked in 5% non-fat dried milk at room temperature for 1 hr and incubated with relevant primary antibodies against MUC1 (Thermo Fisher Scientific, USA), GAPDH (Cell Signaling Technology, Danvers, MA, USA), phosphorylated nuclear factor-κB (NF-κB)/p65 (Cell Signaling Technology, Danvers, MA, USA) and Toll-like Receptor 4 (TLR 4) (Thermo Fisher Scientific, USA) overnight at 4°C. After proper washing, membranes were incubated for 1 hr with HRP-conjugated secondary antibodies. The proteins were visualized on Tanon 5500 Imaging System (Shanghai, China).
Statistical analysis
All data were performed by GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) and SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Kaplan–Meier test was used to analyze survival curves. Data were described as mean with standard deviation or medians and interquartile ranges. Student’s t-test for two groups or one way ANOVA for multiple groups (>2) or Mann–Whitney test was used for comparing data. P-value <0.05 was considered statistically significant.
Results
Paclitaxel could improve survival rates of septic mice
In order to choose the optimal doses and time-points of paclitaxel administration, we set four different doses including 75, 150, 225 and 300 μg/kg and two different time points including immediate administration after CLP and 2 hrs after CLP. All mice were observed during 7 consecutive days and survival curves were shown in Figure 1. Survival rates of mice in CLP group decreased to 55% (11/20) at 24 hrs and 25% (5/20) on the seventh day. Paclitaxel administration of 150 μg/kg 2 hrs after CLP could significantly improve survival rates of CLP mice (Figure 1, P=0.024) compared with other groups of paclitaxel administration (Figure 1, P>0.05). However, immediate administration of 150 μg/kg paclitaxel after CLP did not show significant statistical difference compared with CLP group (Figure 1, P=0.073). The above results indicated that paclitaxel administration of 150 μg/kg 2 hrs after CLP had the best protective effects for septic mice and we employed this method in the following experiments.
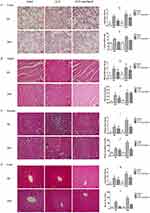
Paclitaxel ameliorates organ injuries especially lung injury in septic mice
HE staining results showed histological structures and relevant changes in different groups both at 6 and 24 hrs (Figure 2A–D). In sham group, tissues from lungs, heart, liver and kidney were typically normal. In CLP group, lung tissues displayed obvious inflammatory cells infiltration, alveolar damages and edema. Cardiac tissues showed loss of myocardial gap, abnormal arrangement of myocardial fibers and cells death and edema. Loss of hepatic sinusoids, infiltration of inflammatory cells and cells death were displayed in liver tissues. Tubular dilatation, tubular cell swelling and hemorrhage were shown in kidney tissues. Similarly, injury scores of corresponding organs increased evidently with statistical significance (Figure 2E, G, I and K, P<0.05). Obviously, organ injuries at 24 hrs were more severe than that at 6 hrs. In addition to this, lung wet/dry ratio (Figure 2F), sera CK (Figure 2H) and creatinine (Figure 2J) as well as ALT (Figure 2L) also increased clearly in accordance with histological changes, results of which were more obvious at 24 hrs than 6 hrs. In the paclitaxel group, histopathologic injuries were abolished in lung, liver and kidney. Protective effects of paclitaxel were notable at 24 hrs in the above organs. However, our results did not show significant protective effects of paclitaxel on heart both at 6and 24 hrs. Changes of lung wet/dry ratio, sera CK and ALT as well as creatine in the paclitaxel group were similar to histological changes. Besides, considering previous similar reports about mitigative effects of paclitaxel on mice models of LPS-induced liver and kidney injury,19,31 our study mainly focused on sepsis-induced acute lung injury.
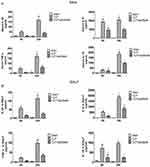
Paclitaxel reduces inflammatory cytokine concentrations in sera and BALF of septic mice
We used ELISA kit to test inflammatory cytokines including TNF-α, IL-1β, IL-6, IL-10 in sera and BALF (Figure 3A and B) and found all the above cytokines increased significantly after CLP, results of which were more obvious at 24 hrs than 6 hrs. Compared with CLP group, paclitaxel administration could significantly decrease levels of TNF-α, IL-1β, IL-6, IL-10 at 6 and 24 hrs both in sera and BALF. However, results of IL-10 concentrations in sera between paclitaxel group and CLP group at 6 hrs did not show statistical difference.
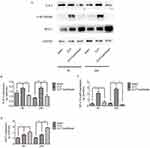
Paclitaxel attenuates TLR 4-NF-κB/p65 activation in sepsis-induced ALI and LPS-stimulated lung type II epithelial cell line
In order to clarify underlying mechanisms of paclitaxel on sepsis-induced lung injury and cytokine production, we detected expression levels of TLR 4 and phosphorylation of NF-κB-p65 at both 6 and 24 hrs after CLP. Results in Figure 4A–D showed that sepsis-induced activation of TLR 4 and phosphorylation of NF-κB/p65 were reduced by paclitaxel, inhibiting effects of which were more evident at 24 hrs. According to the above results, we chose 24 hrs as the optimal time point for subsequent in vivo experiments.
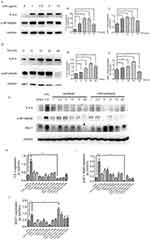
To investigate the effects of paclitaxel on sepsis-related TLR 4 and NF-κB/p65 expression levels in lung type II epithelial cell line, A549 cells were treated with paclitaxel after stimulated with LPS. We first set four different doses of LPS including 1, 2.5, 5, and 10 μg/mL (Figure 5A–C) and four different time points including 6, 12, 24, and 48 hrs (Figure 5D–F). And, we found that TLR 4 and phosphorylation of NF-κB-p65 were best activated after A549 cells were stimulated with 2.5 μg/mL LPS for 24 hrs. Then, we wanted to explore the optimal dose of paclitaxel on A549 cells and six non-lethal doses including 1, 2.5, 5, 10, 15, and 20 nM were set. Results in Figure 5G–J showed that 5 nM paclitaxel could most significantly inhibit activation of TLR 4 and phosphorylation of NF-κB-p65. Therefore, we selected 2.5 μg/mL LPS for 24 hrs and 5 nM paclitaxel for the following in vitro experiments.
The anti-inflammatory effect of paclitaxel depends on MUC1 in sepsis-induced ALI and LPS-stimulated lung type II epithelial cell line
Previous studies have been reported that as MUC1 was a negative regulator of toll-like receptor signaling and upregulation of MUC1 could inhibit activation of NF-κB signaling pathway in inflammatory diseases.22,26 Our study indicated that expression levels of MUC1 were significantly upregulated during inhibitory effects of paclitaxel on activation of TLR 4 and phosphorylation of NF-κB-p65 in both in vitro and in vivo experiments (Figures 4A and 5G); however, we did not know whether MUC1 played a role in this process or not. Therefore, GO203 (specific inhibitor MUC1) and its negative control CP2 were injected intraperitoneally in mice prior to CLP and paclitaxel treatment and changes of therapeutic effects of paclitaxel were observed. As shown in Figure 6A–C, compared with CLP + paclitaxel + CP2 group, inhibitory effects of paclitaxel on TLR 4 and phosphorylation of NF-κB-p65 were reversed in CLP + paclitaxel + GO203 group. The optimal dose of CP2 and GO203 was 20 mg/kg. HE staining showed that evident damages of lung tissues in CLP + paclitaxel + GO203 group compared with CLP + paclitaxel + CP2 group and there was a statistically significant difference in lung injury scores between the two groups (Figure 6D and H). However, liver, kidney and heart tissues did not show evident damages in CLP + paclitaxel + GO203 group (Figure 6E–G and I–K). Furthermore, we used ELISA kit to test inflammatory cytokines including TNF-α, IL-1β, IL-6, IL-10 in sera and BALF of paclitaxel-treated septic mice and found all the above cytokines increased significantly by the administration of MUC1 inhibitor GO203 (Figure 7A and B).
To further verify our conclusions, we transfected A549 cells with MUC1 siRNA1, MUC1 siRNA2, MUC1 siRNA3 and NC siRNA separately. As shown in Figure 8A–D, the inhibitory effect of MUC1 siRNA3 was optimal. Interestingly, we also found expression levels of TLR 4 and phosphorylation of NF-κB-p65 were obviously up-regulated in group of MUC1 siRNA3 compared with other groups. Then, we evaluated whether MUC1 knockdown influenced therapeutic effects of paclitaxel on LPS-stimulated A549 cells. As shown in Figure 8E–H, compared with LPS + paclitaxel + NC siRNA group, inhibitory effects of paclitaxel on TLR 4 and phosphorylation of NF-κB-p65 were reversed in LPS + paclitaxel + MUC1 siRNA3 group. We also tested cytokines from supernatant of A549 cells and found levels of TNF-α, IL-1β, IL-6 and IL-10 significantly increased in LPS + paclitaxel + MUC1 siRNA3 group (Figure 8I). The above results indicated that paclitaxel ameliorated sepsis-induced lung injury through MUC1 in mice and lung type II epithelial cell line A549.
Discussion
Sepsis is a dynamic and heterogeneous syndrome caused by imbalances of the inflammatory network. In sepsis, pro-inflammatory environment triggered by infection caused massive amplification of the inflammatory process which eventually resulted in multiple organ dysfunction of the host.32 Although great progress has been made to explore the host response to infection, no effective new therapeutic drugs exist.33 Paclitaxel, which was widely used as a chemotherapeutic agent, has been reported to have considerable anti-inflammatory effects in relevant diseases including sepsis.17 Qiu Yang et al reported that paclitaxel could alleviate liver injury induced of septic mice by alleviating inflammatory response.31 Another study revealed that paclitaxel could ameliorate LPS-induced kidney injury via suppression of NF-κB activation and inhibition of proinflammatory cytokine production.19 Mirzapoiazova et.al reported that paclitaxel had effects on suppression of endotoxin-induced inflammation.34 However, the above studies mainly focused on LPS injection model which was reported to have some drawbacks. First, it was difficult to draw a firm conclusion of drug effects to some critical illness including sepsis in a mouse model induced by single bacterial infection.35 Second, some investigators questioned that injection of a large number of doses of LPS at one time might cause heart failure and premature death.36 By contrast, CLP mouse model, which could reflect many features of human sepsis, was considered as the optimal sepsis model and most widely used. Our study was the first to adopt CLP model to explore the effects of paclitaxel on sepsis-induced organ damages. We found the administration of 150 μg/kg paclitaxel could significantly improve survival rates of septic mice and decreased levels of cytokines in sera and BALF as well as alleviate organ injuries including lungs, liver and kidney. However, we did not find effective protection of paclitaxel on heart injury. Considering clear mechanisms of mitigative effects of paclitaxel on LPS-induced liver and kidney injuries conducted by previous studies and un-conspicuous protective effects of paclitaxel on heart injury in our CLP model, we paid more attention to clarify mechanisms of effects of paclitaxel on sepsis-induced lung injury which have not been reported before. A variety of studies have reported Toll-like receptor 4 (TLR 4)-NF-κB pathway was a classic signaling pathway which was activated to cause severe inflammation during sepsis.20,37,38
Our data demonstrated that paclitaxel indeed inhibited TLR 4 and NF-κB activation both in lung tissues of septic mice and human lung type II epithelial cell line A549 stimulated by LPS. However, we still did not know the underlying mechanisms of suppression of NF-κB by paclitaxel. Interestingly, we found significant upregulation of MUC1 expression levels during inhibitory effects of paclitaxel on activation of TLR 4 and phosphorylation of NF-κB-p65 in both in vitro and in vivo experiments. MUC1, as the best-characterized number of mucins, is a transmembrane glycoprotein expressed in epithelial cells especially in the respiratory tract and a great number of studies have reported that MUC1 plays an important anti-inflammatory role in response to a variety of infectious insults. Lu et.al used an experimental model of bacterial lung infection and found that MUC1−/− mice exhibited higher levels of IL-8 and TNF-α and greater recruitment of leukocytes in bronchoalveolar lavage fluid compared with MUC+/+ mice.39 Another study conducted by Ueno et.al showed that knockout of MUC1 increased pro-inflammatory cytokine levels in response to various TLR ligands in both airway epithelial cell and macrophages and MUC1 could suppress NF-κB activation induced by TLR ligands including TLR 4.26 Several studies revealed a feedback loop between inflammation and MUC1 in lung epithelial cells. Enhanced production of inflammatory cytokines stimulated by some bacteria or viruses up-regulated MUC1 expression, which in turn suppressed further increase of cytokines.25,40 It has been reported that CT domain was required for the anti-inflammatory activity of MUC1 during TLR activation.26 Therefore, we focused on expression levels of MUC1-CT domain and tried to explore whether MUC1 participated in the suppression of TLR 4-NF-κB signaling pathway by paclitaxel in sepsis-induced lung injury. In accordance with previous studies, we found knockdown of MUC1 could increase expression levels of TLR 4 and phosphorylation of NF-κB/p65 in type II epithelial cell line A549. More importantly, we found inhibition of MUC1 in septic mice and knockdown of MUC1 in A549 cells reversed anti-inflammation of paclitaxel to a great degree. Besides, effects of MUC1 on anti-inflammation of paclitaxel mainly occurred in lung rather than other organs.
To our knowledge, this study was the first to explore anti-inflammatory effects of paclitaxel on septic-induced organ injuries in CLP model. Our study was also the first to find that MUC1 played an important role in the alleviation of paclitaxel on sepsis-induced lung injury in both in vivo and in vitro experiments. However, we had some limitations. The lack of knockout mouse models of MUC1 makes it tough to clarify the function of MUC1 in vivo. Therefore, we chose GO-203 to inhibit dimerization of the MUC1 CT domain which was reported to be required for its anti-inflammatory activity. Besides, our study failed to explain mechanisms of upregulation of MUC1 by paclitaxel and suppression of activated MUC1 to TLR 4-NF-κB signaling pathway which remained unclear before and we were trying to solve these questions in the following experiments in our laboratory.
Conclusion
In conclusion, our study revealed that paclitaxel could alleviate sepsis-induced organ injuries especially lung injury and reduce inflammatory cytokines in CLP mice model. And, mitigative effects of paclitaxel on sepsis-induced lung injury were mainly through activating MUC1 to suppress TLR-4/NF-κB pathway. Paclitaxel might be a potential clinical therapeutic choice for sepsis-induced lung injury. MUC1 might play an important role in sepsis-induced lung injury and needed to be further investigated.
Abbreviations
ALI, acute lung injury; CLP, cecal ligation and puncture; ELISA, enzyme-linked immunosorbent assay; BALF, bronchoalveolar lavage fluid; MODS, multi-organ dysfunction syndrome; PAMP, pathogen-associated molecular pattern; DAMP, danger-associated molecular pattern.
Acknowledgment
This work was supported by Shanghai Shenkang Hospital Development Center of China (grant number SHDC12017116), Program for Outstanding Medical Academic, Shanghai Municipal Committee of Science and Technology (184119500900), Shanghai Municipal Commission of Health and Family Planning (2016ZB0206, ZHYY-ZXYJHZX-1-201702) and Shanghai Jiao Tong University School of Medicine (DLY201803) to Er-Zhen Chen. This work was also supported by Shanghai Municipal Commission of Health and Family Planning (201640089) to Zhi-Tao Yang.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
2. Napolitano LM. Sepsis 2018: definitions and guideline changes. Surg Infect (Larchmt). 2018;19(2):117–125. doi:10.1089/sur.2017.278
3. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi:10.1056/NEJMra1608077
4. Ferguson ND, Kacmarek RM, Chiche JD, et al. Screening of ARDS patients using standardized ventilator settings: influence on enrollment in a clinical trial. Intensive Care Med. 2004;30(6):1111–1116. doi:10.1007/s00134-004-2163-2
5. Fein AM, Calalang-Colucci MG. Acute lung injury and acute respiratory distress syndrome in sepsis and septic shock. Crit Care Clin. 2000;16(2):289–317.
6. Iscimen R, Cartin-Ceba R, Yilmaz M, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–1522. doi:10.1097/CCM.0b013e31816fc2c0
7. Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128(2):525–532. doi:10.1378/chest.128.2.525
8. Kim WY, Hong SB. Sepsis and acute respiratory distress syndrome: recent update. Tuberc Respir Dis (Seoul). 2016;79(2):53–57. doi:10.4046/trd.2016.79.2.53
9. Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi:10.1056/NEJMoa1005372
10. Steingrub JS, Lagu T, Rothberg MB, Nathanson BH, Raghunathan K, Lindenauer PK. Treatment with neuromuscular blocking agents and the risk of in-hospital mortality among mechanically ventilated patients with severe sepsis. Crit Care Med. 2014;42(1):90–96. doi:10.1097/CCM.0b013e31829eb7c9
11. Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. doi:10.1056/NEJMoa051693
12. Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301(22):2362–2375. doi:10.1001/jama.2009.815
13. Truwit JD, Bernard GR, Steingrub J, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–2200. doi:10.1056/NEJMoa1401520
14. Mekhail TM, Markman M. Paclitaxel in cancer therapy. Expert Opin Pharmacother. 2002;3(6):755–766. doi:10.1517/14656566.3.6.755
15. Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23(11):2016–2027. doi:10.1038/sj.onc.1207374
16. Brito DA, Yang Z, Rieder CL. Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J Cell Biol. 2008;182(4):623–629. doi:10.1083/jcb.200805072
17. Zhang D, Yang R, Wang S, Dong Z. Paclitaxel: new uses for an old drug. Drug Des Devel Ther. 2014;8:279–284. doi:10.2147/DDDT.S56801
18. Roberts RL, Nath J, Friedman MM, Gallin JI. Effects of taxol on human neutrophils. J Immunol. 1982;129(5):2134–2141.
19. Zhang D, Li Y, Liu Y, Xiang X, Dong Z. Paclitaxel ameliorates lipopolysaccharide-induced kidney injury by binding myeloid differentiation protein-2 to block Toll-like receptor 4-mediated nuclear factor-kappaB activation and cytokine production. J Pharmacol Exp Ther. 2013;345(1):69–75. doi:10.1124/jpet.112.202481
20. Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13(11):460–469. doi:10.1016/j.molmed.2007.09.002
21. Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25(51):6758–6780. doi:10.1038/sj.onc.1209943
22. Kim KC, Lillehoj EP. MUC1 mucin: a peacemaker in the lung. Am J Respir Cell Mol Biol. 2008;39(6):644–647. doi:10.1165/rcmb.2008-0169TR
23. McAuley JL, Corcilius L, Tan HX, Payne RJ, McGuckin MA, Brown LE. The cell surface mucin MUC1 limits the severity of influenza A virus infection. Mucosal Immunol. 2017;10(6):1581–1593. doi:10.1038/mi.2017.16
24. Dhar P, Ng GZ, Dunne EM, Sutton P. Mucin 1 protects against severe Streptococcus pneumoniae infection. Virulence. 2017;8(8):1631–1642. doi:10.1080/21505594.2017.1341021
25. Li Y, Dinwiddie DL, Harrod KS, Jiang Y, Kim KC. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2010;298(4):L558–L563. doi:10.1152/ajplung.00225.2009
26. Ueno K, Koga T, Kato K, et al. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol. 2008;38(3):263–268. doi:10.1165/rcmb.2007-0336RC
27. Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi:10.1038/nprot.2008.214
28. Chuang CY, Chen TG, Tai YT, et al. Toll-like receptor 2-mediated sequential activation of MyD88 and MAPKs contributes to lipopolysaccharide-induced sp-a gene expression in human alveolar epithelial cells. Immunobiology. 2011;216(6):707–714. doi:10.1016/j.imbio.2010.10.009
29. Shen Y, Zhang FQ, Wei X. Truncated monocyte chemoattractant protein-1 can alleviate cardiac injury in mice with viral myocarditis via infiltration of mononuclear cells. Microbiol Immunol. 2014;58(3):195–201. doi:10.1111/1348-0421.12130
30. Lee CC, Lee RP, Subeq YM, Lee CJ, Chen TM, Hsu BG. Fluvastatin attenuates severe hemorrhagic shock-induced organ damage in rats. Resuscitation. 2009;80(3):372–378. doi:10.1016/j.resuscitation.2008.12.003
31. Yang Q, Zhang D, Li Y. Paclitaxel alleviated liver injury of septic mice by alleviating inflammatory response via microRNA-27a/TAB3/NF-kappaB signaling pathway. Biomed Pharmacother. 2018;97:1424–1433. doi:10.1016/j.biopha.2017.11.003
32. Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8(10):776–787. doi:10.1038/nri2402
33. Cohen J, Vincent J-L, Adhikari NKJ, et al. Sepsis: a roadmap for future research. The Lancet Infectious Diseases. 2015;15(5):581–614. doi:10.1016/S1473-3099(15)70112-X
34. Mirzapoiazova T, Kolosova IA, Moreno L, Sammani S, Garcia JG, Verin AD. Suppression of endotoxin-induced inflammation by taxol. Eur Respir J. 2007;30(3):429–435. doi:10.1183/09031936.00154206
35. Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence. 2014;5(1):213–218. doi:10.4161/viru.27024
36. Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, Sannomiya P. Experimental models of sepsis and their clinical relevance. Shock. 2008;30 Suppl 1:53–59. doi:10.1097/SHK.0b013e318181a343
37. Zhou LF, Zhu Y, Cui XF, Xie WP, Hu AH, Yin KS. Arsenic trioxide, a potent inhibitor of NF-kappaB, abrogates allergen-induced airway hyperresponsiveness and inflammation. Respir Res. 2006;7:146. doi:10.1186/1465-9921-7-146
38. Tak PP, Firestein GS. NF-kappaB_ a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi:10.1172/JCI11830
39. Lu W, Hisatsune A, Koga T, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol. 2006;176(7):3890–3894. doi:10.4049/jimmunol.176.7.3890
40. Kyo Y, Kato K, Park YS, et al. Antiinflammatory role of MUC1 mucin during infection with nontypeable Haemophilus influenzae. Am J Respir Cell Mol Biol. 2012;46(2):149–156. doi:10.1165/rcmb.2011-0142OC
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.