Back to Journals » Journal of Pain Research » Volume 10
P2Y12 and P2Y13 receptors involved in ADPβs induced the release of IL-1β, IL-6 and TNF-α from cultured dorsal horn microglia
Authors Liu P , Yue M, Zhou R, Niu J, Huang D, Xu T, Luo P, Liu X, Zeng J
Received 15 March 2017
Accepted for publication 31 May 2017
Published 26 July 2017 Volume 2017:10 Pages 1755—1767
DOI https://doi.org/10.2147/JPR.S137131
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Pei-Wen Liu,* Ming-Xia Yue,* Rui Zhou, Juan Niu, Du-Juan Huang, Tao Xu, Pei Luo, Xiao-Hong Liu, Jun-Wei Zeng
Department of Physiology, Zunyi Medical College, Guizhou, China
*These authors contributed equally to this work
Objective: P2 receptors have been implicated in the release of neurotransmitter and pro-inflammatory cytokines due to their response to neuroexcitatory substances in the microglia. Dorsal horn P2Y12 and P2Y13 receptors are involved in the development of pain behavior induced by peripheral nerve injury. However, it is not known whether P2Y12 and P2Y13 receptors activation is associated with the expression and the release of interleukin-1B (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) in cultured dorsal spinal cord microglia. For this reason, we examined the effects of ADPβs (ADP analog) on the expression and the release of IL-1β, IL-6, and TNF-α.
Methods and results: In this study, we observed the effect of P2Y receptor agonist ADPβs on the expression and release of IL-1β, IL-6 and TNF-α by using real-time fluorescence quantitative polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA). ADPβs induced the increased expression of Iba-1, IL-1β, IL-6 and TNF-α at the level of messenger RNA (mRNA). ADPβs-evoked increase in Iba-1, IL-1β, IL-6 and TNF-α mRNA expression was inhibited only partially by P2Y12 receptor antagonist MRS2395 or P2Y13 receptor antagonist MRS2211, respectively. Similarly, ADPβs-evoked release of IL-1β, IL-6 and TNF-α was inhibited only partially by MRS2395 or MRS2211. Furthermore, ADPβs-evoked increased expression of Iba-1, IL-1β, IL-6 and TNF-α mRNA, and release of IL-1β, IL-6 and TNF-α were nearly all blocked after co-administration of MRS2395 plus MRS2179. Further evidence indicated that P2Y12 and P2Y13 receptor-evoked increased gene expression of IL-1β, IL-6 and TNF-α were inhibited by Y-27632 (ROCK inhibitor), SB203580 (P38MAPK inhibitor) and PDTC (NF-κb inhibitor), respectively. Subsequently, P2Y12 and P2Y13 receptor-evoked release of IL-1β, IL-6 and TNF-α, were also inhibited by Y-27632, SB203580 and PDTC, respectively.
Conclusion: These observations suggest that P2Y12 and P2Y13 receptor-evoked gene expression and release of IL-1β, IL-6 and TNF-α are associated with ROCK/P38MAPK/NF-κb signaling pathway.
Keywords: glial activation, P2 receptor, NF-kB, p38 mitogen-activated protein kinasez
Introduction
Microglia cells, the primary resident immune cell population in brain and spinal cord, are suggested to be involved in neuron development, neurobehavioral disorders, ischemic injury, neuropathic pain, and some neurodegenerative disease.1 In response to stimuli that disrupt homeostasis, microglia can lead to the excessive induction of neuroactive substances and subsequently, regulate neuronal excitability and synaptic strength.2–4 Some recent evidence indicates that purinergic P2 receptor signaling has been implicated in both the beneficial and toxic effects in microglia.5 P2 purinoceptors can be divided into two classes on the basis of their characteristic molecular structures and their signal transduction mechanisms: the adenosine triphosphate (ATP)-gated ionotropic P2X family and the G protein-coupled metabotropic P2Y receptors.6 In cultured rat retinal microglia, hypoxia-induced interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) release are regulated by P2 receptor.7 Extracellular ATP triggers TNF-α release in rat brain microglia via a P2 receptor, likely to be the P2X7 receptor subtype, by a mechanism that is dependent on both the sustained Ca2+ influx and ERK/P38 cascade.8 More recent studies convince that microglia cells express P2Y purinoceptors, which couple to different G-protein that mediate diverse biological effects through a cascade of downstream intracellular processes. P2Y receptor seems to have a major role in ATP-evoked interleukin-6 (IL-6) production in a mouse microglial cell line, MG-5.9 Uridine 5-diphosphate (UDP) activates P2Y6 receptors, inducing the release of nitric oxide (NO) by microglia that causes astrocyte apoptosis10 Morioka et al, reported that stimulation of spinal microglia P2Y6 receptors induces the production of CCL2 through either phospholipase C-mediated ERK or P38 phosphorylation and the subsequent activation of nuclear factor-κB (NF-κB).11 It is noted that, in the rat peripheral nerve injury model, the increased expression of P2Y12 and P2Y13 receptors in spinal cord microglia results in the P38MAPK phosphorylation and pain behaviors.12 Furthermore, the highest degree of sequence identity is found between P2Y12 and P2Y13 receptors.13 It is well known that some pro-inflammatory cytokines in dorsal spinal cord are involved in the transduction of the “pain” message. However, it is not clear whether P2Y12 and P2Y13 receptor activation can lead to production of some pro-inflammatory cytokines.
Increasing evidence suggests an important role of spinal cord microglia in the genesis of persistent pain, by releasing some pro-inflammatory cytokines.14 Clark et al reported that IL-1β released by spinal microglia in enhanced response states contributes significantly to neuronal mechanisms of chronic pain.15 Furthermore, intrathecal administration of IL-1β induced thermal hyperalgesia by activating the iNOS-NO cascade in the intact rat spinal cord.16 P38MAPK plays a pivotal role in IL-1β-induced spinal sensitization and nociceptive signal transduction.17 In addition, IL-6, another pro-inflammatory cytokine, was up-regulated in rat spinal cord in neuropathic pain produced by lumber 5 ventral root transection.18 Up-regulation of TNF-α in mouse spinal cord contributes to vincristine-induced mechanical allodynia.19 In a rat model of skin/muscle incision and retraction, TNF-α in dorsal horn was increased on the ipsilateral side of spinal dorsal horn.20 Furthermore, Nakanishi et al, indicated that, by using double-staining immunohistochemistry technique, most of the TNF-α was co-expressed in Iba1-positive cells in rat dorsal horn.21 Co-administration of thalidomide (TNF-α synthesis inhibitor) and IL-1ra prevented bortezomib-induced mechanical allodynia.22 Despite all this research in this matter, there has still been little detailed study whether P2Y12 and P2Y13 receptor activation can induce the production and release of these pro-inflammatory cytokines. For this reason, in this study, we observed the effect of P2Y receptor agonist ADPβs on the expression and release of IL-1β, IL-6 and TNF-α by using real-time fluorescence quantitative polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA).
Materials and methods
Purification and culture of microglia
Primary dissociated cultures of dorsal spinal cord were prepared from Sprague-Dawley (SD) rats in accordance with the National Institutes of Health guidelines in a manner that minimized animal suffering and animal numbers. All experiments were carried out in accordance with China animal welfare legislation and were approved by the Zunyi Medical College Committee on Ethics in the Care and Use of Laboratory Animals.
Primary cultures of microglia were prepared from dorsal spinal cord of newborn SD rats, as previously described.23,24 Briefly, a neonatal rat (≤2 days) was anesthetized, and dorsal spinal cord was dissected surgically according to the “open-book” technique under sterile conditions. The dorsal spinal cord was cut into pieces and then incubated in 0.125% trypsin for 30 min at 37°C. Dulbecco’s Modified Eagle’s Medium (DMEM/F12 medium; Gibco, Rockville, MD, USA) containing 10% (v/v) fetal bovine serum (FBS; Gibco) was added to stop the action of typsin (Sigma, St. Louis, MO, USA). A filter is not required for the processes. The cells were collected after centrifugation at 1000 g for 5 min. The supernatant was discarded, and the cells were suspended in complete medium containing DMEM/F12 and 10% (v/v) FBS. Then, confluent glial cell mixed cultures were deprived of fresh medium to induce microglial cell proliferation. After 10–14 days, we put the cell cultured flask on the flattened side and gently banged on the side and tapped at the speed of 45 times/min. We tried to minimize the amount of foam generated when shaking the cell culture flask. The supernatant containing the detached microglial cells was collected and re-seeded for 1 h to allow microglial attachment. After 1 h, the nonadherent cells were removed. The cells were cultured in complete medium containing DMEM with high glucose, 10% (v/v) heat-inactivated FBS and 5% (v/v) heat-inactivated horse serum.
To determine the purity of the primary microglial cultures, cells cultured on glass coverslips were stained for OX-42 and Iba-1 by double immunofluorescence. The cells were fixed in 4% paraformaldehyde for 20 min, washed with 0.01 mol/L phosphate-buffered saline (PBS) 3 times and incubated with 10% donkey serum for 15 min to simultaneously block non-specific binding. Microglia cell cultures were stained with rabbit anti-OX-42 (1:500, Abcam [Cambridge, UK], ab1211) overnight. Subsequently, the cultures were incubated with FITC-conjugated donkey anti-rabbit IgG (1:300, Jackson Immuno-Research, West Grove, PA, USA) for 1 h, followed by incubations with goat anti-Iba-1 (1:500, Abcam, ab5076) overnight. Then, the cultures were incubated with CY3-conjugated donkey anti-goat IgG (1:300, Jackson Immuno-Research) for 1 h. Subsequently, 4′,6-diamidino-2-phenylindole (DAPI; 1:1000, Roche) was applied for 5 min to label all nuclei. At last, we randomly chose 10 visual fields (nearly 50 cells per visual field) to count the percentage of the positive cells by microscopy.
Drug application
ADPβs, MRS2395 (P2Y12 receptor antagonist), Y-27632 (ROCK inhibitor) and SB203580 (P38MAPK inhibitor) were purchased from Sigma. MRS2211 (P2Y13 receptor antagonist) and PDTC (NF-κB inhibitor) were purchased from Abcam. ADPβs, MRS2211 and Y-27632 were dissolved in 0.01M PBS. SB203580 and PDTC were dissolved in 10% dimethyl sulfoxide (DMSO) diluted in 0.01M PBS (vehicle).
According to some previous studies,25–27 microglia cells were pretreated with P2Y12 receptor antagonist MRS2395 (10 μM) and/or P2Y13 receptor antagonist MRS2211 (10 μM) for 20 min to test the effect of receptor inhibition on ADPβs-induced downstream effects. In some previous studies, Y-27632, SB203580 and PDTC at 5–20 μM were frequently used in order to establish the involvement of ROCK, P38MAPK and NF-κB signaling in cellular responses.28–32 Based on these recent experimental reports, in our experiments, cells were exposed to ADPβs (1, 10 and 50 μM for 3 h) with or without pretreatment of Y-27632 (5, 10 and 20 μM for 1 h), SB203580 (10, 20 and 40 μM for 2 h) and PDTC (10, 20 and 40 μM for 4 h), respectively. Afterward, the cells and supernatants were harvested separately and processed according to the different assay protocols.
Real-time fluorescence quantitative PCR (RTFQ-PCR)
The total RNA of cultured dorsal spinal cord microglia cells was also isolated by using Trizol reagent after 3 h of drug administration. RTFQ-PCR is carried out in iCycler IQ Real-Time PCR (RT-PCR) Detection System (BIO-RAD Co., Hercules, CA, USA) with SYBR Green PCR Master Mix (ABI Co., Foster, CA, USA). PCR conditions were as follows: 95°C 30 s 1 Cycle; 95°C 5 s, 60°C 30 s, 40 Cycles. The cycle threshold (Ct) value represents the cycle number at which a fluorescent signal rises statistically above background. The relative quantification of gene expression was analyzed by the 2−ΔΔCt method.
The nucleotide sequences of the primers were synthesized by TaKaRa Biological Engineering Company. Primer preparations were devised according to the sequence searched on GenBank. The nucleotide sequences of the primers used in this experiment were as follows: 1) Iba-1 (genebank: NM-017196.3): F: CTCGATGATCCCAAGTACAGCA; R: CAGCATTCGCTT CAAGGACATA; 2) IL-1β (genebank: NM-031512.2) F: 5′-CCCTGAACTCAACTGTGA AATAGCA-3′; R: 5′-CCCAAGTCAAGGGCTTGGAA-3′. 3) IL-6 (genebank: NM-0,12,589.2): F: ATTGTATGAA CAGCGATGATGCAC; R: CCAGGTA GAAACGGAACTCCAGA; 4) TNF-α (genebank: NM-012675.3) F: 5′-CCTCTTCTCATTCCTGCTC-3′, R: 5′-CTTCTCCTCCTTG TTGGG-3′. 5) IL-10 (NM-25325) F: 5′-GCACTGCTATGTTGCCTGCT-3′; R: 3′ TCAGCTCTCGGAGCATGTG-5. 6) β-actin (GenBank Access NoV01217.1): F: 5′-TGACAGGATGCAGAAGGAG A-3′, R: 5′-TAGAGCCACCAATCCACACA-3′.
ELISA assay
Microglia cells were seeded in 12-well plates pretreated with various antagonists followed by stimulation with ADPβs (10 μM) for 3 h. After stimulation, culture media were collected and centrifuged at 13,000 rpm for 3 min. The amounts of TNF-α, IL-1β, and IL-6 in the culture medium were measured with commercial ELISA kits obtained from Shanghai Hushang Biological Technoligy Co., Ltd., Shanghai, China.
Statistical analysis
All data were presented as mean ± standard deviation. Statistical analysis was performed with one-way analysis of variance followed by Dunnett’s multiple comparison test or Student’s t-test by using SPSS18.0 (IBM Corp., Armonk, NY, USA). Differences at the P<0.05 level were considered statistically significant.
Results
Morphology of cultured dorsal spinal cord microglia cells
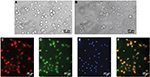
As shown in Figure 1A, phase-contrast microscopy indicated that mixed glia cell cultures reach confluence in ~2 weeks post seeding. The microglia cells are growing on the top of single layer of astrocytes as small rounded cells. In Figure 1B, microglia cells are isolated from the mixed cell culture before the end of the second week by gentle physical shaking and tapping on the flasks. In Figure 1C–F, the cell purity is examined by immunohistochemistry staining for Iba-1 and OX-42, two microglia specific markers. The purity of isolated primary microglia (cultured for 48 h) exceeded 95%, as verified by immunohistochemistry staining for Iba-1 (red) and OX-42 (green). The nuclei are counterstained with DAPI (blue).
Effects of MRS2395 or MRS2211 on the expression of Iba-1, IL-1β, IL-6 and TNF-α mRNA in ADPβs-treated dorsal horn microglia cells
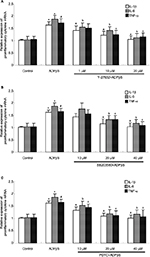
Tatsumi et al reported that intrathecal administration of 2Me-SADP significantly increased immunoreactivity of Iba-1 in rat dorsal spinal cord.12 However, 2Me-SADP was not available. Therefore, we used another potent P2Y receptor agonist ADPβs, a non-hydrolysable ADP analog. A minor but significant increase of Iba-1 mRNA expression was observed after ADPβs (1 μM for 3 h) administration (P<0.05, Figure 2A). Stimulation of microglia cells with 10 μM ADPβs led to a robust increase of Iba-1 mRNA (P<0.01) as shown in Figure 2A. For this reason, we used 10 μM ADPβs in subsequent experiments. To ascertain the role of P2Y12 and P2Y13 receptors in ADPβs-induced increased expression of Iba-1 mRNA, MRS2395 (selective P2Y12 receptor antagonist) and MRS2211 (selective P2Y13 receptor antagonist) were applied. MRS2395 and MRS2211 at 1–50 μM had no effect on Iba-1 mRNA expression in microglia cells at quiescence state. As shown in Figure 2B and C, both MRS2395 (10 and 50 μM) and MRS2211 (10 and 50 μM) show a moderate degree of inhibition with the stimulatory effect of ADPβs on the increased Iba-1 mRNA expression (MRS2395: P<0.05; MRS2211: P<0.05). MRS2395 or MRS2211 at the concentration of 10 and 50 μM show similar results. For this reason, we used 10 μM MRS2395 or MRS2211 in subsequent experiments. At last, microglia cell cultures were co-incubated in MRS2395 (10 μM) plus MRS2211 (10 μM). As a result, we found that ADPβs-induced increased expression of Iba-1 mRNA was nearly abolished (P<0.01, Figure 2D). It appears that ADPβs-induced increased expression of Iba-1 mRNA is mainly regulated by P2Y12 and P2Y13 receptors.
It is reported that P2Y receptor can raise the level of IL-6 in human airway epithelial cells and rat vascular smooth muscle cells.33,34 P2Y12 receptor and IL-1β were time-dependently overexpressed in rat hind paw and lumbar spinal cord following intraplantar complete Freund's adjuvant (CFA) injection.35 For this reason, it is reasonable to presume that P2Y12 and P2Y13 receptor activation may be required for the production of some inflammatory cytokines. Thus, we observed the effect of ADPβs on the gene expression of IL-1β, IL-6 and TNF-α. As shown in Figure 3A–C, we found that ADPβs (10 μM, 3 h) administration increased mRNA levels of IL-1β (P<0.01), IL-6 (P<0.01) and TNF-α (P<0.01). Next, to ascertain the role of P2Y12 and P2Y13 receptors in ADPβs-induced increased gene expression of these inflammatory cytokines, MRS2395 and MRS2211 were used. MRS2395 and MRS2211 at 10 μM had no effect on the expression of these genes in microglia cells at quiescence state. In Figure 3A–C, PCR data demonstrated that both MRS2395 and MRS2211 show a moderate degree of inhibition with stimulatory effect of ADPβs on increased gene expression of IL-1β (P<0.05), IL-6 (P<0.05) and TNF-α (P<0.05). On further study, we found that ADPβs-induced increased expression of IL-1β (P<0.01), IL-6 (P<0.01) and TNF-α (P<0.01) mRNA were abolished in cultures co-incubated in MRS2395 (10 μM, 20 min) plus MRS2211 (10 μM, 20 min). This result suggests that ADPβs-induced increased expression of these genes is mediated by P2Y12 and P2Y13 receptors in cultured dorsal spinal cord microglia cells.
In addition, IL-10 is considered an alternative antinociceptive factor in spinal dorsal horn with experimental pain.36 The production and release of IL-10 were assessed using the RT-PCR and ELISA techniques. Seo et al reported that P2Y1 and P2Y11 receptors are major receptors involved in ADPβs-induced IL-10 expression.37 However, as shown in Figure 3D, contrary to this earlier experimental report, we found that ADPβs (1, 10 and 50 μM) could not enhance the expression of IL-10 mRNA. Similarly, ELISA results also showed that ADPβs (1, 10 and 50 μM) could not enhance IL-10 release from cultured dorsal horn microglia cells. We found that some previous studies support the notion that P2Y1 and P2Y11 receptors are not present in dorsal spinal cord microglia cells.27,38,39 Furthermore, Horváth et al discerned that in inflammatory pain rat models, P2Y12 receptor antagonist did not change the increased level of IL-10 after CFA stimulus.35 From these observations, we conclude that P2Y12 and P2Y13 receptor activation does not lead to increased level of IL-10 in cultured dorsal spinal cord microglia cells.
Effects of MRS2395 or MRS2211 on the release of IL-1β, IL-6 and TNF-α from ADPβs-treated dorsal horn microglia cells
The roles of P2 receptor in microglia pro-inflammatory cytokines release has been reported in some experiments.40–42 For example, in cultured mouse primary microglia, stimulation of P2X7 receptor by ATP or BzATP evoked the mRNA expression and release of pro-inflammatory cytokines IL-6 and TNF-α.40 Sattayaprasert et al reported that in human microglia, RT-PCR analysis showed platelet-activating factor treatment of microglia-induced expression of IL-6.43 However, ELISA assay showed no production of IL-6 was elicited at any time point (1–24 h) for microglial exposures to platelet-activating factor.43 It seems that pro-inflammatory cytokines synthesis and their release are regulated by different signaling pathways, respectively. First, we observed the release of IL-1β, IL-6 and TNF-α from ADPβs-treated dorsal spinal cord microglia cells by using ELISA assay. However, after ADPβs administration, IL-10 release did not happen. Second, we examined the effects of ADPβs on the release of IL-1β, IL-6 and TNF-α in the absence and presence of MRS2395 or MRS2211. In Figure 4, application of 10 μM ADPβs significantly increased IL-1β, IL-6 and TNF-α level in the medium. MRS2395 or MRS2211 alone could not influence the basal concentration of IL-1β, IL-6 and TNF-α. ELISA data demonstrated that both MRS2395 and MRS2211 show a moderate degree of inhibition with the stimulatory effect of ADPβs on the inflammatory cytokines release. On further study, we found that ADPβs-induced release of IL-1β (P<0.01), IL-6 (P<0.01) and TNF-α (P<0.01) were abolished in cultures co-incubated in MRS2395 (10 μM, 20 min) plus MRS2211 (10 μM, 20 min). This result suggests that ADPβs-induced inflammatory cytokines release is primarily mediated by P2Y12 and P2Y13 receptors in cultured dorsal spinal cord microglia cells.
Rock inhibitor, p38MAPK inhibitor, NF-κB inhibitor attenuated gene expression of IL-1β, IL-6 and TNF-α in ADPβs-treated cultured dorsal horn microglia cells
Malaval et al reported activation of RhoA/ROCK1 under the P2Y13 signaling cascade in hepatocytes.44 Several years later, Wang et al reported that deletion of the P2Y13 receptor leads to reduced down-regulated RhoA/ROCKI and NF-κB signaling in P2Y13 receptor (-/-) mice.45 Based on these reports, we hypothesized that ROCK, P38MAPK and NF-κB may contribute to the P2Y12 and P2Y13 receptor-evoked increased expression of IL-1β, IL-6 and TNF-α in dorsal horn microglia cells. Based on these recent experimental reports, in our experiments, cells were exposed to ADPβs (10 μM for 3 h) with or without pretreatment of Y-27632 (1, 10 and 20 μM for 1 h), SB203580 (10, 20 and 40 μM for 2 h) and PDTC (10, 20 and 40 μM for 4 h), respectively. Afterwards, the cells and supernatants were harvested separately and processed according to the different assay protocols. As shown in Figure 5A, Y-27632 at 10 and 20 μM significantly suppressed ADPβs-induced increased expression of IL-1β, IL-6 and TNF-α mRNA, while Y-27632 at 1 μM showed only a slight inhibitory effect on the stimulatory effect of ADPβs. At the same time, SB203580 at 20 and 40 μM significantly suppressed ADPβs-induced increased expression of IL-1β, IL-6 and TNF-α mRNA, while SB203580 at 10 μM showed only a slight inhibitory effect on the stimulatory effect of ADPβs (Figure 5B). In addition, PDTC at 20 and 40 μM similarly suppressed ADPβs-induced increased expression of IL-1β, IL-6 and TNF-α mRNA. PDTC at 10 μM only induced moderate inhibition of the stimulatory effect of ADPβs (Figure 5C). In this part of the experiment, 20 μM Y-27632, 40 μM SB203580 or 40 μM PDTC nearly completely blocked ADPβs-induced increased expression of IL-1β, IL-6 and TNF-α mRNA, which suggest the stimulatory effect of ADPβs is mediated mainly by ROCK/P38MAPK/NF-κB signaling pathway.
Rock inhibitor, p38MAPK inhibitor, NF-κB inhibitor attenuated the release of IL-1β, IL-6 and TNF-α from ADPβs-treated cultured dorsal horn microglia cells
ADPβs at 10 μM was added to the wells, and supernatants were harvested after 3 h and the levels of IL-1β, IL-6 and TNF-α in culture supernatants were determined by ELISA. We found that ADPβs (10 μM for 3 h) administration evoked release of IL-1β (P<0.01), IL-6 (P<0.01) and TNF-α (P<0.01) from dorsal spinal cord microglia. We also exposed cultured microglia cells at quiescence state to Y-27632 (20 μM, 1 h), SB203580 (40 μM, 2 h) and PDTC (40 μM, 4 h), respectively. And we detected no obvious change in the basal level of IL-1β, IL-6 and TNF-α. As shown in Figure 6, we found that P2Y12 and P2Y13 receptor-evoked release of IL-1β, IL-6 and TNF-α was suppressed by Y-27632 (P<0.05). Similarly, the release of IL-1β, IL-6 and TNF-α was also inhibited by SB203580 (P<0.05) and PDTC (P<0.01), respectively. It appears that the activation of P2Y12 and P2Y13 receptors can stimulate the ROCK/P38MAPK and the following NF-κB signaling is required for ADPβs-induced release of IL-1β, IL-6 and TNF-α from cultured dorsal horn microglia.
Discussion
A growing body of literature strongly supports the involvement of extracellular nucleotides and their receptor modulating the origination and maintenance of neuropathologic pain. In addition, it is well known that microglia, the resident macrophages of central nervous system, are involved in practically all aspects of neural development, plasticity, and neuropathic pain. Recently, Tatsumi et al reported that the P2Y12 and P2Y13 receptors, expressed in rat spinal dorsal horn microglia, are likely to have an important role in the origination and maintenance of neuropathologic pain after spared nerve injury.12 It is well known that IL-1β, IL-6 and TNF-α were important cytokines to be implicated in peripheral nerve injury-induced neuropathic pain mechanisms in rodents.46,47 In the present study, we found that the expression of IL-1β, IL-6 and TNF-α mRNA and their release were regulated by P2Y12 and P2Y13 receptors in ADPβs-treated dorsal spinal cord microglia cells.
P2Y receptors are variously coupled to different types of G protein that activate or inhibit various effectors enzymes, including phospholipase C-beta, phospholipase D, phospholipase A2, and adenylyl cyclase.48,49 In cultured rat brain microglia, ADP released from damaged cells and surrounding astrocytes could induce microglia chemotaxis and membrane ruffling through Gαi/o-coupled P2Y12 receptor.50 In sensory neurons, ADP acts at the Gi-coupled P2Y12 and P2Y13 receptors in the modulation of nociceptor sensitivity.51 Soulet et al characterized a Gi-dependent pathway leading to cell proliferation through PI3-kinase and MAP-kinase and a Gi-independent pathway responsible for cytoskeletal changes through Rho and Rho-kinase.52 Furthermore, in human platelet and hepatocytes, P2Y12 and P2Y13 receptor stimulation-induced high specific activation of RhoA/ROCK has also been observed.44,53 In our present experiment, P2Y12 and P2Y13 receptor-evoked production and release of IL-1β, IL-6 and TNF-α were significantly inhibited after pretreatment with Y-27632, SB203580 and PDTC suggesting that ROCK/P38MAPK/NF-κb signaling pathway may be involved in this process. Although our experiment does not directly address the role of Gi-dependent pathway in the production and release of inflammatory cytokines, the present finding alerts us to the possibility that Gi-dependent pathway may not be involved in P2Y12 and P2Y13 receptor-evoked production and release of inflammatory cytokines in cultured dorsal horn microglia cells. Moreover, in the rat peripheral nerve injury model, increased expression of P2Y12 and P2Y13 receptors in spinal cord microglia results in the ROCK/P38MAPK phosphorylation and pain behaviors.12
It is reported that ADP and ADPβs preferentially activate the human P2Y1, P2Y12 and P2Y13 receptor subtypes that are practically insensitive to uridine triphosphate (UTP) and UDP.54–56 Quintas et al suggest that astroglial proliferation induced by ADPβs was mediated by P2Y1 and P2Y12 receptors.57 In cultured adult neural progenitor cells from C57BL/6N mice, ADPβs exerts its function via the P2Y1 and P2Y13 receptors.58 In rat middle meningeal artery, ADPβs caused contraction most likely via P2Y1 or P2Y13 receptors.59 On the other hand, previous research suggests that P2Y1 mRNA were expressed in dorsal horn neurons and astrocytes but not in microglia.38 In our previous experiment, ADP-evoked Ca2+ mobilization was not blocked by MRS2179 (P2Y1 receptor selective antagonist), which also implies that P2Y1 receptor may not be functionally expressed in cultured dorsal spinal cord microglia cells.27 In the present study, we noticed that ADPβs-induced increased expression and release of IL-1β, IL-6 and TNF-α from cultured dorsal spinal cord microglia cells was almost completely blocked after co-incubation of MRS2395 and MRS2211, which suggest that activation of microglia P2Y12 and P2Y13 receptors is required for the production and release of these inflammatory cytokines in response to ADPβs.
P2 receptors have been implicated in the release of pro-inflammatory cytokines by the response to infections, injury, and immunologic challenges in many different cell types.62–64 Koeppen et al even thought all immune and inflammatory cells were regulated by customized purinergic networks of receptors and ectonucleotidases.62 ATP-driven maturation and release of IL-1β are mediated by the P2X7 receptor. In P2X7 receptor (−/−) mice, absence of the P2X7 receptor leads to an inability of peritoneal macrophages to release IL-1β in response to ATP.63 In 1321N1 astrocytoma cells, P2Y6 receptor activation induces a robust secretion of inflammatory mediators IL-6 and IL-8.64 These present findings alert us to the possibility that there may be more than one purinoceptor participate in the expression and release of these pro-inflammatory cytokines under physiological and pathological conditions. In our study, we noticed that ADPβs-induced increased expression of IL-1β, IL-6 and TNF-α mRNA is only partly impaired after MRS2395 or MRS2211 pretreatment, which indicates that both P2Y12 and P2Y13 receptors are involved in this process. Ishimaru et al also showed that, in mice liver macrophages/Kupffer cells, P2Y13 knockdown reduced lipopolysaccharide (LPS)-induced IL-6 production, but by <50%, which also implies that P2Y receptors including P2Y13 and others may be involved in LPS-induced IL-6 production.65
Following peripheral nociceptive activation via nerve injury, microglia cells become activated and release IL-1β, IL-6 and TNF-α, thereby initiating the pain process.66 Spared nerve injury up-regulated IL-1β in the regions in central nervous system is closely associated with pain, memory and emotion, including spinal dorsal horn, hippocampus, prefrontal cortex, nucleus accumbens, and amygdale.67 In CCI rats, the increases in p38MAPK activation and pain behavior were suppressed by blocking IL-6 action with a neutralizing antibody, while they were enhanced by supplying exogenous recombinant rat IL-6 (rrIL-6).68 Peripheral gp120 application into the rat sciatic nerve induced the increased expression of spinal TNF-α (colocalized with GFAP and Iba-1) at 2 weeks.69 From these results, it may be concluded that these pro-inflammatory cytokines are critically involved in the pathogenesis of chronic pain. In the present study, the expression of IL-1β, IL-6 and TNF-α and their release were regulated by P2Y12 and P2Y13 receptors in ADPβs-treated dorsal spinal cord microglia cells. On the other hand, Tatsumi et al reported that the P2Y12 and P2Y13 receptors are likely to have an important role in the origination and maintenance of neuropathologic pain after spared nerve injury. Our results have shown that activation of P2Y12 and P2Y13 receptors cause the release of IL-1β, IL-6 and TNF-α from cultured dorsal spinal cord microglia cells. However, this form of inflammatory cytokines release has happened in in vitro conditions. The next important question is, whether P2Y12 and P2Y13 receptors play a crucial role in neuropathic pain via the transcriptional regulation of IL-1β, IL-6 and TNF-α and their release from spinal microglia awaits resolution.
It is well known that P2Y receptor and its downstream calcium signaling usually leads to the production of some neuroactive substances in some different cell types in nervous system. Our previous study showed that P2Y1 receptor can cause Ca2+ mobilization and glutamate release from spinal dorsal horn astrocyte.70 In addition, we also found that P2Y13 receptor can cause Ca2+ mobilization in cultured spinal dorsal horn microglia cells.27 In the present experiments, MRS2395 or MRS2211 significantly impaired the ability of ADPβs-evoked IL-1β, IL-6 and TNF-α production in microglia cells. Based on these suggestions, a preliminary conclusion was drawn that both Ca2+-dependent and Ca2+-independent pathways may participate in ADPβs-evoked production and release of pro-inflammatory cytokines in cultured dorsal horn microglia cells.
It is well known that transcriptional activities of some pro-inflammatory cytokine in microglia cells may be regulated by MAPK activity. In BV2 microglial cells, after being subjected to oxygen-glucose deprivation, TNF-α and IL-1β expression are inhibited by p38MAPK inhibitor.71 In addition, Ea5 inhibited the activity of p38MAPK, resulting in the decrease expression of IL-6 in N9 microglial cell line.72 Our data showed that ROCK/P38MAPK pathway may be involved in P2Y12 and P2Y13 receptor-induced production and release of these pro-inflammatory cytokines, which implies that ROCK/P38MAPK pathway can be activated in response to the stimulation of P2Y12 and P2Y13 receptors. The report of Tatsumi et al also supports our idea that activation of P2Y12 and P2Y13 receptors and ROCK/P38MAPK downstream signaling pathway leads to the development and maintenance of chronic pain.12
NF-κB is an essential and ubiquitous transcription factor for the expression of many inflammation-related genes, including IL-1β, IL-6 and TNF-α in many different cell types. NF-κB activation and IκB-α degradation are known to be involved in LPS-induced iNOS expression in microglia.73 Gessi et al reported that the activation of μ-opioid receptor potentiates LPS-induced NF-κB promoting an inflammatory phenotype in microglia.74 We also found that ADPβs-evoked production and release of pro-inflammatory cytokines are obvious inhibited by NF-κB inhibitor PDTC, which implies that P2Y12 and P2Y13 receptor activation and NF-κB downstream signaling pathway lead to these effects. In addition, Jeong et al reported that in LPS-stimulated BV2 microglial cell, P38 MAPK plays an important role in the anti-inflammatory effects via the modulation of NF-κB and AP-1 activities.75 Moreover, montelukast could effectively attenuate neuropathic pain in CCI rats by inhibiting the activation of P38MAPK and NF-κB signaling pathways in spinal microglia.76 For this reason, although our experiment does not directly address the role of P38 MAPK in ADPβs-evoked NF-κB activation, the present finding alerts us to the possibility that P38MAPK may be involved in P2Y12 and P2Y13 receptor-evoked NF-κB activation in dorsal spinal cord microglia cells.
In summary, the cell culture experiments reveal that the P2Y12 and P2Y13 receptor subtype is expressed in cultured dorsal spinal cord microglia cells, and participates in ADPβs-evoked expression and release of IL-1β, IL-6, TNF-α from cultured microglia cells. P2Y12 and P2Y13 receptor-mediated ROCK/P38MAPK/NF-kB signaling leads to increased expression and release of IL-1β, IL-6 and TNF-α, which, in turn, may modulate neuronal excitability and synaptic strength. This pathway may be important for physiological as well as pathophysiological events occurring within the spinal cord, where it is implicated in the transduction of the “pain” message.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (No. 31460266 and 31640040). Program for New Century Excellent Talents in University (NCET-13–1070), Scientific Research Foundation for Excellent Talents in Guizhou Province (2012–93). We are very grateful to the other staff of Department of Physiology.
Disclosure
The authors report no conflicts of interest in this work.
References
Eyo UB, Dailey ME. Microglia: key elements in neural development, plasticity, and pathology. J Neuroimmune Pharmacol. 2013;8(3):494–509. | ||
Choi SH, Joe EH, Kim SU, Jin BK. Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. J Neurosci. 2003;23(13):5877–5886. | ||
Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci. 2008;28(9):2221–2230. | ||
Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87(1):10–20. | ||
Crain JM, Watters JJ. Microglial P2 purinergic receptor and immunomodulatory gene transcripts vary by region, sex, and age in the healthy mouse CNS. Transcr Open Access. 2015;3(2). | ||
Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. | ||
Morigiwa K, Quan M, Murakami M, Yamashita M, Fukuda Y. P2 Purinoceptor expression and functional changes of hypoxia-activated cultured rat retinal microglia. Neurosci Lett. 2000;282(3):153–156. | ||
Hide I, Tanaka M, Inoue A, et al. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75(3):965–972. | ||
Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem. 2001;78(6):1339–1349. | ||
Quintas C, Pinho D, Pereira C, Saraiva L, Gonçalves J, Queiroz G. Microglia P2Y6 receptors mediate nitric oxide release and astrocyte apoptosis. J Neuroinflammation. 2014;11:141. | ||
Morioka N, Tokuhara M, Harano S, Nakamura Y, Hisaoka-Nakashima K, Nakata Y. The activation of P2Y6 receptor in cultured spinal microglia induces the production of CCL2 through the MAP kinases-NF-κB pathway. Neuropharmacology. 2013;75:116–125. | ||
Tatsumi E, Yamanaka H, Kobayashi K, Yagi H, Sakagami M, Noguchi K. RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain. Glia. 2015;63(2):216–228. | ||
Marteau F, Le Poul E, Communi D, et al. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64(1):104–112. | ||
Taves S, Berta T, Chen G, Ji RR. Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast. 2013;2013:753656. | ||
Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci. 2010;30(2):573–582. | ||
Sung CS, Wen ZH, Chang WK, et al. Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. J Neurochem. 2005;94(3):742–752. | ||
Sung CS, Wen ZH, Chang WK, et al. Intrathecal interleukin-1beta administration induces thermal hyperalgesia by activating inducible nitric oxide synthase expression in the rat spinal cord. Brain Res. 2004;1015(1–2):145–153. | ||
Wei XH, Na XD, Liao GJ, et al. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Exp Neurol. 2013;241:159–168. | ||
Kiguchi N, Maeda T, Kobayashi Y, Kishioka S. Up-regulation of tumor necrosis factor-alpha in spinal cord contributes to vincristine-induced mechanical allodynia in mice. Neurosci Lett. 2008;445(2):140–143. | ||
Sun Y, Yang M, Tang H, Ma Z, Liang Y, Li Z. The over-production of TNF-α via Toll-like receptor 4 in spinal dorsal horn contributes to the chronic postsurgical pain in rat. J Anesth. 2015;29(5):734–740. | ||
Nakanishi M, Nakae A, Kishida Y, et al. Go-sha-jinki-Gan (GJG) ameliorates allodynia in chronic constriction injury-model mice via suppression of TNF-α expression in the spinal cord. Mol Pain. 2016;12:1–16. | ||
Li ZY, Zhang YP, Zhang J, et al. The possible involvement of JNK activation in the spinal dorsal horn in bortezomib-induced allodynia: the role of TNF-α and IL-1β. J Anesth. 2016;30(1):55–63. | ||
Salter MW, Hicks JL. ATP-evoked increases in intracellular calcium in neurons and glia from the dorsal spinal cord. J Neurosci. 1994;14(3 Pt 2):1563–1575. | ||
Ni M, Aschner M. Neonatal rat primary microglia: isolation, culturing, and selected applications. Curr Protoc Toxicol. 2010; Chapter 12:Unit 12.17. | ||
Wang W, Luo J, Xiang F, Liu X, Jiang M, Liao L, Hu J. Nucleolin down-regulation is involved in ADP-induced cell cycle arrest in S phase and cellapoptosis in vascular endothelial cells. PLoS One. 2014;9(10):e110101. | ||
Voss U, Turesson MF, Robaye B, et al. The enteric nervous system of P2Y13 receptor null mice is resistant against high-fat-diet- and palmitic-acid-induced neuronal loss. Purinergic Signal. 2014;10(3):455–464. | ||
Zeng J, Wang G, Liu X, et al. P2Y13 receptor- mediated rapid increase in intracellular calcium induced by ADP in cultured dorsal spinal cord microglia. Neurochem Res. 2014;39(11):2240–2250. | ||
Khanna V, Jain M, Barthwal MK, et al. Vasomodulatory effect of novel peroxovanadate compounds on rat aorta: role of rho kinase and nitric oxide/cGMP pathway. Pharmacol Res. 2011;64(3):274–282. | ||
Yang LL, Zhou Y, Tian WD, et al. Electromagnetic pulse activated brain microglia via the p38 MAPK pathway. Neurotoxicology. 2016;52:144–149. | ||
Kong L, Liu J, Wang J, et al. Icariin inhibits TNF-α/IFN-γ induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int Immunopharmacol. 2015;29(2):401–407. | ||
Zhou H, Sheng L, Wang H, et al. Anti-β2GPI/β2GPI stimulates activation of THP-1 cells through TLR4/MD-2/MyD88 and NF-κB signaling pathways. Thromb Res. 2013;132(6):742–749. | ||
Wang Q, Zhao Y, Sun M, et al. 2-Deoxy-day-glucose attenuates sevoflurane-induced neuroinflammation through nuclear factor-kappa B pathway in vitro. Toxicol In Vitro. 2014;28(7):1183–1189. | ||
Abbott KL, Loss JR 2nd, Robida AM, Murphy TJ. Evidence that Galpha(q)-coupled receptor-induced interleukin-6 mRNA in vascular smooth muscle cells involves the nuclear factor of activated T cells. Mol Pharmacol. 2000;58(5):946–953. | ||
Cha HJ, Jung MS, Ahn do W, et al. Silencing of MUC8 by siRNA increases P2Y2-induced airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2015;308(6):L495–L502. | ||
Horváth G, Gölöncsér F, Csölle C, et al. Central P2Y12 receptor blockade alleviates inflammatory and neuropathic pain and cytokine production in rodents. Neurobiol Dis. 2014;70:162–178. | ||
Piotrowska A, Kwiatkowski K, Rojewska E, Makuch W, Mika J. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia – Evidence from in vivo and in vitro studies. Neuropharmacology. 2016;108:207–219. | ||
Seo DR, Kim SY, Kim KY, et al. Cross talk between P2 purinergic receptors modulates extracellular ATP-mediated interleukin-10 production in rat microglial cells. Exp Mol Med. 2008;40(1):19–26. | ||
Kobayashi K, Fukuoka T, Yamanaka H, et al. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498(4):443–454. | ||
Barragán-Iglesias P, Pineda-Farias JB, Cervantes-Durán C, et al. Role of spinal P2Y6 and P2Y11 receptors in neuropathic pain in rats: possible involvement of glial cells. Mol Pain. 2014;10:29. | ||
Shieh CH, Heinrich A, Serchov T, van Calker D, Biber K. P2X7-dependent, but differentially regulated release of IL-6, CCL2, and TNF-α in cultured mouse microglia. Glia. 2014;62(4):592–607. | ||
Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, Inoue K. P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J Neurochem. 2010;114(3):810–819. | ||
Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-lnduced release and processing of IL-1beta in microglial cells. Crit Rev Immunol. 2009;29(4):335–345. | ||
Sattayaprasert P, Choi HB, Chongthammakun S, McLarnon JG. Platelet-activating factor enhancement of calcium influx and interleukin-6 expression, but not production, in human microglia. J Neuroinflammation. 2005;2(1):11. | ||
Malaval C, Laffargue M, Barbaras R, et al. RhoA/ROCKI signaling downstream of the P2Y13 ADP-receptor controls HDL endocytosis in human hepatocytes. Cell Signal. 2009;21(1):120–127. | ||
Wang N, Robaye B, Agrawal A, Skerry TM, Boeynaems JM, Gartland A. Reduced bone turnover in mice lacking the P2Y13 receptor of ADP. Mol Endocrinol. 2012;26(1):142–152. | ||
Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. | ||
Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. Microglial regulation of neuropathic pain. J Pharmacol Sci. 2013;121(2):89–94. | ||
Cho YR, Jang HS, Kim W, Park SY, Sohn UD. P2X and P2Y receptors mediate contraction induced by electrical field stimulation in feline esophageal smooth muscle. Korean J Physiol Pharmacol. 2010;14(5):311–316. | ||
Talasila A, Germack R, Dickenson JM. Characterization of P2Y receptor subtypes functionally expressed on neonatal rat cardiac myofibroblasts. Br J Pharmacol. 2009;158(1):339–353. | ||
Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. | ||
Malin SA, Molliver DC. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain. 2010;6:21. | ||
Soulet C, Sauzeau V, Plantavid M, et al. Gi-dependent and -independent mechanisms downstream of the P2Y12 ADP-receptor. J Thromb Haemost. 2004;2(1):135–146. | ||
Hardy AR, Hill DJ, Poole AW. Evidence that the purinergic receptor P2Y12 potentiates potentiates platelet shape change by a Rho kinase-dependent mechanism. Platelets. 2005;16(7):415–429. | ||
von Kügelgen I, Wetter A. Molecular pharmacology of P2Y receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(4–5):310–323. | ||
Communi D, Gonzalez NS, Detheux M, et al. Identification of a novel human ADP receptor coupled to G(i). J Biol Chem. 2001;276(44):41479–41485. | ||
Marteau F, Communi D, Boeynaems JM, Suarez Gonzalez N. Involvement of multiple P2Y receptors and signaling pathways in the action of adenine nucleotides diphosphates on human monocyte-derived dendritic cells. J Leukoc Biol. 2004;76(4):796–803. | ||
Quintas C, Fraga S, Gonçalves J, Queiroz G. Opposite modulation of astroglial proliferation by adenosine 5′-O-(2-thio)-diphosphate and 2-methylthioadenosine-5′-diphosphate: mechanisms involved. Neuroscience. 2011;182:32–42. | ||
Grimm I, Messemer N, Stanke M, Gachet C, Zimmermann H. Coordinate pathways for nucleotide and EGF signaling in cultured adult neural progenitor cells. J Cell Sci. 2009;122(Pt 14):2524–2533. | ||
Haanes KA, Edvinsson L. Expression and characterization of purinergic receptors in rat middle meningeal artery-potential role in migraine. PLoS One. 2014;9(9):e108782. | ||
Ohsawa K, Irino Y, Sanagi T, et al. P2Y12 receptor-mediated integrin-beta1 activation regulates microglial process extension induced by ATP. Glia. 2010;58(7):790–801. | ||
Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia. 2012;60(10):1529–1530. | ||
Koeppen M, Di Virgilio F, Clambey ET, Eltzschig HK. Purinergic regulation of airway inflammation. Subcell Biochem. 2011;55:159–193. | ||
Solle M, Labasi J, Perregaux DG, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276(1):125–132. | ||
Garcia RA, Yan M, Search D, et al. P2Y6 receptor potentiates pro-inflammatory responses in macrophages and exhibits differential roles in atherosclerotic lesion development. PLoS One. 2014;9(10): e111385. | ||
Ishimaru M, Yusuke N, Tsukimoto M, et al. Purinergic signaling via P2Y receptors up-mediates IL-6 production by liver macrophages/Kupffer cells. J Toxicol Sci. 2014;39(3):413–423. | ||
Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10(3):167–184. | ||
Gui WS, Wei X, Mai CL, et al. Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain. 2016;12. pii: 1744806916646784. | ||
Lee KM, Jeon SM, Cho HJ. Interleukin-6 induces microglial CX3CR1 expression in the spinal cord after peripheral nerve injury through the activation of p38 MAPK. Eur J Pain. 2010;14(7):682.e1–12. | ||
Zheng W, Ouyang H, Zheng X, et al. Glial TNFα in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol Pain. 2011;7:40. | ||
Zeng JW, Liu XH, Zhang JH, Wu XG, Ruan HZ. P2Y1 receptor-mediated glutamate release from cultured dorsal spinal cord astrocytes. J Neurochem. 2008;106(5):2106–2118. | ||
Liu Y, Wu XM, Luo QQ, et al. CX3CL1/CX3CR1-mediated microglia activation plays a detrimental role in ischemic mice brain via p38MAPK/PKC pathway. J Cereb Blood Flow Metab. 2015;35(10):1623–1631. | ||
Owona BA, Njayou NF, Laufer SA, Schluesener HJ, Moundipa PF. Entada africana fraction CH2Cl2/MEOH 5% inhibits inducible nitric oxide synthase and pro-inflammatory cytokines gene expression induced by lipopolysaccharide in microglia. BMC Complement Altern Med. 2013;13:254. | ||
More SV, Park JY, Kim BW, et al. Anti-neuroinflammatory activity of a novel cannabinoid derivative by inhibiting the NF-κB signaling pathway in lipopolysaccharide-induced BV-2 microglial cells. J Pharmacol Sci. 2013;121(2):119–130. | ||
Gessi S, Borea PA, Bencivenni S, Fazzi D, Varani K, Merighi S. The activation of μ-opioid receptor potentiates LPS-induced NF-kB promoting an inflammatory phenotype in microglia. FEBS Lett. 2016;590(17):2813–2826. | ||
Jeong YH, Kim Y, Song H, Chung YS, Park SB, Kim HS. Anti-inflammatory effects of α-galactosylceramide analogs in activated microglia: involvement of the p38 MAPK signaling pathway. PLoS One. 2014;9(2):e87030. | ||
Zhou C, Shi X, Huang H, Zhu Y, Wu Y. Montelukast attenuates neuropathic pain through inhibiting p38 mitogen-activated protein kinase and nuclear factor-kappa B in a rat model of chronic constriction injury. Anesth Analg. 2014;118(5):1090–1096. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.