Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Ozonated Water Inhibits Hepatocellular Carcinoma Invasion and Metastasis by Regulating the HMGB1/NF-κB/STAT3 Signaling Pathway
Authors Tang S , Xu B, Pang H , Xiao L, Mei Q, He X
Received 20 October 2022
Accepted for publication 17 January 2023
Published 10 February 2023 Volume 2023:10 Pages 203—215
DOI https://doi.org/10.2147/JHC.S394074
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Shuiying Tang,1 Bihong Xu,2 Huajin Pang,1 Lijun Xiao,1 Quelin Mei,1 Xiaofeng He1
1Division of Vascular and Interventional Radiology, Department of General Surgery, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, People’s Republic of China; 2Interventional Radiology and Pathology, Nanfang Hospital, Department of Pathology, Southern Medical University, Guangzhou, 510515, People’s Republic of China
Correspondence: Xiaofeng He, Email [email protected]
Background: Hepatocellular carcinoma (HCC) is one of the deadliest cancers worldwide. High-mobility group box 1 (HMGB1), a highly conserved chromosome protein, is considered as a potential therapeutic target and novel biomarker because of its regulation in the proliferation and metastasis of HCC. Ozone has been shown to be beneficial in the treatment of cancer. The objective of this study was to explore the effects and molecular mechanism of ozonated water on the proliferation, migration, and invasion of BEL7402 HCC cells.
Materials and Methods: We assessed cell proliferation using a CCK-8 assay kit and flow cytometry; we performed wound healing and transwell assays to evaluate the effects of ozonated water treatment on cell invasion and migration. We determined reactive oxygen species (ROS) values by flow cytometry and used ELISAs to detect cytokines HMGB1, IL-6, and TNF-α. In addition, we assessed mRNA and protein cytokine expressions using RT-qPCR and Western blot.
Results: Ozonated water decreased the viability of the HCC cells; the IC50 of ozonated water at 24 h was approximately 1.5 μg/mL. Compared with control groups, ozone treated cells revealed reduced mobility on wound healing assays and decreased invasion in transwell assays. HMGB1, IL-6, and TNF-α cytokines were found at lower levels in ozone treated cells than in control cells. Ozonated water-induced ROS accumulation. Likewise, the expressions of phosphorylated nuclear factor Kappa B (NF-κB), p65, NF-κB, P-STAT3, IL-6, JAK2, Slug, Twist, vimentin, MMP-2, MMP-9, and HMGB1 were decreased in the treated cells.
Conclusion: Our findings suggest that ozonated water inhibits the proliferation, invasion, and metastasis of HCC cells via regulation of the HMGB1/NF-κB/STAT3 signaling pathway.
Keywords: hepatocellular carcinoma, proliferation, migration, invasion, ozonated water
Introduction
Hepatocellular carcinoma (HCC) has become the most common primary hepatic malignancy; its average survival rates range from 6 to 20 months.1 The worldwide rising incidence of chronic hepatitis B and C infections has led to a proportional increase in the incidence of HCC.2 HCC is the second leading cause of cancer-associated deaths worldwide.3 The life expectancy of patients with HCC depends mainly on the tumor stage at diagnosis. Patients with HCCs diagnosed early have a high 5-year survival rate, provided they undergo effective treatment, but the traditional chemotherapy is ineffective for patients with advanced stage HCC. Effective treatments during the early stages include surgical resection, local ablation, and liver transplantation.4,5 Effective treatments to improve the life expectancy and quality of HCC patients are needed.
Ozone is a strong oxidizer that reacts immediately with antioxidants, polyunsaturated fatty acids (PUFA), proteins, and carbohydrates in physiological environments. Ozone would cause the formation of ROS, lipid hydroperoxide (LOP), and a variable of oxidized antioxidants. Ozone therapy has been proposed as an efficacious adjuvant treatment against diseases such as mucositis, psoriasis, neurovascular disease, and human cancer.6 Scientific literature has published articles on the ability of ozone to cause direct injury to tumor cells in vitro. In 1980, the journal Nature published an article in which ozone exerted selective inhibition of different human tumor cells (lung, breast, and uterine cells) but had little effect on non-tumor cell lines.7 Schulz et al reported that the effective percutaneous intraperitoneal injection rate of ozone in the treatment of rabbit VX2 tumors could reach 50%.8 A non-myometrial invasive bladder rat model using N-methyl-N-nitrosourea (MNU) showed significant tumor growth decreases and superoxide dismutase (SOD) increases in ozone-treated groups, suggesting that ozone could inhibit cancer by increasing SOD and inhibiting the activity of nuclear factor NF-kB.9 However, whether ozonated water inhibits the invasion and metastasis of liver cancer cells remains unexplored.
In this study, we found that ozonated water can inhibit the proliferation, invasion, and metastasis of HCC cells by targeting the HMGB1/NF-kB/STAT3 signaling pathway. Our findings suggest that ozonated water may serve as a potential HCC treatment.
Materials and Methods
Preparation and Concentration of Ozonated Water
Ozonated water is produced by an ozonated water machine (Debaishun, Guangzhou, China). Phosphate buffer solution (PBS) (Gibco; Thermo Fisher Scientific, Waltham, MA, USA) was used in the preparation of ozonated water. We determined the ozonated water concentration quantitatively with an ozonated water concentration meter (HKM, Guangzhou, China).
Cell Culture and Treatment
We obtained HCC cell line (BEL7402) cells from the Clinical Research Laboratory of Nanfang Hospital (Guangzhou, China). All the cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Thermo Fisher Scientific, Virginia, USA) supplemented with 10% fetal bovine serum (Tianjin Kangyuan Biotechnology, China) and 1% penicillin–streptomycin double antibiotics (Hyclone, USA) in a humidified incubator at 37°C with 5% CO2. We subcultured cells at a confluence of 80–90% and treated them with ozonated water for 10 min in the dark.
Inverted Microscope Analysis
We used a Leica XSP-8CA microscope (Leica, Wetzlar, Germany) to observe the morphology of apoptotic cells. After ozone treatment, cells were washed with PBS and placed under a microscope for static observation.
Cell Viability Assay
To validate the cytotoxicity of ozonated water on human HCC cells, we used a Cell Counting Kit-8 (CCK-8, Dojindo, Japan) assay to assess cell viability. The cells treated with ozonated water at different concentrations (2.07, 1.77, 1.27, or 1.13 μg/mL) for 10 min were seeded into 96-well plates. We added 10 μL of the CCK-8 solution and 90 μL of basic DMEM to each well, after culturing 24h. After addition of the solution at each timepoint, we incubated the plate at 37°C for 2 h. We determined the optical density (OD) at 450 nm on a plate reader (Thermo Fisher Scientific), and we calculated the IC50 for the ozone treatment. The cells were then treated with ozonated water at 1.5μg/mL for 10 min were seeded into 96-well plates. We added 10 μL of the CCK-8 solution and 90 μL of basic DMEM to each well, after culturing 24h, 36h, and 48h, After addition of the solution at each timepoint, we incubated the plate at 37°C for 2 h. We determined the optical density (OD) at 450 nm on a plate reader (Thermo Fisher Scientific), and we calculated the viability for the ozone treatment.
Cell Cycle Detection
After treatment with various concentrations of ozone water, cells were harvested, washed twice with PBS, add 500μl70% alcohol, placed in refrigerator at 4°C overnight, and the cells were washed twice with PBS and stained with 400μlPI for 30 min. Then, a flow cytometer (BD Biosciences, San Jose, CA, USA) was used to measure cell cycle.
Wound Healing Assay
We treated BEL7402 cells with different concentrations of ozonated water and inoculated them into a 6-well plate. After 24h, the cells reached 90% confluence. We used the suction head of a 20-μL pipette to draw three vertical marks on the cell monolayer from the center to side of the well and then washed them with PBS three times, added 2 mL of serum-free medium, and cultured them at 37°C. The wound area was measured after 0h, 12h, 24h, and 48h. The experiments were replicated three times.
Transwell Invasion Assays
For these experiments, we selected a plastic insert for a 24-well plate. The insert has a cell-permeable membrane to create a two-chamber system (upper and lower chambers). The pores in the membrane are blocked with a 50-μL gel composed of extracellular matrix (ECM). We placed the BEL7402 cells in the upper chamber on the side of the gel and treated them with ozonated water. We added chemoattractant (serum medium without cells) to the lower chamber on the other side of the gel. Groups of cells in Transwell chambers were cultured in a cell incubator for 24 or 48 h, separately. After the incubation time, we clipped the inserts using tweezers and washed cells twice with PBS. Next, we fixed the cells by volatilization, adding 600 μL of methanol to the lower chamber and leaving it there for 30 min. The inserts were stained in crystal violet solution for 30 min and washed with PBS three times. The remaining cells on the upper chamber were wiped with a cotton bud. We used the inserts directly to observe the cells under the microscope. We calculated the number of invading cells by counting five random fields per chamber at 200X magnification. The experiments were replicated three times.
Measurement of Reactive Oxygen Species
We used a DCFH-DA assay to detect changes in ROS production after ozone treatment. Briefly, cells treated with ozonated water were cultured evenly (106 cells/well) in 60-mm Petri dishes for 24 h. We collected the cells attached to the plate surface and added DCFH-DA in DMEM to them incubating them for 15 min at 37°C. After the incubation period, we triple washed the cells with DMEM to terminate the reactions and assess their magnitude using a flow cytometer.
Enzyme-Linked Immunosorbent Assay
We collected the supernatants of HCC cells and centrifuged them to remove particles before using the samples in assays to determine the levels of HMGB1, interleukin-6 (IL-6), and TNF-α; we used an ELISA kit (Mlbio, China) for these experiments. A blank well was used for the zero setting. We used a plate reader to measure the OD value of each well at a wavelength of 450 nm. We conducted these experiments in triplicate and then calculated mean values.
Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
We extracted total RNA from ozone treated cells and used a reverse transcription kit (Promega Corporation, Madison, WI, USA) to synthesize cDNA following the manufacturer’s instructions. Table 1 lists sequences of primers used for our target genes. The cDNA obtained was used as a template for PCR amplification with a SYBR Premix Ex Taq (TaKara Bio, Otsu, Japan; catalog number, DRR420A), in a thermal cycler (GeneAmp 2400; PE Applied Biosystems, Foster City, CA, USA). All samples were run in triplicate, and we used 2-ΔΔCq method provided by the System software (Applied Biosystems) to quantify the relative mRNA levels. Data were normalized with reference to those of the GAPDH levels in the samples.
 |
Table 1 Primers Used for Real-Time PCR |
Western Blot Analysis
Protein extraction and Western blot analyses were carried out as described.6,7 Briefly, we used RIPA lysis buffer (KeyGEN BioTECH, Jiangsu, China) to isolate proteins and quantified total protein concentrations on a NanoDrop 2000. Next, 100-µg total-protein samples were loaded onto gels and separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After transfer onto PVDF membranes and fixation, we incubated the blots with primary antibodies overnight at 4°C: HMGB1 (dilution, 1:1000; Mouse; Abcam, England), Anti-MMP9 (dilution, 1:1000; Rabbit; Cell Signaling Technology, USA), Anti-MMP2 (dilution, 1:1000; Rabbit; Cell Signaling Technology, USA), Phospho-Stat3 (Tyr705) (dilution, 1:1000; Rabbit; Cell Signaling Technology, USA), Phospho-NF-κB (dilution, 1:1000; Rabbit; Cell Signaling Technology, USA), NF-κB (dilution, 1:1000; Rabbit; Cell Signaling Technology, USA), vimentin (dilution, 1:1000; Rabbit; Cell Signaling Technology, USA), and anti-GAPDH (dilution, 1:25,000; Rabbit; Cell Signaling Technology, USA). The next day, we used HRP-conjugated secondary antibodies (dilution, 1:1000; Rabbit; Cell Signaling Technology, USA) to develop the immunoblots at room temperature for 2h. Signals were detected using the ECL enhanced chemiluminescence substrate (Thermo Fisher Scientific, Shanghai, China). We used an Image Quant RT ECLTM imager (GE Healthcare Life Sciences, Shanghai, China) to capture images. Band intensities were quantified using ImageJ software (GE Healthcare Life Sciences, Shanghai, China).
Statistical Analysis
We performed a statistical analysis using SPSS software version 19.0 (China) and GraphPad Prism 8.0 (China). All the data are expressed as means ± standard errors. The differences between two groups were analyzed by two-tailed Student’s t-test. We assessed associations between groups using one-way analysis of variance (ANOVA) with Dunnett’s test. We considered P values <0.05 as statistically significant and P values <0.01 as highly statistically significant (P < 0.05*P < 0.01**P < 0.001***).
Results
Ozonated Water Inhibits HCC Cell Proliferation
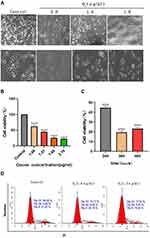
To investigate the proliferation/inhibition effects of ozonated water in HCC cells, we used different concentrations of ozonated water to treat HCC cells. We found that upon treatment with ozonated water, BEL7402 cells became round and small with nuclear shrinkage and their numbers decreased in the culture dish (Figure 1A). The viability assays using the CCK-8 kit showed significant dose- and time-dependent inhibition of proliferation. Moreover, the IC50 value was 1.531 μg/mL (Figure 1B and C). We used ozonated water at concentrations of 1.0 or 1.5 μg/mL for the subsequent experiments. Cell growth cycle is closely related to cell proliferation. We found that number of G1 phase cells in ozone-treated group was higher than in control group (Figure 1D).
Ozonated Water Inhibits HCC Cell (BEL7402) Migration
The migration ability of HCC cells after ozonated water treatment was checked through the wound healing assay. We divided the experiments into three groups: control, ozonated water at 1.0 μg/mL, and ozonated water at 1.5 μg/mL. The HCC cells displayed lower wound healing ability in the ozonated water groups than in the control group after 24 and 48 h (P<0.05). Importantly, the 48-h ozonated water treatments produced a more significant effect (P<0.01) than the 24-h treatments (p < 0.05; Figure 2). We found no effects after the 12-h treatments. These findings suggest that ozonated water can inhibit the migration of BEL7402 cells in a time-dependent manner.
 |
Figure 2 Ozonated water suppresses BELL7402 cell migration. Representative images and quantification of BELL7402 cells in wound healing assays, ×100 *P<0.05, **P<0.01, ***P<0.001, n=3. |
Ozonated Water Inhibits HCC Cell (BEL7402) Invasion
Based on our findings showing that ozonated water inhibits BEL7402 cell migration, we performed transwell assays to assess BEL7402 cell invasion after ozonated water treatments at different concentrations (1.0 μg/mL or 1.5 μg/mL). Our results (Figure 1D) show that the invading cells were significantly fewer in ozonated water groups (P<0.05) than in the control group. The treatment with ozonated water at 1.5 μg/mL showed a more significant effect (P<0.01) than the treatment at 1.0 μg/mL (P<0.05; Figure 3). We found that ozonated water inhibits the invasion of HCC cells in a dose-dependent manner, with higher ozone concentrations causing more cancer invasion inhibition.
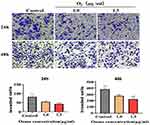 |
Figure 3 Ozonated water suppresses BELL7402 cells invasion. Representative images and quantification of BELL7402 cells in Transwell assay with matrigel, ×200;. *P<0.05, **P<0.01, n=3. |
Epithelial-Mesenchymal Transformation (EMT) and ECM Degradation Decreases After Ozonated Water Treatment
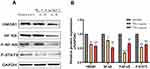
After ozonated water treatment, the cancer cells exhibited a significant reduction in the expression of vimentin and matrix metalloprotein-2 (MMP-2). Moreover, the mRNA levels of Slug and Twist were significantly decreased (Figure 4). These findings confirm that ozonated water inhibits migration and invasion by regulating EMT and ECM degradation.
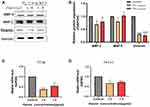 |
Figure 4 Expression of EMT and ECM degradation-related proteins detected using RT-qPCR and Western blotting analyses. *P<0.05, ***P<0.001, ****P<0.0001, n=3. |
Ozonated Water Reduces Cytokine Concentrations
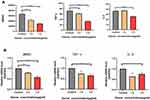
To investigate the association between the ozonated water effects and inflammation, we measured the HMGB1, interleukin-6 (IL-6), and TNF-α levels of cells in the supernatant using an ELISA kit. We found that the levels of HMGB1, interleukin-6 (IL-6), and TNF-α were significantly decreased in cells treated with ozonated water 1.5 μg/mL (Figure 6A).
Ozonated Water Inhibits HCC Cell Features by Promoting ROS Accumulation and HMGB1/NF-κB P65/STAT3 Signaling Pathway Inactivation
Various signals including those in the HMGB1/NF-kB/STAT3 pathway play roles in tumor metastasis. Therefore, we assessed the expression of some of these proteins by Western blot and RT-qPCR. Our results show that different concentrations of ozonated water inhibited HMGB1, NF-kB, p-NF-kB, and p-STAT3 production and that the mRNA levels of, HMGB1, and IL-6 were low in ozonated water-treated cells (Figures 5 and 6B). ROS is known to reduce the expression of HMGB1 cells through a REDOX reaction,10 and our flow cytometry results show that ozonated water significantly increased ROS levels in treated cells (Figure 7). These findings suggest that ozonated water inhibits the HMGB1/NF-κB/STAT3 signaling pathway by accumulating ROS in cancer cells (Figure 8).
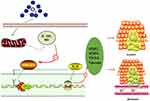 |
Figure 8 Schematic model of the mechanisms by which ozonated water inhibits HCC cell migration and invasion. |
Discussion
In this study, we used different medical concentrations of ozonated water to treat liver cancer cells, and we explored their proliferation, invasion, and metastasis abilities using CCK-8, flow cytometry, wound healing, and Transwell experiments. Our results show that ozonated water inhibited the proliferation, invasion, and migration of liver cancer cells and that these inhibitory effects were proportional to the ozonated water concentrations used.
We explored potential molecular mechanisms by which ozone inhibited the progress of liver cancer cells in this study. HMGB1 is a nuclear damage-related molecule induced by hypoxia, and it is associated with HCC invasion and metastasis. HMGB1 is crucial for HCC pathogenesis. Hypoxia induces HMGB1 expression in human and mouse hepatoma cells, and it induces macrophage infiltration and reprogramming to augment the expression of IL-6. Macrophage-derived IL-6 has been shown to enhance the invasiveness and metastasis of murine HCC cells.11 Extracellular HMGB1 can act as both a chemical attractant of white blood cells and a pro-inflammatory mediator to induce the release of TNF, IL-1, IL-6, and other cytokines from recruited white blood and resident immune cells. Studies have shown that the pro-inflammatory cytokine stimulation activity of HMGB1 depends on the REDOX state of its three cysteines: C23 and C45 must form disulphide bonds within BoxA, the first HMG box domain of HMGB1; and, the unmated C106 in BoxB must be in a mercaptan state. Both the terminal oxidation of these cysteines to sulfonates (CySO3–) with ROS and their complete reduction to thiols (CySH) abrogate the cytokine-stimulating activity of the molecule.12 Thus, ROS abrogates the proinflammatory activity of HMGB1 by terminally oxidizing its cysteines to sulfonates. In this study, we found that ozonated water treatment reduced the concentration of inflammatory cytokines TNF-α, IL-6, and HMGB1 and induced ROS accumulation. This finding is consistent with previous studies’ results.
The NF-κB signaling pathway is a significant regulator of tumor EMT and metastasis. In our study, ozonated water treatment decreased the expression of the NF-κB and p-NF-kB in cells. NF-κB is a nuclear transcription factor, and its pathway includes five components: c-Rel, Rel-B, p50 (NF-κB1), p52 (NF-κB2), and p65 (RelA), which are involved in the survival, growth, and differentiation of cells during cancer and inflammation in the body. NF-κB can regulate the expression of a large number of genes that play a vital regulatory role in tumorigenesis.13 When the NF-κB pathway is activated, dimers p65-p50 and p50-Rel-B are formed in the cytoplasm, which then enter the nucleus to bind to the κB enhancer of target genes, thereby promoting their transcription.14 Many studies have shown that the NF-κB signaling pathway plays an important role in the progression of a variety of tumors such as cervical, liver, gastric, and pancreatic cancers.15,16 NF-κB can activate the expression of Snail and VEGF through ATPIF1 up-regulation and thereby promote the metastasis and angiogenesis of liver cancer cells.17 Song et al reported that HCC metastasis is inhibited by repressing p65/NF-κB activation both in vitro and in vivo.17 PHD finger domain protein 5a (PHF5A) is a highly conserved protein from yeast to humans. PHF5A promotes the invasion and metastasis of liver cancer cells through NF-κB activation. Blocking the NF-κB signaling pathway can weaken the stimulating effect of PHF5A on the invasion and migration of liver cancer cells.18 Approved tyrosine kinase inhibitors (TKIs) for the treatment of liver cancer include sorafenib and regorafenib; their molecular mechanism of action in patients with advanced HCC depends partly on the action of NF-κB. Antibodies inhibit the expression of matrix metalloproteinase-9 (MMP9) and vascular endothelial growth factor (VEGF) by weakening the ERK/NF-κB pathway in HCC cells.19–21 Our research shows that different concentrations of ozonated water in the growth media of liver cancer cells reduce the phosphorylation of NF-κB signal transduction factors in the cells, which indicates that ozonated water may inhibit the invasion and metastasis of liver cancer cells through its effects on the NF-κB signaling pathway.
During liver cancer, NF-κB and STAT3 can regulate each other. NF-κB directly activates IL-6 transcription to produce high levels of the cytokine, and high levels of IL-6 activate NF-κB forming a positive feedback circuit that promotes tumor progression. An epidemiological study determined that elevated serum IL-6 is a reliable predictor of progression from HBV hepatitis to HCC development.22 IL-6 is a major STAT3 activator in HCC. When IL-6 binds to its specific receptor subunit, it can induce the dimerization of the gp130 receptor and the activation of the gp130-related Janus kinase (JAK). After this step, STAT3 is phosphorylated and undergoes homodimerization, leading to nuclear translocation, DNA binding, and gene transcription.23 The overexpression and structural activation of STAT3 are closely associated with the pathogenesis and survival of HCC cells. He et al reported that approximately 60% of HCC patients present STAT3 pY705 in tumor tissues, but not in surrounding healthy tissues.24 In addition, the overexpression of STAT3 pY705 and pS727 in tumor tissues is associated with a poor prognosis and clinicopathological characteristics in patients with HCC including larger tumor sizes, vascular invasion, advanced disease, and liver cirrhosis.25–29 Non-SMC lectin II complex subunit G2 (NCAPG2) is often up-regulated in HCC tumor tissues and indicates a poor prognosis. Overexpression of NCAPG2 can drive HCC proliferation and metastasis by activating STAT3 and NF-κB/miR-188-3p pathways.30 A large amount of evidence indicates that STAT3 may be a promising molecular target for cancer treatment. Studies have shown that STAT3 can regulate the expression of EMT-related transcription factors (Twist, Snail, ZEB1, etc.), thereby affecting the EMT phenotype. In HCC cells, STAT3 binds to the promoter of Twist to mediate its transcriptional activity; the activated Twist then promotes the EMT process and increases the invasion and migration capabilities of cells.31 STAT3 antisense therapy has been proven to reduce the invasiveness of HCC cells by down-regulating the matrix metalloproteinases (MMP)-2 and MMP-9 involved in the digestion of ECM.27 After treating liver cancer cells with different concentrations of ozonated water in this study, we found the level of factor IL-6 to be decreased, and the phosphorylation of STAT3 signal transduction factor and the expression of Twist to be reduced. Thus, ozonated water inhibited the invasion and metastasis of HCC cells by targeting the NF-kB/IL-6/STAT3 signaling pathway.
Many studies have confirmed that the invasion and metastasis of a variety of solid tumors are often accompanied by tumor EMT cells.32–34 EMT refers to the process by which cells lose epithelial cell characteristics and acquire those of mesenchymal cells. This process is accompanied by the loss of cell adhesion, the reorganization of the basic cytoskeleton, and the loss of epithelial cell polarity, resulting in enhanced invasion and migration capabilities. Moreover, tumor recurrence and metastasis are accompanied by changes in the expression of certain molecular markers during the EMT process, such as the down-regulation of E-cadherin and the up-regulation of vimentin.35 Our research showed that vimentin was down-regulated after the treatment of liver cancer cells with ozonated water. Cells undergoing EMT can secrete a large number of cytokines and enzymes to change the composition of the ECM, facilitating its infiltration and distant metastases. The degradation of the ECM plays a key role in the process of metastasis. MMP-2 is a type IV collagenase that can promote ECM degradation and promotes cancer metastasis. Studies have found that HCC cells undergoing EMT can up-regulate the expression of MMP family proteins MMP2, 7, 9; and that they degrade ECM proteins, thereby increasing the infiltration capacity of tumor cells.34 Studies have shown that STAT3 can regulate cancer cell expression of MMP-2.35 STAT3 can be targeted to inhibit the expression of MMP-2 and block the EMT process in HCC cells.36 The results of our experiments suggest that ozonated water inhibits the EMT of liver cancer cells by down-regulating the expression of vimentin, MMP-2, and MMP-9.
In recent years, it has been found that medical ozone plays a certain role in the treatment of COVID-19 pneumonia, intervertebral disc herniation, viral hepatitis, joint pain, wound healing, peripheral artery disease, diabetic foot, oral diseases, and tumors. Most of the biological mechanisms of ozone action have been clarified, which refers to antimicrobial effects, immunoregulation, antioxidant defenses, and epigenetic modification. According to our findings, ozonated water can significantly inhibit the invasion and metastasis of liver cancer cells by inhibiting the HMGB1/NF-ΚB/STAT3 cell pathway and affecting the EMT of liver cancer cells. HMGB1 has been shown to play a role in many cancers, including colorectal, breast, lung, prostate, liver, cervical, and pancreatic, osteosarcoma and leukemia. In HBV-related liver cancer, HMGB1 promotes epithelial–mesenchymal transition and angiogenesis in liver cancer by inducing IL-6/STAT3/miRNA-34a axis, and promotes the progression of liver cancer.37 Therefore, we expect ozone to become a new and effective treatment for liver cancer.
Acknowledgment
The study was supported by Dean Fund of Nanfang Hospital, Southern Medical University (2017B016) and Industrial Technology Research and Development Funds (K1050215).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferenci P, Fried M, Labrecque D, et al. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol. 2010;44:239–245.
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi:10.3322/caac.20107
3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
4. Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21:10327–10335. doi:10.3748/wjg.v21.i36.10327
5. Johnson TM, Overgard EB, Cohen AE, DiBaise JK. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi:10.1016/j.jhep.2011.12.001
6. Smith NL, Wilson AL, Gandhi J, Vatsia S, Khan SA. Ozone therapy: an overview of pharmacodynamics, current research, and clinical utility. Med Gas Res. 2017;7:212–219. doi:10.4103/2045-9912.215752
7. Sweet F, Kao MS, Lee SC, Hagar WL, Sweet WE. Ozone selectively inhibits growth of human cancer cells. Science. 1980;209:931–933. doi:10.1126/science.7403859
8. Schulz S, Haussler U, Mandic R, et al. Treatment with ozone/oxygen-pneumoperitoneum results in complete remission of rabbit squamous cell carcinomas. Int J Cancer. 2008;122:2360–2367. doi:10.1002/ijc.23382
9. Teke K, Ozkan TA, Cebeci OO, et al. Preventive effect of intravesical ozone supplementation on n-methyl-n-nitrosourea-induced non-muscle invasive bladder cancer in male rats. Exp Anim. 2017;66:191–198. doi:10.1538/expanim.16-0093
10. Lotfi R, Herzog GI, DeMarco RA, et al. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J Immunol. 2009;183:5023–5031. doi:10.4049/jimmunol.0900504
11. Jiang J, Wang GZ, Wang Y, Huang HZ, Li WT, Qu XD. Hypoxia-induced HMGB1 expression of HCC promotes tumor invasiveness and metastasis via regulating macrophage-derived IL-6. Exp Cell Res. 2018;367:81–88. doi:10.1016/j.yexcr.2018.03.025
12. Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–1528. doi:10.1084/jem.20120189
13. Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi:10.1038/nri1703
14. Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi:10.1038/nrc780
15. Nomura A, Banerjee S, Chugh R, et al. CD133 initiates tumors, induces epithelial-mesenchymal transition and increases metastasis in pancreatic cancer. Oncotarget. 2015;6:8313–8322.
16. Zhang Y, Zhao Y, Ran Y, Guo J, Cui H, Liu S. Alantolactone exhibits selective antitumor effects in HELA human cervical cancer cells by inhibiting cell migration and invasion, G2/M cell cycle arrest, mitochondrial mediated apoptosis and targeting Nf-kB signalling pathway. J Buon. 2019;24:2310–2315.
17. Song R, Song H, Liang Y, et al. Reciprocal activation between ATPase inhibitory factor 1 and NF-kappaB drives hepatocellular carcinoma angiogenesis and metastasis. Hepatology. 2014;60:1659–1673.
18. Yang Q, Zhang J, Xu S, et al. Knockdown of PHF5A inhibits migration and invasion of HCC cells via downregulating NF-kappaB Signaling. Biomed Res Int. 2019;2019:1621854. doi:10.1155/2019/1621854
19. Chiang IT, Liu YC, Wang WH, et al. Sorafenib inhibits TPA-induced MMP-9 and VEGF expression via suppression of ERK/NF-kappaB pathway in hepatocellular carcinoma cells. Vivo. 2012;26:671–681.
20. Hsu FT, Liu YC, Chiang IT, et al. Sorafenib increases efficacy of vorinostat against human hepatocellular carcinoma through transduction inhibition of vorinostat-induced ERK/NF-kappaB signaling. Int J Oncol. 2014;45:177–188. doi:10.3892/ijo.2014.2423
21. Tsai JJ, Pan PJ, Hsu FT. Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-kappaB activation in hepatocellular carcinoma cells. Oncol Rep. 2017;37:1036–1044. doi:10.3892/or.2016.5328
22. Wong VW, Yu J, Cheng AS, et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766–2770. doi:10.1002/ijc.24281
23. Zhong Z, Wen Z, Darnell JJ. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi:10.1126/science.8140422
24. Feng DY, Zheng H, Tan Y, Cheng RX. Effect of phosphorylation of MAPK and Stat3 and expression of c-fos and c-jun proteins on hepatocarcinogenesis and their clinical significance. World J Gastroenterol. 2001;7:33–36. doi:10.3748/wjg.v7.i1.33
25. Jiang R, Tan Z, Deng L, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54:900–909. doi:10.1002/hep.24486
26. Calvisi DF, Ladu S, Gorden A, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–1128. doi:10.1053/j.gastro.2006.01.006
27. Li WC, Ye SL, Sun RX, et al. Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin Cancer Res. 2006;12:7140–7148. doi:10.1158/1078-0432.CCR-06-0484
28. Ashizawa Y, Kuboki S, Nojima H, et al. OLFM4 Enhances STAT3 activation and promotes tumor progression by inhibiting GRIM19 expression in human hepatocellular carcinoma. Hepatol Commun. 2019;3:954–970. doi:10.1002/hep4.1361
29. He G, Yu GY, Temkin V, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297. doi:10.1016/j.ccr.2009.12.048
30. Meng F, Zhang S, Song R, et al. NCAPG2 overexpression promotes hepatocellular carcinoma proliferation and metastasis through activating the STAT3 and NF-kappaB/miR-188-3p pathways. Ebiomedicine. 2019;44:237–249. doi:10.1016/j.ebiom.2019.05.053
31. Zhang C, Guo F, Xu G, Ma J, Shao F. STAT3 cooperates with Twist to mediate epithelial-mesenchymal transition in human hepatocellular carcinoma cells. Oncol Rep. 20151872;33:1872.
32. Zhao Y, Yao J, Wu XP, et al. Wogonin suppresses human alveolar adenocarcinoma cell A549 migration in inflammatory microenvironment by modulating the IL-6/STAT3 signaling pathway. Mol Carcinog. 2015;54(1):E81–93. doi:10.1002/mc.22182
33. Bai Y, Lu H, Lin C, et al. Sonic hedgehog-mediated epithelial-mesenchymal transition in renal tubulointerstitial fibrosis. Int J Mol Med. 2016;37:1317–1327. doi:10.3892/ijmm.2016.2546
34. Zheng QM, Lu JJ, Zhao J, Wei X, Wang L, Liu PS. Periostin facilitates the epithelial-mesenchymal transition of endometrial epithelial cells through ILK-akt signaling pathway. Biomed Res Int. 2016;(2016):9842619. doi:10.1155/2016/9842619
35. Redmer T. Deciphering mechanisms of brain metastasis in melanoma - The gist of the matter. Mol Cancer. 2018;17:106. doi:10.1186/s12943-018-0854-5
36. Xiong H, Hong J, Du W, et al. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J Biol Chem. 2012;287:5819–5832. doi:10.1074/jbc.M111.295964
37. Shiau DJ, Kuo WT, Davuluri G, et al. Hepatocellular carcinoma-derived high mobility group box 1 triggers M2 macrophage polarization via a TLR2/NOX2/autophagy axis. Sci Rep. 2020;10(1):13582. doi:10.1038/s41598-020-70137-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.