Back to Journals » Psychology Research and Behavior Management » Volume 16
Oxytocin-Receptor Gene Modulates Reward-Network Connection and Relationship with Empathy Performance
Authors Li D , Zhang L, Bai T, Qiu B, Zhu C, Wang K
Received 15 April 2022
Accepted for publication 8 December 2022
Published 7 January 2023 Volume 2023:16 Pages 85—94
DOI https://doi.org/10.2147/PRBM.S370834
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Igor Elman
Dandan Li,1– 3,* Long Zhang,4,* Tongjian Bai,4,* Bensheng Qiu,5 Chunyan Zhu,1– 3 Kai Wang1,2,4,6,7
1School of Mental Health and Psychological Sciences, Anhui Medical University, Hefei, People’s Republic of China; 2Institute of Artificial Intelligence, Hefei Comprehensive National Science Center, Hefei, People’s Republic of China; 3Research Center for Translational Medicine, Second Hospital of Anhui Medical University, Hefei, People’s Republic of China; 4Department of Neurology, First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 5Hefei National Laboratory for Physical Sciences at the Microscale and the Centers for Biomedical Engineering, University of Science and Technology of China., Hefei, People’s Republic of China; 6Anhui Province Key Laboratory of Cognition and Neuropsychiatric Disorders, Hefei, People’s Republic of China; 7Collaborative Innovation Center of Neuropsychiatric Disorders and Mental Health, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chunyan Zhu; Kai Wang, Email [email protected]; [email protected]
Introduction: Empathy traits are highly heritable and linked with reward processing. It is implicated that common variations of the oxytocin-receptor gene (OXTR) play a modulatory effect on empathic performance. However, it is unclear about the neural substrates underlying the modulatory effect of the OXTR genotype on empathic performance. This study aimed to characterize the modulatory effect of common OXTR variations on reward-circuitry function and its relationship with empathy.
Methods: Based on the seed of the nucleus accumbens (NAcc; a key hub of reward circuitry), we examined differences in spontaneous local activity and functional connectivity between OXTR rs2268493 genotype groups and their relationship with empathic performance among 402 high-homogeneity participants.
Results: Comparing with C carriers (CC/CT) group, the individuals with the rs2268493 TT genotype exhibited lower functional connectivity of the right NAcc with the medial prefrontal cortex (mPFC) and inferior frontal gyrus. Similarly lower functional connectivity was found between the left NAcc and mPFC. Consequently, no significant difference was found in the spontaneous local activity of NAcc.
Discussion: Our findings suggested that common OXTR variations have a modulatory effect on the connection of the NAcc with the hub of empathic networks (mPFC and IFG), which may provide insight on the neural substrate underlying the modulatory effect of OXTR on empathic behavior.
Keywords: empathy, oxytocin-receptor gene, reward network, RS-fMRI, rs2268493
Introduction
Empathy is the ability to resonate with others’ intentions and thoughts, which is crucial for facilitating prosocial behavior and driving mental health.1–3 Current health professional evidence suggests that higher levels of empathy are correlated strongly with better life satisfaction, interpersonal skills, and mental state,4 but also associated with core features of several mental diseases, such as autism spectrum disorder (ASD) and schizophrenia.5–8 According to the dual nature of empathy, it could be categorized into two components — cognitive and emotional.9–11 Cognitive empathy is the ability to assess the reasons behind emotional states of oneself and other people.12 Emotional empathy refers to sharing the emotional state of another, which is closely related to emotional contagion or empathic arousal.9,12
It is widely believed that the empathy is a heritable trait in humans, confirmed by strong evidence from studies of twins and offspring.13–15 These genetic studies provided convincing evidence of a link between gene variations and risk with aberrant affective and empathic responses.16,17 The oxytocin-receptor gene (OXTR) is the significant empathic gene, and exerts its effects by oxytocin (OXT).18–20 OXT, a neurotransmitter, has a crucial role in social trust/altruism and emotional empathy.21,22 Oxytocin in combination with OXTR then modulates social behavior.23 Common variations of OXTR have demonstrated the impact on social performance.24 Risk alleles of OXTR single-nucleotide polymorphisms (SNPs) for ASD, a social dysfunction disorder, have been linked with poor social interaction.25–27 For example, compared with individuals homozygous for the C allele of 2268493 (CT/CC), individuals with the rs2268493 TT allele (risk allele) present low perceived ability in maternal care and dispositional empathy via modulation of OXT.28 OXTR polymorphisms exhibit individual differences in empathic communication.29 They are distributed in social–empathic brain circuits, which implicate the key role of the brain network in the modulation of OXTR on empathic performance.22,30
The social–affective network, especially the inferior frontal gyrus (IFG), fusiform gyrus, and medial prefrontal cortex (mPFC), are thought to be critical for the empathy processing.17,31–33 For example, increased activity in the IFG has been demonstrated during the condition of emotional empathy, but not cognitive empathy.9 Conversely, individuals with impaired cognitive empathy have reduced hemodynamic responses in the mPFC.34 Recently, besides social–affective processing, reward processing has also been linked with empathy.35,36 During social interactions, stimuli are often predictors of rewards for us and others. Effective social interaction and empathy need the ability to compute the value of stimuli that predict rewards for others.37 Deficits in reward processing may relate to empathic disturbance.36
Decades of neuroimaging research have identified regions involved in different reward processes, including the nucleus accumbens (NAcc), ventral striatum, and orbitofrontal cortex.38–41 NAcc is considered a hub of the reward circuit,42 receiving 5-hydroxytryptamine and further reinforcing properties of social interaction. Evidence suggests that oxytocin mediates 5-hydroxytryptamine release in the NAcc and thereby regulates social reward.43 Also, the OXTR risk alleles are closely associated with impaired reward-circuitry function, including the NAcc and PFC, in response to reward anticipation.28,44
Importantly, the OXTR risk allele rs2268493 TT has confirmed decreased activation in the reward circuitry.45 Intriguingly, the local function of NAcc during empathy tasks can be modulated by OXTR polymorphisms.46 These studies support the key role of reward process in empathy.33 However, recent theories have emphasized that reward is not attributable to single brain regions, but critically depends on interconnected brain networks.47 Resting-state functional magnetic resonance imaging (RS-fMRI), which enables the identification of spontaneous neural activity and provides an available tool to depict interconnected brain networks.47 Taken together, the exploration of core seed target (especially NAcc) functional connections is associated with clarifying the relationship between OXTR genetic variation and brain empathy processing.
In the present study, we aimed to examine the modulatory effect of common OXTR variations on reward-circuitry function and their relationship with empathy. Considering the key role of the NAcc in reward, functional connectivity with the seed of the NAcc was used to depict the reward network. Based on the aforementioned findings, we hypothesized that carriers with the OXTR risk genotype rs2268493 TT may show lower empathy performance, as well as reduced reward-circuitry function. The reward-network function may be positively associated with empathy performance.
Methods
Participants
Initially, 641 healthy young college students in the same year at Anhui Medical University were recruited. The average age of the participants was 20.7 years, and their ages ranged from 19 to 23 years. Exclusion criteria were head trauma, metal implants, history of neurological or psychiatric disorders, drug or alcohol abuse, and having first-degree relatives with psychiatric disorders.
Procedures
This experiment was approved by the Ethics Committee at Anhui Medical University. Participants provided written informed consent prior to the experiment, in accordance with the Declaration of Helsinki. A total of 641 eligible participants completed the Chinese version of the Interpersonal Reactivity Index (IRI-C) questionnaire, which is frequently used in measuring empathy. Of these participants, rs2268493 genotype data were available for 530. With available behavior (IRI-C) and genotype data for the participants, 446 completed MRI scanning. After excluding poor MRI data (resulting from artifacts or excessive head motion), 402 participants were included in the final analysis of functional connectivity (Figure 1). Considering the strong impact of singular values on correlation analyses, we excluded singular data defined as greater than three standard deviations from the mean. Ten participants (three participants in the CT/CC group in and seven participants in the TT group) were excluded when performing correlation analysis because of singular data for mean connectivity. Finally, there were 392 participants (102 in the CT/CC group and 290 in the TT group) included in the correlation analysis for the subscales of the IRI-C.
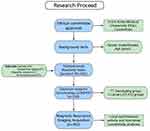 |
Figure 1 Study flowchart. |
Measures
All participants completed the IRI-C questionnaire, which is a 28-item instrument to measure multiple dimensions of trait empathy. Items are measured on a five-point Likert scale (scores 0–4). The IRI-C includes cognitive and affective subscales, both scored on four seven-item subscales: cognitive aspects (perspective taking [PT] and fantasy [FS]) and affective processes (empathic concern [EC] and personal distress [PD]).
Oxytocin-Receptor Genotyping
Participants had blood taken to obtain DNA using standard procedures. The genotyping of all participants was determined in comparison with control DNA and confirmed by sequencing in the SNP pattern. The genotype of OXTR rs2268493 is TT, CT, and CC. According to previous studies’ grouping,28,48 we classified the genotypes of OXTR rs2268493 into TT and C carrier (CT/CC) groups. Finally, there were 297 participants in the TT group and 105 in the CT/CC group.
Image-Data Acquisition
Image scanning was performed with a 3 T scanner (Discovery GE750w) at the University of Science and Technology of China. During fMRI scanning, participants were told to remain awake with their eyes relaxed and closed and not to think of anything. Functional images were acquired composed of 217 echo-planar imaging volumes with parameters of repetition time 2400 ms, echo time 30 ms, flip angle 90°, matrix size 64×64, field of view 192×192 mm2, slice thickness 3 mm, and 46 continuous slices (one voxel, 3×3×3 mm3). High spatial resolution T1-weighted anatomic images with 188 slices were also acquired in sagittal orientation (TR 8.16 ms, TE 3.18 ms, flip angle 12°, field of view 256×256 mm2, slice thickness 1 mm, and voxel size 1×1×1 mm3).
Functional Data Preprocessing
We preprocessed functional neuroimaging data using the Data Processing Assistant for Resting-State Functional MR Imaging (DPARSF) toolkit.49 Data were preprocessed in the following steps: removing first five volumes, slice-timing correction, realignment, spatial normalizing based on the unified segmentation of structural images, regressing out 24 Friston motion parameters, high white-matter signal, cerebrospinal fluid signal and global signals, spatial smoothing (Gaussian kernel 4×4×4 mm), and filtering with a temporal band-pass of 0.01–0.1 Hz.
Local Spontaneous Activity and Functional Connectivity Analyses
After preprocessing, the filtered time series was converted to a frequency domain using fast Fourier transform. The square root of the power for each frequency was calculated to obtain amplitude values. The amplitude of low-frequency fluctuation (ALFF) was calculated as the sum of the amplitude values in the 0.01–0.1 Hz low-frequency power range. To reduce the global effects of variability across participants, the ALFF was normalized by the mean within-brain ALFF value for each participant. In the present study, we focused on the ALFF within the NAcc region. The NAcc was defined with the human Brainnetome atlas,50 a new brain atlas built upon connectional architecture. Functional connectivity based on the seed of the NAcc was calculated using DPARSF software. For each individual, Pearson’s correlation coefficients were computed between the mean time series of seed and time series of each voxel for the rest of the whole brain. To improve the normality, the correlation coefficients were converted to z values using Fisher’s r–z transformation and the results displayed as connectivity maps for each participant.
Statistical Analysis
We used SPSS 26.0 for statistical analysis of the data. The  and t tests were used to compare demographic data and cognitive performance, such as sex, age, IRI-C subscale scores, and local spontaneous activity (ALFF) between the two genotype groups (TT vs CT/CC). Voxel-wise two-sample t tests were used to quantitatively compare differences in NAcc functional connectivity between the two genotype groups within the gray-matter mask with sex as covariate. Statistical maps were corrected using the Gaussian random-field method at the threshold for voxel P≤0.001 and cluster P≤0.05. Pearson’s correlation analyses were applied to explore the associations between NAcc connectivity and IRI-C subscale scores. Significance was set at P≤0.05 (two-tailed), with no correction.
and t tests were used to compare demographic data and cognitive performance, such as sex, age, IRI-C subscale scores, and local spontaneous activity (ALFF) between the two genotype groups (TT vs CT/CC). Voxel-wise two-sample t tests were used to quantitatively compare differences in NAcc functional connectivity between the two genotype groups within the gray-matter mask with sex as covariate. Statistical maps were corrected using the Gaussian random-field method at the threshold for voxel P≤0.001 and cluster P≤0.05. Pearson’s correlation analyses were applied to explore the associations between NAcc connectivity and IRI-C subscale scores. Significance was set at P≤0.05 (two-tailed), with no correction.
Results
Genotype Effects on Behavior Performance
The demographics and range of scores on the IRI-C subscales are listed in Table 1. Genotype distributions of OXTR rs2268493 were divided into CT/CC and TT groups in the current study. There were differences between the two genotype groups (P≤0.05). There was no significant difference in cognitive IRI-C and its subdomain scores (FS, PT) between the CT/CC and TT groups (P>0.05). There was a significant difference in affective IRI-C between the groups (P=0.046). This difference was mainly derived from the subdomain of PD (P=0.004).
 |
Table 1 Demographic and behavioral information for participants with available IRI data grouped by rs2268493 genotype (mean ± SD) |
Genotype Effects on ALFF and Functional Connectivity in NAcc
There were 402 participants in the final RS-fMRI analysis (CT/CC group 105, TT group 297). No significant difference was found between the genotype groups for head motion indexed by frame-wise displacement (Table 1).51 There was no significant difference between the groups in ALLF of the bilateral NAcc (Figure 2 and Table 1). Participants in the TT group exhibited lower functional connectivity of the right NAcc with the medial mPFC (peak voxel MNI coordinates x=9, y=60, z=−6, t=−4.44, cluster size 37) and higher functional connectivity of the right NAcc with the IFG (peak voxel MNI coordinates x=51, y=15, z=21; t=4.18, cluster size 26) than the CT/CC group (Figure 3A and B). Participants in the TT group also exhibited lower functional connectivity of the left NAcc with the mPFC (peak voxel MNI coordinates x=9, y=60, z=−3; t=−4.45, cluster size 48; Figure 4A and B).
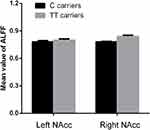 |
Figure 2 Genotype effects on ALFF in the NAcc. There was no significant difference in ALLF of the bilateral NAcc between C carriers and TT groups in rs2268493 (P>0.05 for both). |
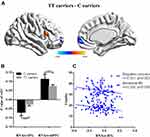 |
Figure 3 Genotype effects on functional connectivity in the RNAcc. Participants in the TT group exhibited lower functional connectivity of the RNAcc with the medial prefrontal cortex (mPFC) and higher functional connectivity of the RNAcc with the inferior frontal gyrus (IFG) than the CC/CT group (P<0.05: Figure 3A and B). Functional connectivity for the RNAcc with the IFG was significantly correlated with emotional IRI score (R=−0.205, P<0.05), especially the subscore of empathic concern (R=−0.301, P<0.01; Figure 3C). |
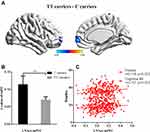 |
Figure 4 Genotype effects on functional connectivity in the LNAcc. Participants in the TT group exhibited lower functional connectivity of the LNAcc with the medial prefrontal cortex (mPFC, P<0.05; Figure 4A and B). The functional connectivity between the LNAcc and mPFC were significantly correlated with the cognitive IRI score (R=0.151, P=0.01) and Fantasy subscore (R=0.134, P<0.05; Figure 4C). |
Correlation Analyses
Functional connectivity for the right NAcc with the IFG was significantly correlated with emotional IRI-C score (R=−0.205, P<0.05, low power), especially the empathic concern subscore (R=−0.301, P<0.01, medium power; Figure 3C). Functional connectivity between the left NAcc and mPFC was significantly correlated with cognitive IRI score (R=0.151, P=0.01, low power) and Fantasy subscore(R=0.134, P<0.05, low power; Figure 4C).
Discussion
The current investigation aimed to examine the modulatory effect of common OXTR variations on reward-circuitry function and its relationship with empathy using resting-state functional connectivity within healthy individuals. Our results demonstrated that individuals with the rs2268493 TT genotype exhibited lower functional connectivity for right NAcc–mPFC, right NAcc–IFG, and left NAcc–mPFC than the rs2268493 CT/CC genotype. Associations were demonstrated between performance on the emotional/cognitive IRI-C and reward-network function, suggesting that the OXTR gene (rs2268493) modulated empathy performance through functional reward-network connections between the NAcc and mPFC regions.
Consistently with previous findings, allelic variations in rs2268493 were associated with social cognition, including reward and emotional processing.28,52 Damiano et al (2014) found decreased activation in reward circuitry during reward tasks among carriers of the rs2268493 TT allele. Similarly, a previous study also reported an interaction between the rs2268493 T allele and perceived maternal care during emotion processing.28 Importantly, both social–affective and reward processing contribute to normal empathy, hence it is predictable that carriers of the rs2268493 TT allele may have empathic impairments.53 Indeed, the rs2268493 TT allele is frequently reported in patients with empathic dysfunction, such as thos with ASD and schizophrenia.26 Our study further confirmed the OXTR polymorphism is associated with reward-network connection and related to empathy performance.
Our results suggested abnormal NAcc-mPFC and NAcc-IFG resting-state functional connectivity in individuals with the rs2268493 TT genotype. The mPFC and IFG are recognized as integral parts of empathic neural activity.54 The mPFC has frequently been confirmed to have aberrant structure and function in patients with empathic impairment.31 Evidence from structural studies suggests that bilateral mPFC damage abolishes empathic response and insight.33,47 Functionally, mPFC activity shows increased functional connectivity with affective pain regions during emotional empathy.55 In addition, IFG is another region involved in empathy processing. Significant activation of the right IFG is associated with performance on the IRI-C empathy questionnaire.9 Interestingly, the IFG plays a preferential role in emotional empathy, but not cognitive empathy.31 It should be noted that our findings have similar connections between emotional empathy andthe IFG, mainly due to the functional connectivity for the right IFG correlating with emotional IRI-C score, but not cognitive ones.
Although empathy is intimately related to the reward network, we did not find gene-regulating empathy function in the rs2268493 genotype. There was no ALFF difference in the bilateral NAcc among the rs2268493 subgroups. On the contrary, our results indicated that the NAcc had a modulatory effect on empathic activities by connections with the empathic-related region.8,56 This result underscored an interaction between OXTR variation and empathy by means of modulating intranetwork connections of the reward network, independently of the effects of local activity.
Our findings showed that right-connection NAcc–IFG was significantly correlated with emotional IRI-C scores and left-connection (NAcc-mPFC) linked with cognitive IRI-C. The nature of hemisphere lateralization may be behind this discrepancy. Substantial evidence support cognitive and emotional activity preferentially depending on the left and right hemispheres, respectively. This is very similar to the neural mechanisms underlying the two types of empathy (cognitive and emotional).46 Hemisphere lateralization for the two types has been implicated in previous studies.16 The involvement of the left mPFC, left amygdala, and left anterior insula are related to cognitive empathy, and affective empathy is mediated by the right TPJ and right IFG.54,57,58 It is notable that this assumption is based on the left-hemisphere dominance of participants. Research on a large scale has shown 87.7%–94.8% right-sided domination in China’s population; therefore, most of the participants enrolled were left hemisphere–dominant. The left NAcc-mPFC may preferentially be involved in cognitive empathy processing.
We observed a curious phenomenon whereby all the C-carriers were male, which has never been seen in other related studies,28,44,45,59 most likely due to ethnicity differences. The rs2268493 gene polymorphisms were first reported among Han Chinese. Although we compared the differences in NAcc functional connectivity between the two genotype groups with sex as a covariate, we still need to admit that the sex imbalance had some impact on our results, and follow-up research should be conducted to fully understand rs2268493 gene specificity and comprehensively across different ethnic groups.
We also admit to several additional limitations in this study. Based on the behavioral and imaging research findings, we chose only OXTR rs2268493. Multiple polymorphisms in the OXTR gene have demonstrated relationships with reward–empathy processing. However, examination based on one SNP may not provide a comprehensive understanding of the variance in behavioral and neuroimaging phenotypes, hence inevitably attenuating the generalizability of our results. Moreover, the correlation between the neural features of right NAcc–IFG and left NAcc-vmPFC functional connectivity and IRI-C subscores achieved statistical significance (at the 0.05 level) without correction in our study. This low effect size may have been caused by the high homogeneity of the enrolled students (same race, similar educational backgrounds and ages). Future studies should verify gene–brain interactions of reward networks contributing to the effect of empathy in patients with typical disorders, especially ASD.
Conclusion
The current study provided evidence of the modulatory effect in common OXTR variations on reward-circuitry function and its relationship with empathy in a highly homogeneous population. Our results suggest that OXTR rs2268493 has a modulatory effect on the connection of NAcc with hubs of empathic networks (mPFC and IFG), which was closely related with empathic performance. Those with the rs2268493 TT genotype exhibited lower functional connectivity of the right NAcc with the mPFC and IFG, and similarly lower functional connectivity between the left NAcc and mPFC. These findings may provide insight into the neural substrates underlying the modulatory effect of OXTR on empathic behavior.
Ethics Statement
The subjects were recruited from Anhui Medical University, Anhui Province, China. This study was approved by the Research Ethics Committee of Anhui Medical University. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Acknowledgments
We thank all the participants enrolled in our study. We also thank the Information Science Laboratory Center of USTC for the measurement services.
Funding
This study was funded by the Natural Science Foundation of China (91432301, 31571149, 81171273, and 91232717 to KW) and the Basic and Clinical Collaborative Research Enhancement Programme of Anhui Medical University (2022xkjT016).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci. 2012;35(1):1–23. doi:10.1146/annurev-neuro-062111-150536
2. Blair RJ. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn. 2005;14(4):698–718. doi:10.1016/j.concog.2005.06.004
3. Singer T, Lamm C. The social neuroscience of empathy. Ann N Y Acad Sci. 2009;1156:81–96. doi:10.1111/j.1749-6632.2009.04418.x
4. Pikhart M, Sommerlad A, Huntley J, Livingston G, Rankin KP, Fancourt D. Empathy and its associations with age and sociodemographic characteristics in a large UK population sample. PLoS One. 2021;16(9):e0257557. doi:10.1371/journal.pone.0257557
5. Jones AP, Happe FG, Gilbert F, Burnett S, Viding E. Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. J Child Psychol Psychiatry. 2010;51(11):1188–1197. doi:10.1111/j.1469-7610.2010.02280.x
6. Bonfils KA, Lysaker PH, Minor KS, Salyers MP. Empathy in schizophrenia: a meta-analysis of the Interpersonal Reactivity Index. Psychiatry Res. 2017;249:293–303. doi:10.1016/j.psychres.2016.12.033
7. Groen Y, den Heijer AE, Fuermaier ABM, Althaus M, Tucha O. Reduced emotional empathy in adults with subclinical ADHD: evidence from the empathy and systemizing quotient. ADHD Attention Deficit and Hyperactivity Disorders. 2017;10(2):141–150. doi:10.1007/s12402-017-0236-7
8. Banzhaf C, Hoffmann F, Kanske P, et al. Interacting and dissociable effects of alexithymia and depression on empathy. Psychiatry Res. 2018;270:631–638. doi:10.1016/j.psychres.2018.10.045
9. Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132(Pt 3):617–627. doi:10.1093/brain/awn279
10. Dziobek I, Rogers K, Fleck S, et al. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET). J Autism Dev Disord. 2008;38(3):464–473. doi:10.1007/s10803-007-0486-x
11. Shamay-Tsoory SG. The Neural Bases for Empathy. Neuroscientist. 2010;17(1):18–24. doi:10.1177/1073858410379268
12. Chrysikou EG, Thompson WJ. Assessing cognitive and affective empathy through the interpersonal reactivity index: an argument against a two-factor model. Assessment. 2016;23(6):769–777. doi:10.1177/1073191115599055
13. Nishitani S, Ikematsu K, Takamura T, Honda S, Yoshiura KI, Shinohara K. Genetic variants in oxytocin receptor and arginine-vasopressin receptor 1A are associated with the neural correlates of maternal and paternal affection towards their child. Horm Behav. 2017;87:47–56. doi:10.1016/j.yhbeh.2016.09.010
14. Toccaceli V, Fagnani C, Eisenberg N, Alessandri G, Vitale A, Stazi MA. Adult empathy: possible gender differences in gene-environment architecture for cognitive and emotional components in a large Italian twin sample. Twin Res Hum Genet. 2018;21(3):214–226. doi:10.1017/thg.2018.19
15. Driscoll CA, Barr CS. Studying longitudinal trajectories in animal models of psychiatric illness and their translation to the human condition. Neurosci Res. 2016;102:67–77. doi:10.1016/j.neures.2015.08.001
16. Ma Y, Hu N, Liu G, Chen X. State attachment moderates the maternal-related neural responses to infant faces. Int J Psychophysiol. 2020;147:83–92. doi:10.1016/j.ijpsycho.2019.10.007
17. Takeuchi H, Taki Y, Sassa Y, et al. Regional gray matter volume is associated with empathizing and systemizing in young adults. PLoS One. 2014;9(1):e84782. doi:10.1371/journal.pone.0084782
18. Paloyelis Y, Doyle OM, Zelaya FO, et al. A spatiotemporal profile of in vivo cerebral blood flow changes following intranasal oxytocin in humans. Biol Psychiatry. 2016;79(8):693–705. doi:10.1016/j.biopsych.2014.10.005
19. Donaldson ZR. Oxytocin, Vasopressin, and the Neurogenetics of Sociality. Science. 2008;5903:900–904.
20. Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in Oxytocin Receptor Density in the Nucleus Accumbens Has Differential Effects on Affiliative Behaviors in Monogamous and Polygamous Voles. J Neurosci. 2009;29(5):1312–1318. doi:10.1523/JNEUROSCI.5039-08.2009
21. Jurek B, Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev. 2018;98(3):1805–1908. doi:10.1152/physrev.00031.2017
22. Ebert A. Oxytocin and Social Cognition. Curr Top Behav Neurosci. 2017;1(35):375–388.
23. Biggs LM, Hammock EAD. Oxytocin via oxytocin receptor excites neurons in the endopiriform nucleus of juvenile mice. Sci Rep. 2022;12(1):11401. doi:10.1038/s41598-022-15390-5
24. Fujiwara T, Kofuji T, Akagawa K. Disturbance of the reciprocal-interaction between the OXTergic and DAergic systems in the CNS causes atypical social behavior in syntaxin 1A knockout mice. Behav Brain Res. 2021;413:113447. doi:10.1016/j.bbr.2021.113447
25. Yrigollen CM, Han SS, Kochetkova A, et al. Genes controlling affiliative behavior as candidate genes for autism. Biol Psychiatry. 2008;63(10):911–916. doi:10.1016/j.biopsych.2007.11.015
26. Napoli A, Warrier V, Baron-Cohen S. Genetic variation in the oxytocin receptor (OXTR) gene is associated with Asperger Syndrome. Molecular Autism. 2014;5(14).
27. Campbell DB, Datta D, Jones ST, et al. Association of oxytocin receptor (OXTR) gene variants with multiple phenotype domains of autism spectrum disorder. J Neurodev Disord. 2011;3(2):101–112. doi:10.1007/s11689-010-9071-2
28. Antonucci LA, Pergola G, Passiatore R, et al. The interaction between OXTR rs2268493 and perceived maternal care is associated with amygdala–dorsolateral prefrontal effective connectivity during explicit emotion processing. Eur Arch Psychiatry Clin Neurosci. 2020;270(5):553–565. doi:10.1007/s00406-019-01062-5
29. Schneiderman I, Kanat-Maymon Y, Ebstein RP, Feldman R. Cumulative risk on the oxytocin receptor gene (OXTR) underpins empathic communication difficulties at the first stages of romantic love. Soc Cogn Affect Neurosci. 2014;9(10):1524–1529. doi:10.1093/scan/nst142
30. Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biol Psychiatry. 2016;79(3):194–202. doi:10.1016/j.biopsych.2015.07.020
31. Oliver LD, Vieira JB, Neufeld RWJ, Dziobek I, Mitchell DGV. Greater involvement of action simulation mechanisms in emotional vs cognitive empathy. Soc Cogn Affect Neurosci. 2018;13(4):367–380. doi:10.1093/scan/nsy013
32. Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100(9):5497–5502. doi:10.1073/pnas.0935845100
33. Jacobs-Brichford E, Manson KF, Roitman JD. Effects of chronic cannabinoid exposure during adolescence on reward preference and mPFC activation in adulthood. Physiol Behav. 2019;199:395–404. doi:10.1016/j.physbeh.2018.12.006
34. Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. J Child Psychol Psychiatry. 2011;52(6):631–644. doi:10.1111/j.1469-7610.2010.02349.x
35. O’Connell G, Christakou A, Haffey AT, Chakrabarti B. The role of empathy in choosing rewards from another’s perspective. Front Hum Neurosci. 2013;7:174. doi:10.3389/fnhum.2013.00174
36. Bellebaum C, Brodmann K, Thoma P. Active and observational reward learning in adults with autism spectrum disorder: relationship with empathy in an atypical sample. Cogn Neuropsychiatry. 2014;19(3):205–225. doi:10.1080/13546805.2013.823860
37. Lockwood PL, Apps MAJ, Roiser JP, Viding E. Encoding of vicarious reward prediction in anterior cingulate cortex and relationship with trait empathy. J Neurosci. 2015;35(40):13720–13727. doi:10.1523/JNEUROSCI.1703-15.2015
38. Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DG. Neural correlates of reward in autism. Br J Psychiatry. 2008;192(1):19–24. doi:10.1192/bjp.bp.107.036921
39. Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res. 2010;3(2):53–67. doi:10.1002/aur.122
40. Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord. 2012;42(2):147–160. doi:10.1007/s10803-011-1221-1
41. Kohls G, Schulte-Ruther M, Nehrkorn B, et al. Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci. 2013;8(5):565–572. doi:10.1093/scan/nss033
42. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048–1054. doi:10.1038/nn1327
43. Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi:10.1038/nature12518
44. Glenn AL, Lochman JE, Dishion T, Powell NP, Boxmeyer C, Qu L. Oxytocin Receptor Gene Variant Interacts with Intervention Delivery Format in Predicting Intervention Outcomes for Youth with Conduct Problems. Prev Sci. 2018;19(1):38–48. doi:10.1007/s11121-017-0777-1
45. Damiano CR, Aloi J, Dunlap K, et al. Association between the oxytocin receptor (OXTR) gene and mesolimbic responses to rewards. Mol Autism. 2014;5(1):7. doi:10.1186/2040-2392-5-7
46. Luo S, Li B, Ma Y, Zhang W, Rao Y, Han S. Oxytocin receptor gene and racial ingroup bias in empathy-related brain activity. NeuroImage. 2015;110:22–31. doi:10.1016/j.neuroimage.2015.01.042
47. Kawakubo H, Matsui Y, Kushima I, Ozaki N, Shimamura T. A network of networks approach for modeling interconnected brain tissue-specific networks. Bioinformatics. 2019;35(17):3092–3101. doi:10.1093/bioinformatics/btz032
48. Davis C, Patte K, Zai C, Kennedy JL. Polymorphisms of the oxytocin receptor gene and overeating: the intermediary role of endophenotypic risk factors. Nutr Diabetes. 2017;7(5):e279–e279. doi:10.1038/nutd.2017.24
49. Chao-Gan Y, Matlab A. Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13. doi:10.3389/fnsys.2010.00013
50. Fan L, Li H, Zhuo J, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26(8):3508–3526. doi:10.1093/cercor/bhw157
51. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi:10.1006/nimg.2002.1132
52. Kawamura Y, Liu X, Akiyama T, et al. The association between oxytocin receptor gene (OXTR) polymorphisms and affective temperaments, as measured by TEMPS-A. J Affect Disord. 2010;127(1–3):31–37. doi:10.1016/j.jad.2010.04.014
53. Cataldo I, Azhari A, Esposito G. A review of oxytocin and arginine-vasopressin receptors and their modulation of autism spectrum disorder. Front Mol Neurosci. 2018;11:27. doi:10.3389/fnmol.2018.00027
54. De Carli P, Bakermans-Kranenburg MJ, Parolin L, et al. A walk on the dark side: TMS over the right inferior frontal gyrus (rIFG) disrupts behavioral responses to infant stimuli. Soc Neurosci. 2019;14(6):697–704. doi:10.1080/17470919.2019.1574891
55. Meyer ML, Masten CL, Ma Y, et al. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Soc Cogn Affect Neurosci. 2013;8(4):446–454. doi:10.1093/scan/nss019
56. Werlen E, Shin SL, Gastambide F, et al. Amphetamine disrupts haemodynamic correlates of prediction errors in nucleus accumbens and orbitofrontal cortex. Neuropsychopharmacology. 2020;45(5):793–803. doi:10.1038/s41386-019-0564-8
57. Li Y, Zhang T, Li W, Zhang J, Jin Z, Li L. Linking brain structure and activation in anterior insula cortex to explain the trait empathy for pain. Hum Brain Mapp. 2019;41(4):1030–1042. doi:10.1002/hbm.24858
58. Shimada K, Kasaba R, Fujisawa TX, Sakakibara N, Takiguchi S, Tomoda A. Subclinical maternal depressive symptoms modulate right inferior frontal response to inferring affective mental states of adults but not of infants. J Affect Disord. 2018;229:32–40. doi:10.1016/j.jad.2017.12.031
59. Francis SM, Kim SJ, Kistner-Griffin E, Guter S, Cook EH, Jacob S. ASD and Genetic Associations with Receptors for Oxytocin and Vasopressin-AVPR1A, AVPR1B, and OXTR. Front Neurosci. 2016;10:516. doi:10.3389/fnins.2016.00516
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
