Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Overview of Clinical Trials on Type 2 Diabetes Mellitus: A Comprehensive Analysis of the ClinicalTrials.gov Database
Authors Long J, Liang R, Zheng Q, Yuan G, Xin Z, Chen X, Lai F , Liu Y
Received 21 October 2020
Accepted for publication 7 January 2021
Published 26 January 2021 Volume 2021:14 Pages 367—377
DOI https://doi.org/10.2147/DMSO.S288065
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Jianyan Long,1,* Ruiming Liang,1,* Qiuyi Zheng,1,2 Gang Yuan,3 Ziyi Xin,4 Xinwen Chen,2 Fenghua Lai,2 Yihao Liu1,2
1Clinical Trials Unit, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510080, People’s Republic of China; 2Department of Endocrinology, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510080, People’s Republic of China; 3Phase I Clinical Trial Center, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510080, People’s Republic of China; 4Department of Medical Records, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510080, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yihao Liu
Clinical Trials Unit, Department of Endocrinology, The First Affiliated Hospital of Sun Yat-Sen University, No. 58, ZhongShan Road 2, Guangzhou, Guangdong 510080, People’s Republic of China
Tel +86-20-87755766
Email [email protected]
Fenghua Lai
Department of Endocrinology, The First Affiliated Hospital of Sun Yat-Sen University, No. 58, ZhongShan Road 2, Guangzhou, Guangdong 510080, People’s Republic of China
Tel +86-20- 87332200
Email [email protected]
Purpose: A better understanding of the current features of type 2 diabetes mellitus (T2DM)-related clinical trials is important for improving designs of clinical trials and identifying neglected areas of research. It was hypothesized that the trial registration policy promoted the designs of T2DM-related trials over the years. Therefore, this study aimed to present a comprehensive overview of T2DM-related clinical trials registered in the ClinicalTrials.gov database.
Methods: T2DM-related clinical trials registered in the ClinicalTrials.gov database were searched and assessed the characteristics of the relevant trials. We searched PubMed and Google Scholar for the publication statuses of the primary completed trials.
Results: Overall, 5117 T2DM-related trials were identified for analysis. Of the interventional trials, 71.5% had a primary treatment purpose while only 8.9% were prevention or health service. There were more interventional trials registered prior to patient recruitment between 2012 and 2019 than between 2004 and 2011 (44.6% vs 19.9%, P< 0.001). The period between 2012 and 2019 also had more trials that enrolled < 100 participants (59.2% vs 50.9%), were single-center studies (60.7% vs 50.6%), had non-randomized allocations (11.3% vs 6.3%), were open-label (49.2% vs 45.6%), and had smaller sample sizes than the period between 2004 and 2011 (all P< 0.001). The five-year cumulative publication rates after primary completion of the trials were < 40%.
Conclusion: Although the ClinicalTrials.gov database did not include all clinical trials, the trials registered in the ClinicalTrials.gov database still accounted for most of the clinical studies. Encouragingly, more interventional trials were registered prior to patient recruitment over the years. The majority of T2DM-related clinical trials focused on drug-related treatment, and trials regarding prevention in T2DM should be promoted. More attention should be paid to improve the publication and dissemination of clinical trials results.
Keywords: clinical trials, type 2 diabetes mellitus, ClinicalTrials.gov, publication status
Introduction
Diabetes is a chronic disease with an estimated global prevalence of 450 million.1 The public health toll of diabetes is on an upward trajectory, with its prevalence estimated to increase to 623 million by 2045; approximately 90% of these are type 2 diabetes mellitus (T2DM) patients.2 The health, social, and economic burdens caused by T2DM and its complications present a major challenge to healthcare systems worldwide.3
T2DM is a complex endocrine and metabolic disorder. Genetic and environmental factors, including varying degrees of insulin resistance, dysfunction of pancreatic β cells and α cells, and other endocrine disturbances, interact and cause organs damage.4–7 Over the past two decades, many treatment options have been introduced, and the overall quality of life of T2DM patients has improved. However, due to the heterogeneity of the etiologies and complications of T2DM, glycemic control and complications prevention in T2DM are still challenging.8–10 A large number of clinical trials have been conducted worldwide to improve the management of T2DM.
Clinical trials, especially well-designed randomized clinical trials (RCTs), are the foundation of evidence-based medicine and the driving force behind the development of clinical medicine. In 2004, the International Committee of Medical Journal Editors (ICMJE) advocated that clinical trials should be registered in a public registry before participants were recruited to ensure transparency of the process.11,12 ClinicalTrials.gov, a web-based registry maintained by the National Library of Medicine (NLM) and National Institutes of Health (NIH), was created to provide the public and healthcare providers with easy access to information about clinical trials. Currently, the ClinicalTrials.gov database provides the most comprehensive information about ongoing and completed clinical studies worldwide.13 However, despite the availability of information regarding ongoing and completed clinical trials, a thorough evaluation of T2DM-related trials is yet to be conducted, and physicians still lack a comprehensive understanding of clinical trials on T2DM.
A better understanding of the current features of T2DM-related clinical trials is important for improving designs of clinical trials and identifying neglected areas of research, which will in turn improve the translatability of results into benefits for patients. Hence, we conducted this research to present a comprehensive overview of the features of T2DM-related clinical trials registered in the ClinicalTrials.gov database and to evaluate the publication statuses of these trials.
Methods
Search and Selection of Relevant Registered Trials
An established research protocol was developed in advance. On July 1, 2020, we searched the ClinicalTrials.gov database for relevant trials using the search term “type 2 diabetes mellitus” or “non-insulin dependent diabetes” or “T2DM”. All available results were downloaded as XML files. Subsequently, all the data were imported into an Excel form to facilitate further data selection, classification, and management. Two investigators (JL and RL) independently screened the “condition”, “brief title”, and “official title” of the trials. For each potentially eligible trial, the full document was retrieved and independently assessed for inclusion (JL and RL). Any discrepancies were resolved by consensus, and those unresolved through consensus were reviewed by a third investigator (FL). Trials started between 2004 and 2019 were included in our study. Trials with withdrawn, unknown, terminated, and expanded access statuses and trials included non-T2DM participants were excluded. Finally, all included clinical trials were classified manually in duplicate by two independent investigators (JL and RL). Inconsistencies were resolved by consensus, and those unresolved through consensus were classified by a third investigator (FL). This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. Patient consent was not required in this study.
Data Extraction
A data extraction form was developed by a senior investigator (YL). Three investigators (JL, RL, and FL) were pre-trained in a pilot-testing phase to calibrate extraction criteria. The following variables were extracted by two investigators (JL and RL) independently using a standardized data extraction form: age of participants, sample size, study design, primary purpose of the trial, types of interventions, types of therapeutic drugs, region where the study was performed, centers, funding sources, start date, status of trial, duration of trial, and results of primary completed trials. Any disagreements were resolved by consensus, and those unresolved through consensus were reviewed by a third investigator (FL). If an industry was listed as the lead funder, the trial was classified as being funded by the industry. If the NIH was listed as the lead funder, the trial was considered NIH-funded.14 The time to primary completion was defined as the time from the start of the trial to the time the primary endpoint was reached. The duration of the trial was defined as the time from the start of the trial to the completion of the trial.
Search for the Publication Statuses of Included Trials
Two investigators (JL and RL) independently searched for peer-reviewed publications of trials in a stage of primary completion by using a standardized strategy. The “publications” field in the ClinicalTrials.gov database was identified and used to search for potentially matching publications. We then searched PubMed and Google Scholar by using brief titles and registration numbers in all the fields. The search for the publication statuses of the trials was updated and finalized by July 5, 2020. Publication was confirmed by matching the study characteristics outlined in the ClinicalTrials.gov database with the description in the published manuscript. The earliest article that reported primary outcome results was chosen if multiple publications were obtained from the same registered trial. Study protocols, commentaries, interim analyses, and other non-relevant publication types were excluded. A third investigator (FL) independently reconfirmed the selection and conducted a publication search for the studies that were found to be unpublished by the first two investigators. Differences were resolved by consensus.
Statistical Analysis
The year 2012, which was the mid timepoint of 2004–2019, was chosen as the cutoff to compare the characteristics of interventional trials. The number (percentage) of categorical variables and the median (interquartile range) of continuous variables were calculated. The χ2 test was used to compare categorical variables. Kaplan–Meier analysis was used to analyze the cumulative publication rates after primary completion of the trials. Trials that did have not a “completed” status were excluded from the analysis. All statistical tests were performed using SAS version 9.4 software (SAS, institute, Cary, NC), and a two-sided P value <0.05 was considered statistically significant.
Results
Distribution of T2DM-Related Clinical Trials
A total of 7823 registered clinical trials were retrieved from the Clinicaltrials.gov database; 2706 of the clinical trials were started before 2004 and after 2019. Trials that had withdrawn (n=120), unknown (n=670), and terminated (n=361) statuses and trials that included non-T2DM participants (n=769) were excluded. A total of 5117 clinical trials were eligible for analysis, including 794 (15.5%) observational trials and 4323 (84.5%) interventional trials (Figure 1). Inter-rater agreement for selecting the trials for full-document review was excellent with a kappa of 0.92 (95% CI =0.91–0.94). The distribution of the eligible trials by year according to the time of registration was summarized in Figure 2. Overall, the number of registered T2DM-related clinical trials has increased over the years. The number of trials registered between 2004 and 2009 increased rapidly but decreased slightly after 2009. Since 2012, the number of T2DM-related clinical trials registered each year has remained stable.
 |
Figure 1 Flow chart of trial selection. |
 |
Figure 2 Distribution of the eligible clinical trials according to the registered year. |
Characteristics of the Interventional and Observational Trials
Table 1 presents the characteristics of the interventional and observational trials. Fewer children were enrolled in the interventional trials than in the observational trials (3.3% vs 22.2%). More interventional trials than observational trials were registered before patient recruitment (33.2% vs 23.7%), had fewer than 100 participants (55.3% vs 27.9%), and were mainly focused on drug-related therapy (63.3% vs 52.6%) (all P<0.001). Most T2DM-related clinical trials were conducted in the United States/Canada/Europe (70.6% of the interventional trials and 63.8% of the observational trials). More interventional trials than observational trials were multiple-center studies (33.9% vs 20.8%) and were funded by industries (54.5% vs 46.2%) (both P<0.001). Most of the included trials had a primary completed status (83.3% of the interventional trials and 78.0% of the observational trials). After primary completion of the trials, interventional trials had more results available in the ClinicalTrials.gov database (28.5% vs 8.3%) and more publications (31.8% vs 19.5%) than observational trials (both P<0.001).
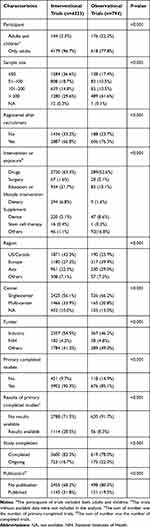 |
Table 1 Characteristics of Interventional and Observational Trials |
Trend of Changes in the Characteristics of the Interventional Trials
The characteristics of the T2DM-related interventional trials registered between 2004–2011 and 2012–2019 are listed in Table 2. More of the interventional trials registered between 2012 and 2019 were registered before patient recruitment than those registered between 2004 and 2011 (44.6% vs 19.9%); more of the trials registered between 2012 and 2019 also had fewer than 100 participants (59.2% vs 50.9%), were single-center studies (60.7% vs 50.6%), had non-randomized allocations (11.3% vs 6.3%), and were open-label (49.2% vs 45.6%) (all P<0.001). The proportion of intervention trials conducted in Asia increased from 17.7% to 26.1% during the two periods (P<0.001). Of all the interventional trials, 71.5% had a primary treatment purpose while only 8.9% were prevention or health service. The proportion of intervention trials that focused on health services or preventive measures increased from 6.7% to 10.7% during the two periods (P<0.001).
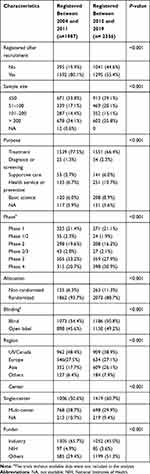 |
Table 2 Trend of Changes in the Characteristics of Interventional Trials Registered Between Two Temporal Subsets |
Distribution of Antidiabetic Drugs
Figure 3 shows the summary of the common antidiabetic drugs studied in T2DM-related clinical trials and the trend of changes that occurred during the two periods between 2004–2011 and 2012–2019. Insulin, metformin, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1RAs), and sodium glucose cotransporter-2 (SGLT-2) inhibitors were the antidiabetic drugs most commonly studied in the trials. The trials focused less on α-glucosidase inhibitors and sulfonylureas. The proportion of trials that focused on GLP-1RAs and SGLT-2 inhibitors increased rapidly from 2012 to 2019. The proportion of trials that focused on thiazolidinediones (TZDs) shrunk over time.
Publication Status of Primary Completed Trials
The one-year, three-year, and five-year cumulative publication rates since trial primary completion were 4.3%, 26.0%, and 33.1%, respectively (Figure 4). Table 3 shows the detailed characteristics of the completed intervention trials according to publication status; 82.8% of the published trials reported positive outcomes. More published trials than unpublished trials enrolled more than 100 participants (57.2% vs 38.3%), were multi-center studies (43.5% vs 32.4%), had randomized allocations (95.9% vs 89.6%), and used blinding methods (57.2% vs 51.9%) (all P<0.001). Published trials had more results available in the ClinicalTrials.gov database than unpublished trials (44.7% vs 24.4%, P<0.001).
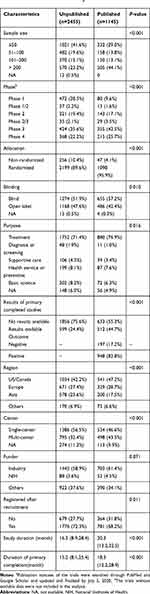 |
Table 3 Characteristics of Completed Interventional Trials According to the Publication Statusa |
 |
Figure 4 Cumulative publication rate curve since trial primary completion. Kaplan–Meier analysis was used. Trials with a “completed” status were included in the analysis. |
Discussion
The aim of this study was to present a comprehensive overview of the T2DM-related clinical trials registered in the ClinicalTrials.gov database. To the best of our knowledge, this is the first comprehensive assessment of the characteristics of T2DM-related clinical trials. Our results showed that the T2DM-related clinical trials were mostly intervention trials. The interventional trials registered between 2012 and 2019 had smaller sample sizes, included more single-center studies, had more non-randomized allocations, and had more open-label studies than those registered between 2004 and 2011. The T2DM-related clinical trials mainly focused on drug-related therapy rather than preventive strategy. The proportion of trials that involved GLP-1RAs and SGLT-2 inhibitors increased rapidly over the years. The five-year cumulative publication rates after primary completion of the trials were lower than 40%.
The results of the present study showed that more than 80% of T2DM-related clinical trials were interventional trials, a proportion that was similar to that of other chronic disease trials.15,16 Registration of clinical trials has improved significantly since the ICMJE proposed the guideline that clinical trials should be registered in a public registry before participants are recruited.17,18 The results of the present study showed that there were more trials registered prior to patient recruitment between 2012 and 2019 than those registered between 2004 and 2011. However, the design of T2DM-related interventional clinical trials between 2012 and 2019 differed from those between 2004 and 2011. Firstly, the sample sizes of the intervention trials between 2012 and 2019 were generally small. This could probably be due to the rapid development and application of new antidiabetic drugs in the past decade19 and the increased number of exploratory clinical trials that focused on the safety and efficacy of the new drugs. The new drugs were more expensive than the traditional antidiabetic drugs, and the financial burden on researchers or clinicians limited the recruitment of participants in trials. Another possible reason might be that the small-sample trials were less registered in the early years but forced to be registered in the later years due to the trial publication policy. Secondly, the proportion of multi-center trials was small. A potential reason for this may be that more trials were conducted in Asia between 2012 and 2019. There were huge differences in health policies and inequality in economic development across the Asian region,20,21 which may limit the cooperation of staff and administration in multiple centers. Finally, the proportion of clinical trials that involved blinding and randomized designs shrunk. With the development of evidence-based medicine, well-designed RCTs play an important role in the establishment of health policies and in the decision-making of clinicians. A lack of randomization and blinding greatly increased the risk of bias in the results of trials.22 Therefore, more attention should be paid to the design of T2DM-related clinical trials.
Drug-related therapy, especially antidiabetic drug therapy, has always been a hot topic in T2DM-related clinical trials. In the past 20 years, many antidiabetic drugs have been introduced, and this influenced the goal/objective of drug-related clinical trials.23 In the present study, the proportion of clinical trials on insulin and metformin was more than 40%. The number of clinical trials on TZDs decreased significantly after 2012, whereas the number of clinical trials on GLP-1RAs and SGLT-2 inhibitors increased rapidly, a finding which was similar to the results of another study.24 TZDs were introduced in the late 1990s. Rosiglitazone was discontinued in Europe and its use was restricted in the USA in 2008 after reports of an association with cardiovascular risk.25 Pioglitazone was discontinued in 2011 in some European countries pending enquires into a possible risk of bladder cancer.26 Therefore, clinical trials on TZDs decreased significantly between 2012 and 2019. GLP-1RAs were introduced in 2005, and the first SGLT-2 inhibitor, dapagliflozin, was approved in 2013 after the Food and Drug Administration issued its recommendation for the treatment of T2DM. Currently, clinical trials were conducted with special interest in their influence on the cardiovascular system and on nephropathy.27,28
The American Diabetes Association and the International Diabetes Federation emphasized that diabetes prevention should be the focus of future research.29,30 Previous studies demonstrated that lifestyle intervention and health education implementation may delay the onset of diabetes in high-risk persons.31–33 Therefore, additional research was needed to assess the effectiveness of prevention strategies in clinical practice and standardize their implementation.34,35 We found that most T2DM clinical trials in the ClinicalTrials.gov database focused on drug-related therapy, while only small percentages were primarily concerned with prevention, health services research, supportive care, diagnosis or screening. Although the ideal proportion of trials focused on prevention has not been established, the current T2DM-related clinical trials appeared to be inadequate for expanding and refining preventive strategies into the community setting.
The systematic reporting and publication of clinical research results provide a reliable basis for evidence-based medicine, facilitate the establishment of health policies, aids clinicians in decision-making, and promote the development of public health and clinical medicine.36 Following the announcement of the ICMJE trial registration policy in 2004, some organizations subsequently enacted laws and policies requiring the systematic reporting of aggregate results. In the USA, the Food and Drug Administration Amendments Act (FDAAA) of 2007 established a legal mandate requiring those responsible for initiating clinical trials to report summary results for certain trials.37 In 2016, the department of Health and Human Services promulgated regulations to implement, clarify, and expand legal requirements under FDAAA for trial results information submission.38 The Chinese Trial Registration and Publication Collaboration issued the second statement to implement the publication ethic of clinical trials in 2011.39 In June 2017, the ICMJE published a statement supporting data-sharing policy for clinical trials.40 However, in the present study, more than 70% of trials had no results available in the ClinicalTrials.gov database and the five-year cumulative publication rates of T2DM-related clinical trials after primary completion were lower than 40%. Previous studies showed that publication rates among completed trials registered in ClinicalTrials.gov were less than 50%.41 Selective publication was an important factor that affects the publication of biomedical research.42 If trial results put either investigators/sponsors at financial risk or trial results contradicted investigators’ beliefs, publications may be delayed or suppressed.43 In addition, researchers, reviewers, and editors were generally more enthusiastic about positive or equivalence trials and less excited about negative trials.11 As 82.8% of the published trials in the present study reported positive results, our study results were in accordance with this opinion.
The limitations of our study should be addressed. Firstly, the ClinicalTrials.gov database does not include all clinical trials, and investigators may use other worldwide registries to fulfill the ICMJE-advocated mandatory registration guideline. However, the trials registered in the ClinicalTrials.gov database still account for most of the clinical studies in the World Health Organization International Clinical Trials Registry Platform. Secondly, the data for all clinical trials in the database were reported by researchers and the NLM cannot verify the validity of all trial information in the ClinicalTrials.gov database. Finally, the data of all trials in the ClinicalTrials.gov database were not always complete and up to date.
Conclusion
In summary, this study presented the first comprehensive overview of T2DM-related clinical trials registered in the ClinicalTrials.gov database. Our results indicated that there were more interventional trials registered prior to patient recruitment over the years. The majority of T2DM-related clinical trials focused on drug-related treatment, and trials regarding prevention in T2DM should be promoted. More attention should be paid to improve the publication and dissemination of clinical trials results.
Abbreviations
FDAAA, Food and Drug Administration Amendments Act; GLP-1RAs, glucagon-like peptide-1 receptor agonists; ICMJE, International Committee of Medical Journal Editors; NIH, National Institutes of Health; NLM, National Library of Medicine; RCTs, randomized clinical trials; SGLT-2, sodium glucose cotransporter-2; TZDs, thiazolidinediones; T2DM, type 2 diabetes mellitus.
Data-Sharing Statement
The data will be made available at reasonable request from the corresponding author (YL).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Research Fund of Medical Science and Technology of Guangdong Province (A2020132).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
2. Holman N, Young B, Gadsby R. Current prevalence of type 1 and type 2 diabetes in adults and children in the UK. Diabet Med. 2015;32(9):1119–1120. doi:10.1111/dme.12791
3. GBD DALYs, HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1603–1658. doi:10.1016/S0140-6736(16)31460-X.
4. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–1346. doi:10.1016/S0140-6736(05)61032-X
5. Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–362. doi:10.1038/nature03711
6. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627–631. doi:10.1038/nature09253
7. Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–871. doi:10.1016/j.cell.2012.02.017
8. Mata-Cases M, Rodriguez-Sanchez B, Mauricio D, et al. The association between poor glycemic control and health care costs in people with diabetes: a population-based study. Diabetes Care. 2020;43(4):751–758. doi:10.2337/dc19-0573
9. Satirapoj B, Pratipanawatr T, Ongphiphadhanakul B, Suwanwalaikorn S, Benjasuratwong Y, Nitiyanant W. Real-world evaluation of glycemic control and hypoglycemic events among type 2 diabetes mellitus study (REEDS): a multicentre, cross-sectional study in Thailand. BMJ Open. 2020;10(2):e031612. doi:10.1136/bmjopen-2019-031612
10. Stolar M. Glycemic control and complications in type 2 diabetes mellitus. Am J Med. 2010;123(Suppl 3):S3–S11. doi:10.1016/j.amjmed.2009.12.004
11. De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the international committee of medical journal editors. N Engl J Med. 2004;351(12):1250–1251. doi:10.1056/NEJMe048225
12. Hartung DM, Zarin DA, Guise JM, McDonagh M, Paynter R, Helfand M. Reporting discrepancies between the ClinicalTrials.gov results database and peer-reviewed publications. Ann Intern Med. 2014;160(7):477–483. doi:10.7326/M13-0480
13. Zwierzyna M, Davies M, Hingorani AD, Hunter J. Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005. BMJ. 2018;361:k2130. doi:10.1136/bmj.k2130
14. Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, Califf RM. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med. 2015;372(11):1031–1039. doi:10.1056/NEJMsa1409364
15. Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307(17):1838–1847. doi:10.1001/jama.2012.3424
16. Buffel DVC, Dechartres A, Battin C, Ravaud P, Boutron I. Exclusion of patients with concomitant chronic conditions in ongoing randomised controlled trials targeting 10 common chronic conditions and registered at ClinicalTrials.gov: a systematic review of registration details. BMJ Open. 2016;6(9):e012265. doi:10.1136/bmjopen-2016-012265
17. De Angelis CD, Drazen JM, Frizelle FA, et al. Is this clinical trial fully registered? A statement from the international committee of medical journal editors. Ann Intern Med. 2005;143(2):146–148. doi:10.7326/0003-4819-143-2-200507190-00016
18. Zarin DA, Tse T, Williams RJ, Rajakannan T. Update on trial registration 11 years after the ICMJE policy was established. N Engl J Med. 2017;376(4):383–391. doi:10.1056/NEJMsr1601330
19. Bull RI, Petersen SS, Juel J, Hansen MR, Andersen NH, Pareek M. Novel antidiabetic drugs and cardiovascular complications. Ugeskr Laeger. 2018;180(26).
20. Kumar P, Larrison C, Rodrigues SB, McKeithen T. Assessment of general practitioners’ needs and barriers in primary health care delivery in Asia Pacific region. J Family Med Prim Care. 2019;8(3):1106–1111. doi:10.4103/jfmpc.jfmpc_46_19
21. Tham TY, Tran TL, Prueksaritanond S, Isidro JS, Setia S, Welluppillai V. Integrated health care systems in Asia: an urgent necessity. Clin Interv Aging. 2018;13:2527–2538. doi:10.2147/CIA.S185048
22. Wood L, Egger M, Gluud LL, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601–605. doi:10.1136/bmj.39465.451748
23. Tahrani AA, Bailey CJ, Del PS, Barnett AH. Management of type 2 diabetes: new and future developments in treatment. Lancet. 2011;378(9786):182–197. doi:10.1016/S0140-6736(11)60207-9
24. Rocha S, Ribeiro D, Fernandes E, Freitas MA. Systematic review on anti-diabetic properties of chalcones. Curr Med Chem. 2020;27(14):2257–2321. doi:10.2174/0929867325666181001112226
25. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi:10.1056/NEJMoa072761
26. Hillaire-Buys D, Faillie JL, Montastruc JL. Pioglitazone and bladder cancer. Lancet. 2011;378(9802):1543–1545. doi:10.1016/S0140-6736(11)61662-0
27. Deedwania P, Acharya T. Cardiovascular protection with anti-hyperglycemic agents. Am J Cardiovasc Drugs. 2019;19(3):249–257. doi:10.1007/s40256-019-00325-9
28. El MC, Riachy R, Khalil AB, Eid A, Azar S. SGLT2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors in diabetes and microvascular complications: a review. Int J Endocrinol. 2020;2020:1762164. doi:10.1155/2020/1762164
29. Fonseca VA, Kirkman MS, Darsow T, Ratner RE. The American diabetes association diabetes research perspective. Diabetes Care. 2012;35(6):1380–1387. doi:10.2337/dc12-9001
30. Alberti KG, Zimmet P, Shaw J. International diabetes federation: a consensus on type 2 diabetes prevention. Diabet Med. 2007;24(5):451–463. doi:10.1111/j.1464-5491.2007.02157.x
31. Gong Q, Zhang P, Wang J, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing diabetes prevention outcome study. Lancet Diabetes Endocrinol. 2019;7(6):452–461. doi:10.1016/S2213-8587(19)30093-2
32. Jiang L, Johnson A, Pratte K, Beals J, Bullock A, Manson SM. Long-term outcomes of lifestyle intervention to prevent diabetes in American Indian and Alaska native communities: the special diabetes program for Indians diabetes prevention program. Diabetes Care. 2018;41(7):1462–1470. doi:10.2337/dc17-2685
33. Hill-Briggs F. 2018 Health care & education presidential address: the American diabetes association in the era of health care transformation. Diabetes Care. 2019;42(3):352–358. doi:10.2337/dci18-0051
34. Dutton GR, Lewis CE. The look AHEAD trial: implications for lifestyle intervention in type 2 diabetes mellitus. Prog Cardiovasc Dis. 2015;58(1):69–75. doi:10.1016/j.pcad.2015.04.002
35. Lakey WC, Barnard K, Batch BC, Chiswell K, Tasneem A, Green JB. Are current clinical trials in diabetes addressing important issues in diabetes care? Diabetologia. 2013;56(6):1226–1235. doi:10.1007/s00125-013-2890-4
36. Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM. Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ. 2012;344(jan03 1):d7292. doi:10.1136/bmj.d7292
37. Hirsch L. Trial registration and results disclosure: impact of US legislation on sponsors, investigators, and medical journal editors. Curr Med Res Opin. 2008;24(6):1683–1689. doi:10.1185/03007990802114849
38. Zarin DA, Tse T, Williams RJ, Carr S. Trial reporting in ClinicalTrials.gov - the final rule. N Engl J Med. 2016;375(20):1998–2004. doi:10.1056/NEJMsr1611785
39. Liu X, Li Y, Jiang L, et al. To promote good research practice, good publication practice and good translation by implementing research ethic and publication ethic–the explanation document for the second statement of Chinese clinical trial registration and publication collaboration[Chinese]. Chin J Evid Based Med. 2011;11(07):726–732.
40. Taichman DB, Sahni P, Pinborg A, et al. Data sharing statements for clinical trials - a requirement of the international committee of medical journal editors. N Engl J Med. 2017;376(23):2277–2279. doi:10.1056/NEJMe1705439
41. Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis. PLoS Med. 2009;6(9):e1000144. doi:10.1371/journal.pmed.1000144
42. Decullier E, Lheritier V, Chapuis F. Fate of biomedical research protocols and publication bias in France: retrospective cohort study. BMJ. 2005;331(7507):19. doi:10.1136/bmj.38488.385995.8F
43. Blumenthal D, Campbell EG, Anderson MS, Causino N, Louis KS. Withholding research results in academic life science. Evidence from a national survey of faculty. JAMA. 1997;277(15):1224–1228. doi:10.1001/jama.277.15.1224
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

