Back to Journals » OncoTargets and Therapy » Volume 12
Overexpression of TRMT12 may independently predict poor overall survival in patients with head and neck squamous cell carcinoma
Authors Wang K , Zheng M, Ren Y
Received 14 April 2019
Accepted for publication 13 August 2019
Published 5 September 2019 Volume 2019:12 Pages 7269—7279
DOI https://doi.org/10.2147/OTT.S212200
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Kai Wang1, Mengmeng Zheng1, Yuan Ren1,2
1Department of Otorhinolaryngology Head and Neck Surgery, Ningbo Medical Center Lihuili Hospital, Ningbo, Zhejiang, People’s Republic of China; 2Department of Otorhinolaryngology Head and Neck Surgery, Ningbo Medical Center Lihuili Eastern Hospital, Ningbo, Zhejiang, People’s Republic of China
Correspondence: Yuan Ren
Department of Otorhinolaryngology Head and Neck Surgery, Ningbo Medical Center Lihuili Eastern Hospital, 1111 Jiangnan Road, Ningbo 315000, Zhejiang, People’s Republic of China
Tel +86 1 596 893 2131
Email [email protected]
Purpose: The TRMT12 is a novel oncogene involved in breast cancer. However, the association between TRMT12 and head and neck squamous cell carcinoma (HNSCC) remains unclear.
Materials and methods: The levels of TRMT12 mRNA in HNSCC and normal tissues were analyzed using data from the Cancer Genome Atlas-HNSC. The expression of TRMT12 was also determined using quantitative real-time polymerase chain reaction in 165 paired HNSCC and adjacent normal tissues, which were used as the validation cohort.
Results: TRMT12 expression was significantly increased in HNSCC tissues versus normal tissues. High TRMT12 expression was significantly associated with human papillomavirus infection, perineural invasion, tumor invasion, lymphatic metastasis, and clinical stage. Moreover, elevated TRMT12 expression was correlated with unfavorable overall survival (hazard ratio [HR]: 1.711, 95% confidence interval [CI]: 1.141–2.567, P=0.009) and recurrence-free survival (HR: 1.648, 95% CI: 1.060–2.563, P=0.027) in HNSCC patients.
Conclusion: TRMT12 was significantly upregulated in HNSCC tissues, which may be attributed to both genetic and epigenetic alterations. Increased TRMT12 expression may be involved in the progression and metastasis of HNSCC, and serve as an independent biomarker of poor prognosis in HNSCC with respect to overall survival and recurrence-free survival.
Keywords: tRNA methyltransferase 12 homolog, bioinformatics, head and neck squamous cell carcinoma, prognosis, survival
Introduction
Head and neck cancer is the sixth most common type of cancer and fifth leading cause of cancer-related death worldwide. The highest prevalence is reported in south-central Asia, northern America, and central Europe.1 Head and neck squamous cell carcinoma (HNSCC) accounts for approximately 90% of all head and neck cancer cases. According to the International Agency for Research on Cancer, the global annual incidence of HNSCC is more than 1 million cases.2 Although excessive use of tobacco and alcohol are the primary etiological factors of HNSCC, there is increasing molecular and epidemiological evidence supporting the causal role of human papillomavirus (HPV) infection in HNSCC, especially for oropharyngeal cancer.3 Despite great achievements based on manifold interdisciplinary treatment approaches, the survival rate of HNSCC patients has not improved significantly in the previous decades.3 Notably, the 5-year survival rate of patients with HNSCC is <50%.1 Traditional prognostic factors, such as tumor–node–metastasis stage, histological grade, positive surgical margins, and perineural invasion, are currently used to predict the outcome of HNSCC.4 However, these factors are inadequate to predict the prognosis of individual patients, due to the heterogeneous molecular mechanisms and tumor behaviors linked to HNSCC.5 Therefore, identification of biomarkers associated with clinical outcomes of HNSCC may facilitate proper therapeutic decision-making and improve prognosis. Nevertheless, thus far, research studies have not successfully identified clinically useful biomarkers of prognosis in HNSCC.
The TRMT12 gene is the human homolog to the yeast TRM12 gene,6 and is a tRNA methyltransferase catalyzing the third step in the biochemical pathway to form wybutosine. The latter is a hypermodified guanosine at the 3-prime position adjacent to the anticodon of phenylalanine tRNA, stabilizing codon-anticodon interactions during decoding on the ribosome.7 A recent report revealed that overexpression of TRMT12 in both breast cancer tissues and cell lines may lead to disruption of the wybutosine biochemical pathway. This results in hypomodified phenylalanine tRNA which may ultimately lead to translational errors in cancer cells.8 However, the expression profile of TRMT12 and its role in other types of cancer (including HNSCC) remain unclear.
The Cancer Genome Atlas (TCGA) Data Portal – established by the National Cancer Institute’s Center for Cancer Genomics and the National Human Genome Research Institute – contains DNA, RNA, protein, and survival data related to various types of cancer, including HNSCC (http://cancergenome.nih.gov/).9 In the present study, the expression profile, clinical significance, prognostic value in terms of overall survival (OS) and recurrence-free survival (RFS), and mechanism of dysregulation of TRMT12 in HNSCC were investigated through data mining from the TCGA.
Materials and methods
Data mining – HNSCC
The level-3 data of primary HNSCC in the TCGA cohort (Project Id: TCGA-HNSC) were obtained using the University of California Santa Cruz Xena browser (https://xenabrowser.net/). Via this approach, TRMT12 RNA-sequencing data, TRMT12 Illumina Human Methylation 450K data, and details of the clinicopathological characteristics of patients (ie, sample type, age at initial pathologic diagnosis, gender, alcohol history, tobacco smoking history, anatomic neoplasm subdivision, HPV status by p16 testing, neoplasm histologic grade, pathologic T, pathologic N, pathological stage, OS status, and OS time) were collected and downloaded for secondary analysis. The RNA-sequencing data was normalized based on the values of reads per kilobase of transcript per million mapped reads. A total of 517 HNSCC patients with integrated TRMT12 expression and OS data were divided into high and low TRMT12 expression groups. This classification was performed according to the maximum Youden index based on receiver operating characteristic curves for the detection of death in HNSCC patients. Accordingly, there were 339 and 178 patients in the high and low TRMT12 expression groups, respectively. The mean β values of five CpG probes (cg11829072, cg25375711, cg26547947, cg22853986, and cg00846483) mapping 200 bp downstream of the transcription start sites of TRMT12 were defined as the level of methylation of the TRMT12 promoter. TRMT12 genetic alterations reported in TCGA-HNSC were examined using the cBio-Portal for Cancer Genomics (http://www.cbioportal.org/).10
Collection of tissue specimens
To validate the findings of the bioinformatics analysis, 165 HNSCC tissues with adjacent non-tumorous tissues were collected from the Ningbo Medical Centre Lihuili Hospital (Ningbo, China) and the Affiliated Tumor Hospital of Xiangya Medical School (Changsha, China) between February 2004 and November 2018. None of the patients received treatment prior to surgical excision. The final diagnosis was histopathologically confirmed by two pathologists according to the clinical practice guidelines established by the National Comprehensive Cancer Network. Tumors were determined according to the seventh edition of the tumor–node–metastasis staging system produced by the International Union Against Cancer. All specimens were preserved in RNA-fixer Reagent (Bioteke, Beijing, China) at −80 °C. The clinical and survival data of all patients were collected. The median follow-up period was 24 months (interquartile range: 14–43 months). During the follow-up, 13 patients were lost and 70 patients expired.
This study was approved by the Human Research Ethics Committee of Ningbo Medical Centre Lihuili Hospital (Ningbo, China) and the Affiliated Tumor Hospital of Xiangya Medical School (Changsha, China). All patients provided written informed consent prior to their participation in this study.
Extraction of total RNA and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the frozen tissues using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the instructions provided by the manufacturer. Reverse transcription and qRT-PCR was performed as previously described.11 In the present study, the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase was used as an endogenous control to normalize the cycle threshold (Ct) value. The primers were synthesized by Huada Biotech (Shen Zhen, China) and their sequences were as follows: TRMT12, forward: 5′-GGGAGAGACGCTTCCAGAG-3′ and reverse: 5′-GAGCTGCGTGAGCATACAGG-3′; and glyceraldehyde 3-phosphate dehydrogenase, forward: 5′- CCATGGAGAAGGCTGGGG -3′ and reverse: 5′- CAAAGTTGTCATGGATGACC -3′. The PCR conditions were as follows: 95 °C for 10 min, 45 amplification cycles at 95 °C for 20 s, 55 °C for 35 s, and 70 °C for 30 s. The expression of TRMT12 was calculated using the ΔCt method. All experiments were performed in triplicate.
Statistical analysis
All statistical analyses were performed using the IBM SPSS, version 20.0 (IBM Corp., Armonk, NY, USA) and R 3.5.1 software (https://www.r-project.org/), which were also used to generate figures. Independent Student’s t-tests were applied to identify correlations between TRMT12 expression and clinical features of HNSCC. Receiver operating characteristic (ROC) curve was used to evaluate the diagnostic value of TRMT12 for HNSCC. OS and RFS were compared between the high and low TRMT12 expression groups using Kaplan–Meier curves, and log-rank tests were performed to evaluate the difference between the survival curves. A univariate cox proportional hazards model was utilized to determine TRMT12 expression and established important clinicopathologic features associated with OS or RFS. Hazard ratios (HR) with 95% confidence intervals (CI) were obtained for each variable. A multivariate cox proportional hazards model was utilized to verify the correlations between TRMT12 expression and survival along with significant clinical features identified in the univariate analysis. The correlation between TRMT12 expression and methylation was tested using the Spearman’s rank correlation coefficient. A P<0.05 denoted statistical significance.
Results
TRMT12 expression is upregulated in HNSCC versus normal head and neck tissue
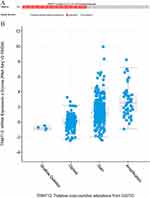
Using mRNA-sequencing data from the Xena browser, we reviewed the expression of TRMT12 in 520 HNSCC tissues and 44 head and neck normal tissues in TCGA-HNSC. By comparing with the normal tissue, we found that the expression of TRMT12 was significantly upregulated in HNSCC patients in the TCGA database (Figure 1A and B, P=3.79E−10). The qRT-PCR analysis of the 165 paired HNSCC samples confirmed that the expression of TRMT12 was significantly upregulated in HNSCC tissues versus adjacent normal tissues (P=3.85E−30, Figure 1C).
Correlation between TRMT12 expression and clinicopathological features of HNSCC patients
In addition, we analyzed the relationship between TRMT12 expression and clinicopathological features of HNSCC patients in the TCGA database. TRMT12 expression was upregulated in HPV-negative patients versus HPV-positive patients (8.426±0.528 vs 7.686±1.277, respectively, P=0.001, Table 1). Perineural invasion-positive patients presented with significantly higher levels of TRMT12 expression versus perineural invasion-negative patients (8.487±0.752 vs 8.231±0.861, respectively, P=0.003, Table 1). In addition, the expression of TRMT12 was higher in those with an advanced T stage (T1/2: 8.203±0.807 vs T3/4: 8.421±0.800, P=0.004, Table 1). Moreover, TRMT12 expression increased in patients with lymph node metastasis versus those without lymph node metastasis (N1–N3: 8.451±0.809 vs N0: 8.201±0.785, respectively, P=0.002, Table 1). Significantly elevated levels of TRMT12 expression were found in patients with an advanced clinical stage (I + II: 8.073±0.852 vs III + IV: 8.409±0.783, P=2.28E−04, Table 1). Subsequently, by analyzing the clinicopathological characteristics of 165 patients in our validation cohort, we observed that the differences in TRMT12 expression were related to history of alcohol consumption (P=0.015, Table 2), tumor invasion (P=0.005, Table 2), lymphatic metastasis (P=0.026, Table 2), and clinical stage (P=0.001, Table 2). However, TRMT12 expression was not related to other clinicopathological features.
 |
Table 1 Association between TRMT12 expression and clinicopathological characteristics of HNSCC patients in the TCGA database |
 |
Table 2 Association between TRMT12 expression and clinicopathological characteristics of HNSCC patients in the validation cohort |
The diagnostic value of TRMT12 for HNSCC
We assessed the diagnostic value of TRMT12 for HNSCC using ROC curve analysis, which is a synthesized index that reflects the accuracy of diagnostic test. An area under the ROC curve (AUC) close to 1.0 signifies that the test has almost perfect diagnostic value. The maximum Youden index was used as a cut-off point. We found TRMT12 expression yielded an AUC of 0.760 (95% CI: 0.712–0.808), a sensitivity of 57.7% and a specificity of 97.7% (Figure 2).
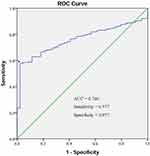 |
Figure 2 The receiver operating characteristic (ROC) curve. The arrow points to the intercept. |
High TRMT12 expression is an independent risk factor of OS in HNSCC patients
A total of 517 HNSCC patients, with complete TRMT12 expression and OS data in the TCGA-HNSC, were enrolled to investigate the prognostic value of TRMT12. Based on the best performing threshold, the Kaplan–Meier analysis showed that high TRMT12 expression was associated with worse OS (log-rank P=2.26E−05, Figure 3A). In addition, 152 HNSCC patients with sufficient OS data were analyzed to confirm this finding. The Kaplan–Meier analysis confirmed that high TRMT12 expression was associated with shorter OS (log-rank P=0.01, Figure 3B) in our validation cohort. The subgroup analyses were performed according to TRMT12 significantly associated clinicopathological features. By generating Kaplan–Meier curves of OS, we showed that high TRMT12 expression significantly affected the OS in the following HNSCC patients: HPV-negative (log-rank P=7.8E−04, Figure 4A and B), perineural invasion-negative (log-rank P=0.015, Figure 4C and D), lymph node metastatic (log-rank P=0.002, Figure 4E and F), and advanced clinical stage (log-rank P=0.003, Figure 4G and H). However, subgroup analysis indicated that high TRMT12 expression was associated with significantly worse OS in both early T stage (log-rank P=0.023, Figure 4I) and advanced T stage (log-rank P=0.002, Figure 4J) patients. Subsequently, a Cox proportional hazards model was performed to assess the independent risk factors of OS in HNSCC patients. In the univariate model, age (HR: 1.318, 95% CI: 1.003–1.731, P=0.047), gender (HR: 1.349, 95% CI: 1.014–1.796, P=0.047), perineural invasion (HR: 2.135, 95% CI: 1.516–3.007, P=1.42E−05), pathologic stage (HR: 1.754, 95% CI: 1.203–2.558, P=0.004), and TRMT12 expression (HR: 1.910, 95% CI: 1.409–2.591, P=3.11E−05) were significantly associated with OS. After adjustment of the prognostic risk factors, the subsequent multivariate analysis confirmed that high TRMT12 expression (HR: 1.711, 95% CI: 1.141–2.567, P=0.009), perineural invasion positive (HR: 1.984, 95% CI: 1.387–2.838, P=1.76E−04), and advanced pathologic stage (HR: 1.830, 95% CI: 1.104–3.035, P=0.019) were independent prognostic factors of poor OS in HNSCC patients. All independent risk factors and their HR with 95% CI are listed in Table 3.
 |
Table 3 Univariate and multivariate analyses of overall survival in HNSCC patients |
High TRMT12 expression independently predicted poor RFS among HNSCC patients
Using the TCGA dataset that contained follow-up data of HNSCC recurrence in 425 patients, we produced Kaplan–Meier curves to determine whether TRMT12 expression was associated with recurrence in such patients. As shown in Figure 5, the log-rank test of the Kaplan–Meier curves in TCGA-HNSC indicated that high TRMT12 expression was markedly related to worse RFS versus low TRMT12 expression (P=0.005). The univariate Cox proportional hazards analysis showed that alcohol consumption (HR: 1.809, 95% CI: 1.130–2.896, P=0.014), advanced stage (HR: 2.302, 95% CI: 1.249–4.242, P=0.007), and high TRMT12 expression (HR: 1.864, 95% CI: 1.255–2.769, P=0.002) were associated with worse RFS in HNSCC patients (Table 4). Subsequently, the multivariate Cox proportional hazards analysis confirmed that advanced stage (HR: 2.036, 95% CI: 1.095–3.785, P=0.025) and high TRMT12 expression (HR: 1.648, 95% CI: 1.060–2.563, P=0.027) were independent prognostic factors of poor RFS (Table 4).
 |
Table 4 Univariate and multivariate analyses of recurrence-free survival in HNSCC patients |
 |
Figure 5 Kaplan–Meier analysis of 435 HNSCC patients with recorded TRMT12 expression and RFS data. High TRMT12 expression was significantly associated with worse RFS in HNSCC patients. |
TRMT12 upregulation was related to DNA amplification and promoter hypomethylation in HNSCC
To further investigate the mechanism of TRMT12 dysregulation in HNSCC, we examined its genetic and epigenetic alterations in TCGA-HNSC using the sequencing and copy number alterations data of 504 HNSCC patients in TCGA-HNSC. Our analysis revealed that 53 HNSCC patients (11%) had genetic alterations of TRMT12, with amplification being the predominant type of alteration (Figure 6A). Further plot analysis using the cBio-Portal for Cancer Genomics showed that amplification was associated with increased TRMT12 expression in HNSCC (Figure 6B). Subsequently, we analyzed the expression of TRMT12 and the methylation status of its promoter using data from 520 HNSCC patients. We observed a strong negative correlation (Pearson’s r = –0.639, P=6.16E−61) between TRMT12 expression and promoter methylation (Figure 7).
Discussion
Progress achieved in the fields of genetics and molecular biology has promoted the rapid development of personalized treatment and management of cancer.5 the identification of crucial biomarkers may contribute to improved individualized treatment and prognosis of HNSCC.12 Rodriguez was the first to report the high expression of TRMT12 in breast cancer.8 However, few studies have investigated TRMT12 expression in other cancers, including HNSCC. In the present study, using bioinformatics analysis based on TCGA-HNSC data and our validation patient cohort, we initially determined the expression profile of TRMT12 in HNSCC. The results showed that HNSCC tissues exhibited significantly elevated TRMT12 expression compared with normal head and neck tissues, indicating its potential function as an oncogene in this setting.
Furthermore, we assessed the clinical significance of TRMT12 expression in HNSCC using the TCGA cohort and validation cohort. Increasing evidence has shown that HPV infection is an independent pathogenic factor of HNSCC.13,14 HPV-positive and -negative HNSCC patients differ in terms of the molecular mechanisms underlying their respective oncogenic processes and clinical outcomes.15,16 HPV-positive patients are more susceptible to radiation and chemotherapy with a favorable prognosis versus HPV-negative patients.17 In the TCGA cohort, we observed that TRMT12 expression was significantly elevated in HNSCC patients without HPV infection versus those with HPV infection. This finding indicated that TRMT12 may play an important role in HNSCC carcinogenesis, specifically in HPV-negative patients. Additionally, data obtained from the TCGA showed that high TRMT12 expression was related to perineural invasion, tumor invasion, lymphatic metastasis, and advanced pathologic stage. This is consistent with the findings observed in the validation cohort for the expression of TRMT12 in HNSCC patients, suggesting that TRMT12 may be involved in the progression and metastasis of this type of cancer.
In the present study, we also constructed ROC curves and calculated the AUC to determine the diagnostic value of TRMT12 expression for HNSCC. Although the AUC and sensitivity were not encouraging, the specificity was close to 1.0, signifying that TRMT12 expression might have excellent diagnostic accuracy for HNSCC combining with other high sensitivity biomarkers.
Although surgical resection remains the mainstay treatment for HNSCC, adjuvant therapy (ie, immunotherapy, radiotherapy, and chemotherapy) have achieved great progress in recent years, especially in patients with advanced HNSCC.5,18 Due to tumor heterogeneity and resistance to chemotherapy, the prediction of high-risk HNSCC patients and application of precision therapy remain challenges for both clinicians and pathologists.19 Therefore, the use of reliable predictive biomarkers is urgently required to guide treatment selection in HNSCC patients. In the present study, by generating Kaplan–Meier curves of OS, we observed that high TRMT12 expression was significantly associated with unfavorable OS in both the TCGA and validation cohorts. Subsequent univariate and multivariate cox proportional hazards analyses showed that high TRMT12 expression was an independent prognostic factor of poor OS. These findings imply that TRMT12 upregulation may serve as a predictive biomarker of patient prognosis. Interestingly, subgroup analyses showed that high expression of TRMT12 was associated with worse OS in the following patients: HPV-negative, perineural invasion-negative, lymph node metastatic, and advanced clinical stage. These findings demonstrated the specific prognostic role of TRMT12 expression and its potential contribution to the development of precision therapy against HNSCC.
Despite aggressive, site-specific, multimodal therapy, a considerable proportion of HNSCC patients develop locoregional recurrence, which adversely impacts treatment and survival. Hence, recurrence is an important factor in the choice of treatment.12,20 In this study, we also investigated the association of TRMT12 expression with recurrence in HNSCC patients using the TCGA cohort. The Kaplan–Meier analysis showed that elevated TRMT12 expression was significantly correlated with unfavorable RFS. Subsequently, univariate and multivariate Cox proportional hazards analyses confirmed that high TRMT12 expression was an independent factor of unfavorable RFS in HNSCC patients. This evidence indicated that TRMT12 upregulation may be useful in treatment stratification and surveillance for recurrence of HNSCC.
Subsequently, the potential underlying mechanisms of TRMT12 upregulation in HNSCC were investigated. DNA amplification is an important form of copy number variation, which refers to a form of genomic structural variation. This results in abnormal gene copy numbers.21 Moreover, DNA amplification is an important influential factor for the expression of both protein-coding and non-coding genes, affecting the activity of various signaling pathways in numerous types of cancer.22,23 In this study, we performed an analysis of TRMT12 DNA amplification and expression data in TCGA-HNSC using the cBio-Portal. We found that DNA amplification was associated with elevated TRMT12 expression. We further examined the potential association between the methylation status of the TRMT12 promoter and its mRNA expression. As a major form of epigenetic modification, aberrant methylation in the promoter region is associated with gene expression in numerous types of cancer,24,25 including HNSCC.26 The results revealed that promoter hypomethylation was significantly associated with increased TRMT12 expression. These findings indicated that both genetic and epigenetic alterations contribute to the dysregulation of TRMT12 in HNSCC.
Conclusion
The present findings demonstrated that TRMT12 was significantly upregulated in HNSCC tissues, and this upregulation may be attributed to both genetic and epigenetic alterations. The increased TRMT12 expression may be involved in the progression and metastasis of HNSCC. Additionally, elevated TRMT12 expression was an independent biomarker of poor prognosis in HNSCC with respect to OS and RFS. However, the mechanism through which TRMT12 functions as an oncogene in HNSCC remains to be determined.
Acknowledgment
This research was supported by grants from the Ningbo Health Branding Subject Fund (PPXK2018-02).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shield KD, Ferlay J, Jemal A, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67(1):51–64. doi:10.3322/caac.21384
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Gooi Z, Chan JY, Fakhry C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope. 2016;126(4):894–900. doi:10.1002/lary.25767
4. Huang SH, O’Sullivan B. Overview of the 8th edition TNM classification for head and neck cancer. Curr Treat Options Oncol. 2017;18(7):40. doi:10.1007/s11864-017-0484-y
5. Kaidar-Person O, Gil Z, Billan S. Precision medicine in head and neck cancer. Drug Resist Updat. 2018;40:13–16. doi:10.1016/j.drup.2018.09.001
6. Kalhor HR, Penjwini M, Clarke S. A novel methyltransferase required for the formation of the hypermodified nucleoside wybutosine in eucaryotic tRNA. Biochem Biophys Res Commun. 2005;334(2):433–440. doi:10.1016/j.bbrc.2005.06.111
7. Noma A, Suzuki T. Ribonucleome analysis identified enzyme genes responsible for wybutosine synthesis. Nucleic Acids Symp Ser (Oxf). 2006;(50):65–66.
8. Rodriguez V, Chen Y, Elkahloun A, Dutra A, Pak E, Chandrasekharappa S. Chromosome 8 BAC array comparative genomic hybridization and expression analysis identify amplification and overexpression of TRMT12 in breast cancer. Genes Chromosomes Cancer. 2007;46(7):694–707. doi:10.1002/gcc.20454
9. Weinstein JN, Collisson EA; Mills GB, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45(10):1113–1120. doi:10.1038/ng.2764
10. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi:10.1158/2159-8290.CD-12-0095
11. Shen Z, Hao W, Zhou C, et al. Long non-coding RNA AC026166.2-001 inhibits cell proliferation and migration in laryngeal squamous cell carcinoma by regulating the miR-24-3p/p27 axis. Sci Rep. 2018;8(1):3375. doi:10.1038/s41598-018-21659-5
12. Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3305–3313. doi:10.1200/JCO.2015.62.0963
13. Bussu F, Ragin C, Boscolo-Rizzo P, et al. HPV as a marker for molecular characterization in head and neck oncology: looking for a standardization of clinical use and of detection method(s) in clinical practice. Head Neck. 2019;41(4):1104–1111. doi:10.1002/hed.25591
14. Tsai SC, Huang JY, Lin C, Liaw YP, Lin FC. The association between human papillomavirus infection and head and neck cancer: a population-based cohort study. Medicine (Baltimore). 2019;98(7):e14436. doi:10.1097/MD.0000000000014436
15. Koneva LA, Zhang Y, Virani S, et al. HPV integration in HNSCC correlates with survival outcomes, immune response signatures, and candidate drivers. Mol Cancer Res. 2018;16(1):90–102. doi:10.1158/1541-7786.MCR-17-0153
16. Saada-Bouzid E, Peyrade F, Guigay J. Molecular genetics of head and neck squamous cell carcinoma. Curr Opin Oncol. 2019;31(3):131-137. doi:10.1097/CCO.0000000000000536
17. Brakenhoff RH, Wagner S, Klussmann JP. Molecular patterns and biology of HPV-associated HNSCC. Recent Results Cancer Res. 2017;206:37–56. doi:10.1007/978-3-319-43580-0_3
18. Schuler PJ, Laban S, Doescher J, Bullinger L, Hoffmann TK. Novel treatment options in head and neck cancer. Oncol Res Treat. 2017;40(6):342–346. doi:10.1159/000477254
19. Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the Phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838–3845. doi:10.1200/JCO.2016.68.1478
20. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386–396. doi:10.1016/j.mayocp.2015.12.017
21. Liang L, Fang JY, Xu J. Gastric cancer and gene copy number variation: emerging cancer drivers for targeted therapy. Oncogene. 2016;35(12):1475–1482. doi:10.1038/onc.2015.209
22. Liu J, Wu Y, Wang Q, Liu X, Liao X, Pan J. Bioinformatic analysis of PFN2 dysregulation and its prognostic value in head and neck squamous carcinoma. Future Oncol. 2018;14(5):449–459. doi:10.2217/fon-2017-0348
23. Tian C, Zhou S, Yi C. High NUP43 expression might independently predict poor overall survival in luminal A and in HER2+ breast cancer. Future Oncol. 2018;14(15):1431–1442. doi:10.2217/fon-2017-0690
24. Heng J, Zhang F, Guo X, et al. Integrated analysis of promoter methylation and expression of telomere related genes in breast cancer. Oncotarget. 2017;8(15):25442–25454. doi:10.18632/oncotarget.16036
25. Zhou C, Li J, Li Q. CDKN2A methylation in esophageal cancer: a meta-analysis. Oncotarget. 2017;8(30):50071–50083. doi:10.18632/oncotarget.18412
26. Zhou C, Ye M, Ni S, et al. DNA methylation biomarkers for head and neck squamous cell carcinoma. Epigenetics. 2018;13(4):398–409. doi:10.1080/15592294.2018.1465790
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.