Back to Journals » OncoTargets and Therapy » Volume 10
Overexpression of the cancer stem cell marker CD117 predicts poor prognosis in epithelial ovarian cancer patients: evidence from meta-analysis
Authors Yang B, Yan X, Liu L, Jiang C, Hou S
Received 9 March 2017
Accepted for publication 25 April 2017
Published 13 June 2017 Volume 2017:10 Pages 2951—2961
DOI https://doi.org/10.2147/OTT.S136549
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Bikang Yang,1,* Xuebing Yan,2,* Liguo Liu,3,* Chunyu Jiang,4 Shuping Hou1
1Department of Gynecology and Obstetrics, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 2Department of General Surgery, Shanghai Tenth People’s Hospital Affiliated to Tongji University, 3Department of General Surgery, 4Deparment of Radiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Purpose: Cancer stem cells have recently been identified as a key driving factor for tumor metastasis and chemoresistance. CD117 is a well-established cancer stem cell marker, but its clinical significance in epithelial ovarian cancer (EOC) remains controversial. Therefore, we aimed to identify correlations between CD117 expression and clinical features and outcomes in EOC patients in this meta-analysis.
Materials and methods: A literature search was performed in the PubMed, Cochrane Library, Web of Science, EMBASE, and OVID databases to identify eligible studies. Correlations between CD117 expression and clinicopathological parameters and overall survival or disease-free survival were analyzed. A subgroup analysis was then performed, which was classified by patient ethnicity and age at diagnosis, study sample size, and tumor histological type.
Results: A total of seven studies enrolling 1,247 EOC patients were included in this meta-analysis. Our results demonstrated that CD117 expression was significantly correlated with age (pooled odds ratio [OR] =1.67, 95% confidence interval [CI] =1.05–2.66), International Federation of Gynecology and Obstetrics stage (pooled OR =1.99, 95% CI =1.31–3.02), tumor differentiation grade (pooled OR =2.46, 95% CI =1.48–4.10), and histological type (pooled OR =1.85, 95% CI =1.05–3.26). EOC patients with high CD117 expression had significantly worse OS (hazard ratio [HR] =1.39, 95% CI =1.03–1.90) than patients with low CD117 expression. However, no significant correlation was found between CD117 expression and disease-free survival (HR =1.31, 95% CI =0.79–2.17). In subgroup analysis, CD117 was identified as a significant prognostic factor for overall survival in European patients (HR =1.59, 95% CI =1.13–2.23), younger patients (<60 years) (HR =1.59, 95% CI =1.10–2.30), studies with sample sizes >200 (HR =1.84, 95% CI =1.32–2.56), and the mixed histological types (HR =1.47; 95% CI =1.08–2.00).
Conclusion: Our meta-analysis suggests that CD117 is associated with EOC progression and can serve as a promising prognostic predictor for EOC patients. However, larger scale multicenter clinical trials are still needed to further validate our results.
Keywords: epithelial ovarian cancer, CD117, cancer stem cell, prognosis
Introduction
Worldwide, epithelial ovarian cancer (EOC) is a common gynecologic malignancy.1 In the USA, there will be an estimated 22,440 new EOC cases and 14,080 EOC-specific deaths in 2017.2 Currently, combined radical surgery and platinum-based adjuvant chemotherapy is standard treatment for EOC patients. With the rapid development of biotechnology, numerous breakthroughs have been achieved in the clinical management of EOC, such as precancerous screening programs, personalized neoadjuvant chemotherapy, and immunotherapy.3 Despite these encouraging advances, the overall 5-year relative survival rate of EOC patients is only around 46%, with 73% and 29% of patients experiencing regional and distant metastases, respectively.2 This clinical dilemma may largely reflect a lack of reliable biomarkers for early diagnosis, prognostic prediction, and targeted therapy. Recent studies have provided an increasing number of molecular candidates that have been proven to be involved in EOC development.4,5 However, most of them failed to be further developed as reliable indicators for clinical management owing to insufficient validation studies. Therefore, it is of great practical significance to conduct a comprehensive evidence-based analysis to clearly confirm their clinical utility in EOC.
Cancer stem cells (CSCs) are a distinct subpopulation of cancer cells known for their self-renewal and ability to generate heterogeneity.6 Recently, accumulating studies have identified CSCs as a crucial driving factor for EOC tumorigenesis and metastasis.7 Moreover, emerging evidence also suggests that CSCs contribute to chemotherapy resistance in EOC patients, thereby leading to worse disease-free survival (DFS).8,9 Experimental studies have revealed that CSC-induced malignant characteristics may be conditioned by the activation of oncogenic signaling pathways (such as Wnt signaling) or the epithelial–mesenchymal transition pathway.10,11 Specific cell surface markers, including CD44 and CD133, have been extensively investigated as a means of detecting CSCs, not only in EOC but also in other malignancies.12,13 In addition to CD44 and CD133, CD117 is commonly used to identify CSCs among EOC cells. CD117 is a type III receptor tyrosine kinase that regulates cell proliferation, apoptosis, and adhesion by binding to its ligand-stem cell factor.14 Recent studies have found that CD117 is abnormally overexpressed in various human malignancies and that it may play a crucial role in carcinogenesis and metastasis.15,16 With regard to its role in EOC, Zhang et al isolated highly tumorigenic and chemoresistant CSCs from primary human EOC tissues and characterized them as CD44+CD117+.17 Using serum-free medium, Yan et al identified CD117+CD133+ CSCs among the human EOC cell line HO8910 and found that this subpopulation was more likely to develop resistance to conventional chemotherapy agents including cisplatin, doxorubicin, and mitoxantrone.18 Therefore, CD117 is a crucial CSC marker in EOC that has the potential to be an effective therapeutic target.
Despite its role in promoting EOC development, the clinical significance of CD117 expression for EOC patients remains controversial. Some studies have indicated that CD117 is an adverse prognostic factor, while other studies found that CD117 expression is unrelated to clinical outcome or may even serve as a favorable prognostic factor.19–22 Thus, it is essential to make a comprehensive and objective evaluation of its clinical significance for EOC patients. To achieve this aim, we performed a meta-analysis of seven studies enrolling 1,247 EOC patients to identify correlations between CD117 and clinical features and patient prognosis. This effort will not only provide a novel insight into the clinical significance of CD117 expression in EOC, but also be of great benefit to biomarker-directed precision medicine for this disease.
Materials and methods
Search strategy
The online electronic databases PubMed, Cochrane Library, Web of Science, EMBASE, and OVID were searched for papers regarding clinical correlations between CD117 expression and EOC patients. The deadline for the literature search was December 1, 2016. The search terms used were as follows: “CD117,” “c-kit,” “tyrosine-protein kinase Kit,” “ovarian cancer,” “ovarian carcinoma,” “epithelial ovarian cancer,” “ovarian neoplasm,” “prognosis,” “prognostic,” and “survival.” Other potentially eligible publications were identified by reviewing references from the retrieved papers.
Selection criteria
The inclusion criteria for selecting studies for this meta-analysis were as follows: 1) all patients enrolled in the studies were pathologically confirmed as having EOC; 2) full-length papers provided sufficient data to evaluate CD117 expression and its correlation with patient features and/or prognosis; 3) CD117 expression was detected in the resected tumor tissues instead of other specimen types such as serum; 4) CD117 expression was detected using immunohistochemistry (IHC) instead of Western blot or reverse transcription-polymerase chain reaction; and 5) hazard ratios (HRs) and 95% confidence intervals (CIs) were available, or survival curves and/or P-values were given. No limitation was set for the minimum number of enrolled patients during the inclusion process. The exclusion criteria were as follows: 1) studies irrelevant to the research subject; 2) studies that did not involve clinical investigations and only contained cellular assays in vitro and/or in vivo; 3) reviews or letters without original data; 4) studies lacking accurate information about pathological diagnosis, clinicopathological parameters, or survival data; 5) follow-up periods <2 years; and 6) studies containing duplicate/unextracted clinical data.
Data extraction
All data were extracted and verified independently by two investigators based on the above selection criteria. The general data of the selected studies included first author and publication year. The clinical data of enrolled patients in each study included total number, age at diagnosis, country, follow-up time, histologic type, and International Federation of Gynecology and Obstetrics (FIGO) disease stage. Additionally, other essential details of each study were also extracted, including experimental methods (eg, IHC); IHC cutoff score; ratio of patients with high/low CD117 expression; type of survival analysis; and HRs and corresponding 95% CIs.
Statistical analysis
All statistical analyses were performed using the STATA Software v12.0 (STATACorp, College Station, TX, USA). Correlations between CD117 expression and clinicopathological parameters, including age FIGO stage, tumor differentiation grade, and histological type, were evaluated using odds ratio (OR) and 95% CI, while correlations with overall survival (OS) and DFS were evaluated using HR and 95% CI. An observed HR >1 suggested that high CD117 expression was associated with poor OS and/or DFS, while a 95% CI >1 indicated that the association was statistically significant. In cases where the HR and 95% CI were not provided directly by the authors, they were estimated using Engauge Digitizer Software v4.1 according to the Kaplan–Meier survival curve data. A random-effect or fixed-effect model was determined by the heterogeneity test across the studies, which was evaluated by the I2 value. A random-effect model was applied for significant heterogeneity, which was characterized as an I2 value of >50%, while a fixed-effect model was applied for the remaining cases. Publication bias was evaluated using Begg’s and Egger’s tests. For all analyses, a P-value <0.05 was considered statistically significant.
Results
Characteristics of the included studies
As shown in Figure 1, a total of 127 potentially relevant papers were initially identified from the electronic databases. Then, 51 papers were excluded owing to duplicate studies, and an additional 60 papers were excluded owing to irrelevant content (27), nonoriginal papers (8), or cellular assays alone (25). Finally, nine papers were excluded owing to insufficient or unextracted clinical data (8) or inconsistent experimental methods (1). The remaining seven papers were used in our meta-analysis.
  | Figure 1 Flowchart of the literature search process. |
The characteristics of these seven papers are summarized in Table 1.19,21–26 A total of 1,247 EOC patients from seven countries (Germany, Norway, Serbia, Finland, Austria, Canada, and the USA) were enrolled in our meta-analysis. Patient age ranged from 19 to 89 years, and the longest follow-up time was 420 months. Two papers provided complete HRs and 95% CIs in their survival analysis, while the other five papers did not, and so data were estimated by our researchers. Three papers used both OS and DFS in evaluating the prognostic value of CD117 expression in EOC patients, while the other four papers only used OS.
Correlations between CD117 expression and clinicopathological parameters
As shown in Figure 2 and Table 2, using the fixed-effect model, our meta-analysis indicated that CD117 expression was significantly correlated with FIGO stage (III/IV vs I/II, pooled OR =1.99, 95% CI =1.31–3.02, P=0.519, I2=0.00%) and histological type (serous vs mixed, pooled OR =1.85, 95% CI =1.05–3.26, P=0.212, I2=35.6%). Meanwhile, using the random-effect model, our meta-analysis demonstrated that CD117 expression was also correlated with age (>60 years vs <60 years, pooled OR =1.67, 95% CI =1.05–2.66, P=0.063, I2=71.0%) and tumor differentiation grade (poor vs well, pooled OR =2.46, 95% CI =1.48–4.10, P=0.008, I2=71.2%).
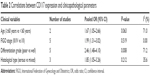  | Table 2 Correlations between CD117 expression and clinicopathological parameters |
Correlations between CD117 expression and patient prognosis
As shown in Figure 3A, using the random-effects model (I2=69.2%), our meta-analysis based on seven papers revealed that high CD117 expression was significantly correlated with poor OS in EOC patients (HR =1.39; 95% CI =1.03–1.90). However, when analyzing data from three papers (Figure 3B), we used the same model (I2=70.4%) to demonstrate that there was no statistically significant association between CD117 expression and DFS in EOC patients (HR =1.31; 95% CI =0.79–2.17).
To further identify whether CD117 could be a reliable prognostic biomarker for EOC patients, a subgroup analysis was performed based on patient ethnicity, age at diagnosis, sample size, and histological type. As shown in Table 3, CD117 expression was found to be significantly associated with poor OS in European patients (HR =1.59, 95% CI =1.13–2.23), younger patients (<60 years, HR =1.59, 95% CI =1.10–2.30), studies with sample sizes >200 (HR =1.84, 95% CI =1.32–2.56), and patients with mixed histological type (HR =1.47; 95% CI =1.08–2.00), but not in non-European patients (HR =0.83, 95% CI =0.52–1.34), older patients (≥60 years, HR =0.95, 95% CI =0.54–1.67), studies with sample sizes <200 (HR =1.13, 95% CI =0.95–1.34), and patients with serous type (HR =1.37, 95% CI =0.84–2.24).
  | Table 3 Prognostic significance of CD117 in subgroup analysis |
Publication bias analysis
The Begg’s and Egger’s tests were employed to evaluate publication bias in our meta-analysis, and the results are presented as funnel plots in Figure 4 and Table 4. No significant publication bias was observed in our meta-analysis regarding the correlation between CD117 expression and clinical features and patient OS/DFS (Begg’s test, Figure 4; Egger’s test, all P>0.05, Table 4).
  | Table 4 Egger’s test of funnel plot asymmetry |
Sensitivity analysis
To estimate the bias caused by the limited number of papers included in our meta-analysis, a sensitivity analysis was performed, and the results are displayed in Figure 5. We found that the pooled HRs of OS and DFS were not significantly changed owing to the omission of any single paper, demonstrating the statistical robustness of our results.
  | Figure 5 Sensitivity analyses of the studies assessing overall survival (A) and disease-free survival (B). |
Discussion
Recent studies have shown that CSC markers are promising therapeutic targets and prognostic biomarkers for EOC patients, largely owing to their driving role in metastasis and chemotherapy resistance.7 To further validate the clinical implications of the CSC marker, a meta-analysis based on previous literature was used to objectively assess correlations between its expression and clinical features/outcome in EOC patients. For example, in a meta-analysis enrolling 1,051 EOC patients, high CD133 expression was suggested to be associated with advanced tumor stage and reduced 2-year OS rate.27 A similar result was observed in another meta-analysis enrolling 2,161 EOC patients, which demonstrated CD44 expression was positively correlated with TNM stage and 5-year OS rate.28 With regard to CD117, to the best of our knowledge, no comprehensive evidence-based analysis is currently available to evaluate its clinical significance in EOC patients, although controversial correlations with clinical outcomes have been found in several recent studies.19,22 Therefore, it is necessary to perform a meta-analysis to identify whether CD117 can serve as a predictive biomarker for the clinical management of EOC patients.
In this study, a meta-analysis enrolling 1,247 EOC patients was carried out to assess correlations between CD117 expression and clinical features and postoperative survival. We found that CD117 expression was significantly correlated with FIGO stage and tumor differentiation grade, suggesting that CD117 might be involved in the malignant progression of EOC. This speculation can be partly supported by a previous study that demonstrated that CD117 enhanced the tumorigenicity of EOC cells by conferring a CSC phenotype.29 Additionally, other mechanistic investigations have revealed that CD117 might contribute to EOC development by inducing epithelial–mesenchymal transition, a well-established molecular event that is involved in cell invasion and metastasis.30,31 Chene et al recently even have detected high expression of CD117 in the premalignant lesions of EOC, suggesting its potential role as a diagnostic marker for early ovarian tumorigenesis.32 Collectively, these data suggested that CD117 might serve not only as a helpful indicator for EOC progression but also as a potential target for EOC treatment.
Given its positive correlation with EOC development, it is reasonable to propose that CD117 expression might have some prognostic significance for EOC patients. Based on the extracted survival data from the seven included studies, our meta-analysis demonstrated that CD117 overexpression is significantly associated with worse postoperative OS, suggesting that it is an adverse prognostic factor for EOC patients. However, this conclusion is inconsistent with two included studies, which indicated that CD117 expression was uncorrelated or positively correlated with postoperative OS.21,22 Therefore, we subsequently performed a subgroup meta-analysis to further identify correlations between CD117 expression and the OS of EOC patients. We found that CD117 expression was significantly correlated with OS in European patients but not in non-European patients, suggesting that its prognostic value may vary with ethnicity. This is not the first study to report the prognostic inconsistency of CSC markers in EOC patients within different ethnicities. For example, Liu et al recently reported that the CSC marker aldehyde dehydrogenase is correlated with OS and DFS in Asian EOC patients, but not in non-Asian EOC patients.33 In addition to CSC markers, prognostic inconsistency caused by patient ethnicity has also been found for other molecular biomarkers, such as Matrix metalloproteinase-9 (MMP-9), a well-established molecule that regulates cell invasion and migration.34 Based on these data, we further hypothesized that CD117 may be a specific prognostic biomarker for European EOC patients. However, large-scale multicenter retrospective studies will be required to validate this hypothesis. In addition, we found that CD117 is a significant prognostic factor in younger patients, implying that its prognostic significance may also depend on age at diagnosis. Moreover, a significant correlation between CD117 expression and OS was only observed in studies enrolling >200 patients, strongly supporting the necessity of utilizing large sample sizes in future clinical validations. Finally, we noted that the prognostic impact of CD117 was contradictory in two included studies only enrolling patients with stage III and IV disease, indicating that its clinical utility for late-stage EOC also requires more related validations.21,26
Chemotherapy resistance is a major threat to EOC prognosis, and accumulating evidence suggests a close link between chemoresistance and the presence of CSCs.35,36 Thus, detecting CSC markers in EOC patients can help predict benefits of chemotherapy to some degree. For example, Conic et al have recently found that EOC patients with positive CD117 expression were more likely to resist paclitaxel/carboplatin treatment, resulting in a higher recurrence than patients negative for CD117 expression.19 However, by analyzing CSCs isolated from EOC patients, Ayub et al found that CD117 was uncorrelated with platinum resistance and OS, although it was identified as a significant factor affecting recurrence.21 In our meta-analysis of the three available studies, we found that CD117 expression was uncorrelated with DFS in EOC patients. DFS is a widely used criteria for evaluating chemotherapy response; thus, our results suggested that CD117 may not be a predictive factor for assessing the chemotherapy benefits for EOC patients, which is somewhat inconsistent with our knowledge about CSC markers. However, considering the limited number of studies (n=3) included in our analysis, we are unable to make a definite conclusion with regard to that, and therefore strongly advocate for more clinical validations based on large-scale samples.
It must be noted that there are several inherent limitations to our meta-analysis. First, the number of included studies was relatively small, which can be attributed to two reasons: 1) we restricted studies to those published in English, which may have omitted potentially eligible studies published in other languages. Furthermore, compared with other CSC markers, such as CD133 and CD44, CD117 is relatively poorly investigated, not only in EOC but also in other cancer types. Therefore, relevant studies were limited. 2) There are various inherent factors affecting study heterogeneity, such as patient selection, experiment procedures, and staining evaluation. This deficiency is expected to be improved by stricter selection criteria upon sufficient available studies in our following work. Finally, a considerable part of the patient survival data was not provided in the included studies, and therefore was estimated by a digitizing software, which may also be partly responsible for analytical deviations in our study.
Conclusion
In conclusion, our findings demonstrated that CD117 expression is positively correlated with advanced FIGO stage and tumor differentiation grade in EOC patients. In addition, we also found that EOC patients with high CD117 expression have significantly worse OS than those with low CD117 expression. Collectively, these data suggest that CD117 is not only a promising therapeutic target but also a potential prognostic indicator for EOC patients. Larger scale multicenter clinical trials are strongly advocated to further validate our meta-analysis.
Acknowledgments
This study was supported by funding from the Shanghai Health and Family Planning Commission (No 201640370). We are deeply appreciative of Professor Shu-ping Hou for her crucial guidance in study design and manuscript preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. | ||
Suh DH, Kim M. Major clinical research advances in gynecologic cancer in 2015. J Gynecol Oncol. 2016;27(6):e53. | ||
Mills K, Fuh K. Recent advances in understanding, diagnosing, and treating ovarian cancer. F1000Research. 2017;6:84. | ||
Bai H, Cao D, Yang J, Li M, Zhang Z, Shen K. Genetic and epigenetic heterogeneity of epithelial ovarian cancer and the clinical implications for molecular targeted therapy. J Cell Mol Med. 2016;20(4):581–593. | ||
Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16(1):41. | ||
Ozakpinar OB, Maurer AM, Ozsavci D. Ovarian stem cells: from basic to clinical applications. World J Stem Cells. 2015;7(4):757–768. | ||
Ren F, Shen J, Shi H, Hornicek FJ, Kan Q, Duan Z. Novel mechanisms and approaches to overcome multidrug resistance in the treatment of ovarian cancer. Biochim Biophys Acta. 2016;1866(2):266–275. | ||
Walters Haygood CL, Arend RC, Straughn JM, Buchsbaum DJ. Ovarian cancer stem cells: Can targeted therapy lead to improved progression-free survival? World J Stem Cells. 2014;6(4):441–447. | ||
Tomao F, Papa A, Strudel M, et al. Investigating molecular profiles of ovarian cancer: an update on cancer stem cells. J Cancer. 2014;5(5):301–310. | ||
Chen X, Zhang J, Zhang Z, Li H, Cheng W, Liu J. Cancer stem cells, epithelial-mesenchymal transition, and drug resistance in high-grade ovarian serous carcinoma. Hum Pathol. 2013;44(11):2373–2384. | ||
Thapa R, Wilson GD. The importance of CD44 as a stem cell biomarker and therapeutic target in cancer. Stem Cells Int. 2016;2016:2087204. | ||
Schmohl JU, Vallera DA. CD133, selectively targeting the root of cancer. Toxins (Basel). 2016;8(6): pii: E165. | ||
Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13(3): 205–220. | ||
Zhao F, Chen Y, Wu Q, Wang Z, Lu J. Prognostic value of CD117 in cancer: a meta-analysis. Int J Clin Exp Pathol. 2014;7(3):1012–1021. | ||
Giusca SE, Caruntu ID, Cimpean AM, et al. Tryptase-positive and CD117 positive mast cells correlate with survival in patients with liver metastasis. Anticancer Res. 2015;35(10):5325–5331. | ||
Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68(11):4311–4320. | ||
Yan HC, Fang LS, Xu J, et al. The identification of the biological characteristics of human ovarian cancer stem cells. Eur Rev Med Pharmacol Sci. 2014;18(22):3497–3503. | ||
Conic I, Stanojevic Z, Jankovic Velickovic L, et al. Epithelial ovarian cancer with CD117 phenotype is highly aggressive and resistant to chemotherapy. J Obstet Gynaecol Res. 2015;41(10):1630–1637. | ||
Stemberger-Papic S, Vrdoljak-Mozetic D, Ostojic DV, et al. Expression of CD133 and CD117 in 64 serous ovarian cancer cases. Coll Antropol. 2015;39(3):745–753. | ||
Ayub TH, Keyver-Paik MD, Debald M, et al. Accumulation of ALDH1-positive cells after neoadjuvant chemotherapy predicts treatment resistance and prognosticates poor outcome in ovarian cancer. Oncotarget. 2015;6(18):16437–16448. | ||
Tonary AM, Macdonald EA, Faught W, Senterman MK, Vanderhyden BC. Lack of expression of c-KIT in ovarian cancers is associated with poor prognosis. Int J Cancer. 2000;89(3):242–250. | ||
Huang R, Wu D, Yuan Y, et al. CD117 expression in fibroblasts-like stromal cells indicates unfavorable clinical outcomes in ovarian carcinoma patients. PloS One. 2014;9(11):e112209. | ||
Lassus H, Sihto H, Leminen A, et al. Genetic alterations and protein expression of KIT and PDGFRA in serous ovarian carcinoma. Br J Cancer. 2004;91(12):2048–2055. | ||
Brustmann H. Immunohistochemical detection of human telomerase reverse transcriptase (hTERT) and c-kit in serous ovarian carcinoma: a clinicopathologic study. Gynecol Oncol. 2005;98(3):396–402. | ||
Khalifeh I, Munkarah AR, Schimp V, Morris R, Lawrence WD, Ali-Fehmi R. The impact of c-kit and ki-67 expression on patients prognosis in advanced ovarian serous carcinoma. Int J Gynecol Pathol. 2005;24(3):228–234. | ||
Zhou Q, Chen A, Song H, Tao J, Yang H, Zuo M. Prognostic value of cancer stem cell marker CD133 in ovarian cancer: a meta-analysis. I Int J Clin Exp Med. 2015;8(3):3080–3088. | ||
Lin J, Ding D. The prognostic role of the cancer stem cell marker CD44 in ovarian cancer: a meta-analysis. Cancer Cell Int. 2017;17:8. | ||
Luo L, Zeng J, Liang B, et al. Ovarian cancer cells with the CD117 phenotype are highly tumorigenic and are related to chemotherapy outcome. Exp Mol Pathol. 2011;91(2):596–602. | ||
Zhang R, Zhang P, Wang H, et al. Inhibitory effects of metformin at low concentration on epithelial-mesenchymal transition of CD44(+)CD117(+) ovarian cancer stem cells. Stem Cell Res Ther. 2015;6:262. | ||
Cao L, Shao M, Schilder J, Guise T, Mohammad KS, Matei D. Tissue transglutaminase links TGF-beta, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene. 2012;31(20):2521–2534. | ||
Chene G, Ouellet V, Rahimi K, et al. Expression of stem cell markers in preinvasive tubal lesions of ovarian carcinoma. Biomed Res Int. 2015;2015:808531. | ||
Liu S, Liu C, Min X, et al. Prognostic value of cancer stem cell marker aldehyde dehydrogenase in ovarian cancer: a meta-analysis. PloS One. 2013;8(11):e81050. | ||
Li LN, Zhou X, Gu Y, Yan J. Prognostic value of MMP-9 in ovarian cancer: a meta-analysis. Asian Pac J Cancer Prev. 2013;14(7):4107–4113. | ||
Deng J, Wang L, Chen H, et al. Targeting epithelial-mesenchymal transition and cancer stem cells for chemoresistant ovarian cancer. Oncotarget. 2016;7(34):55771–55788. | ||
Wang X, Li X, Fu X, et al. Eliminating ovarian cancer stem cells: a potential therapeutic target for ovarian cancer chemoresistance. Curr Protein Pept Sci. 2015;16(4):270–278. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.