Back to Journals » Cancer Management and Research » Volume 13
Overexpression of Nucleolin is a Potential Prognostic Marker in Endometrial Carcinoma
Authors Lin Q, Ma X, Hu S, Li R, Wei X, Han B, Ma Y, Liu P , Pang Y
Received 7 December 2020
Accepted for publication 5 February 2021
Published 25 February 2021 Volume 2021:13 Pages 1955—1965
DOI https://doi.org/10.2147/CMAR.S294035
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chien-Feng Li
Qianhan Lin,1,2,* Xiaoxue Ma,1,2,* Shunxue Hu,3 Rui Li,1,2 Xuan Wei,1,2 Bing Han,1 Yanhui Ma,1 Peishu Liu,1,2,4 Yingxin Pang1,2,4
1Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Jinan, 250012, Shandong, People’s Republic of China; 2Key Laboratory of Gynecologic Oncology of Shandong Province, Jinan, 250012, Shandong, People’s Republic of China; 3Department of Pathology, Qilu Hospital of Shandong University, Jinan, 250012, Shandong, People’s Republic of China; 4Shandong Engineering Laboratory for Urogynecology, Jinan, 250012, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peishu Liu
Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Jinan, 250012, Shandong, People’s Republic of China
Tel +8618560081988
Email [email protected]
Yingxin Pang
Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Jinan, 250012, Shandong, People’s Republic of China
Tel +8618560081127
Email [email protected]
Purpose: Nucleolin (NCL) is a multifunctional protein with oncogenic properties. NCL expression levels have been linked to the outcomes of various malignancies, but the clinical value of NCL in patients with endometrial carcinoma (EC) remains unclear. Here, the expression of NCL in EC tissues and its associations with patient outcomes were assessed.
Patients and Methods: Data on NCL mRNA expression in EC and adjacent nonneoplastic tissues from The Cancer Genome Atlas (TCGA) were analyzed. In addition, NCL protein expression in 82 endometroid endometrial adenocarcinoma tissues and 15 non-malignant tissues was detected by immunohistochemistry.
Results: Elevated NCL expression was markedly correlated with serous endometrial carcinoma (P< 0.001), advanced stage (P=0.029), and grade 3 (P< 0.001). High NCL levels were associated with poorer overall survival (OS) and disease-free survival (DFS) compared with intermediate or low NCL levels (OS: P=0.001, DFS: P=0.006). The multivariate Cox proportional hazards model showed that NCL expression was an independent poor prognostic factor for DFS (HR=1.282, CI=1.027– 1.601, P=0.028). A similar correlation between high expression levels of NCL and unfavorable DFS was found in endometrioid endometrial adenocarcinoma (HR=1.411, CI=1.083– 1.840, P=0.011). Positive extra-nuclear NCL expression (HR=3.377, 95% CI=1.029– 11.186, P=0.046) and low nuclear NCL expression (HR=0.233, 95% CI=0.068– 0.796, P=0.020) were independent prognostic factors for DFS in endometrioid endometrial adenocarcinoma.
Conclusion: Heterotopic NCL is a potential prognostic biomarker for EC. Inhibiting the distribution of NCL from the nucleus to the cytoplasm and membrane may be a promising therapeutic strategy to improve outcomes in patients with EC with high NCL expression.
Keywords: nucleolin, endometrial carcinoma, prognostic marker, TCGA
Introduction
Endometrial carcinoma (EC) is one of the most common gynecologic cancers, with an estimated 65,620 new cases and 12,590 related deaths in the United States in 2020.1 Moreover, the incidence rate and mortality rate of EC have steadily increased over the past decade. Risk stratification for EC is based on tumor stage, tumor grade and histological type according to the Federation International of Gynecology and Obstetrics (FIGO) staging system, and the mainstay treatment for high-risk EC is staging surgery in combination with adjuvant chemotherapy and radiotherapy. Another promising therapeutic approach for EC is immunotherapy, which targets several biological pathways. For example, blockade of immune checkpoints such as programmed cell death-1 (PD-1) and its ligand (PD-L1 or B7-H1) mainly targets patients with microsatellite instability-high (MSI-H)/mismatch repair (MMR)-deficient tumors,2,3 which represent approximately 30% of primary ECs.4 However, the clinical applications of immunotherapy have been relatively limited, and more clinical trials are required. Despite progress in EC treatment, survival time and quality of life have not significantly improved.5 The different disease outcomes of patients with similar clinicopathological characteristics may in part reflect molecular heterogeneity in tumor invasion and metastasis.6
Although knowledge of the molecular alterations involved in EC is expanding, many questions remain unanswered. Therefore, it is important to explore new molecular markers and identify potential therapeutic targets. Nucleolin (NCL), a multifunctional protein, is a member of the most abundant non-ribosomal phosphoprotein family in the nucleolus and plays important roles in ribosome formation, rRNA processing and mRNA stabilization.7 Several studies have found that NCL may be associated with tumor development and progression.8–12 The NCL protein is usually localized in the nucleus in normal tissues, and its abnormal expression in the cytoplasm and cell membrane in malignant tumors typically predicts poor prognosis. Abnormal expression of NCL has been related to poor survival in a variety of cancers, including breast cancer, gastric cancer, liver cancer, acute myeloid leukemia, non-small cell lung cancer, and pancreatic ductal adenocarcinoma.13–18 The mechanisms underlying this association may include regulation of cancer cell proliferation, apoptosis, angiogenesis, invasion and metastasis.8,9,18–25 Thus, NCL may be a promising target for anti-tumor therapy, and in recent years, progress has been made in aptamers, polypeptides and immune agents targeting NCL.8,9,26–30
The role of NCL in EC has not been clearly established. To assess the potential of NCL as a prognostic factor for EC, we examined NCL expression in EC tissues at the mRNA and protein levels. Moreover, the relationships between NCL expression levels and clinicopathological characteristics and the prognostic value of NCL in EC were evaluated. Our results indicate that NCL overexpression may serve as a prognostic biomarker in EC.
Patients and Methods
Patients and Tissue Samples
RNA-seq expression data (downloaded count format) and clinical data of 575 cases were downloaded in October 2019 from the official website for the Uterine Corpus Endometrial Carcinoma (UCEC) project of The Cancer Genome Atlas (TCGA) by utilizing GDC-client.exe software. When multiple samples were available for the same patient, only the sample with the highest gene expression was retained. Accordingly, twenty-three normal tissue samples and different samples of the same patient were excluded, as were patients with follow-up periods of less than 30 days or longer than 8 years. Patients in whom cancer recurred or who died within 30 days of surgery were also excluded. Finally, 494 patients in the TCGA dataset were included in this analysis. NCL expression was normalized using the R package “DESeq2”.
Paraffin sections of 82 endometrioid endometrial adenocarcinoma tissues and 15 non-malignant tissues collected from February 2010 to December 2014 were selected from the Department of Pathology of Qilu Hospital, Cheelloo College of Medicine, Shandong University. The study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University, and all samples were completely anonymized. Follow-up data of all patients were obtained by interview or telephone.
Immunohistochemical Analysis
Tissue sections with a thickness of 4 μm were cut from the tissue blocks and placed on slides. The sections were dewaxed with xylene and rehydrated in a series of concentrations of ethanol. Endogenous peroxidase activity was blocked by treatment with hydrogen peroxide, and the sections were incubated with goat serum for 30 min to block non-specific antigens. The sections were then incubated with nucleolin antibody (1:100, ab129299, Abcam, Cambridge, UK) overnight at 4°C. After successive incubation with biotin-labeled goat anti-rabbit IgG polymer for 30 min and horseradish peroxidase-conjugated streptomycin, positive signals were detected using a DAB substrate (Beijing Zhongshan Jinqiao Biotechnology, Beijing, China) following the manufacturer’s recommendations and quantified by Image-Pro Plus software (Media Cybernetics, USA). Stained tissue specimens were evaluated and scored by two blinded gynecologic pathologists. Strong positive staining was defined as a brown signal; medium staining as a yellow-brown reaction; and weak staining as a light-yellow reaction. The staining intensity was graded as follows: 0 = no staining; 1 = weak staining; 2 = moderate staining; 3 = strong staining. The percentage of tumor cells stained was graded according to the following criteria: 0 = no staining; 1 = ≤10% positive tumor cells; 2 = 11–50% positive tumor cells; 3 = 51–80% positive tumor cells; 4 = ≥ 81% positive tumor cells. The staining score of nuclear NCL was calculated as the sum of the staining intensity grade and staining percentage grade, with a range of 0 to 7. A score of 0 was defined as negative expression, scores of 1–3 as 1+, scores of 4–5 as 2+, and scores of 6–7 as 3+. To facilitate statistical evaluation, nuclear NCL expression levels were further reclassified according to a three-level semi-quantitative scheme: negative expression, expression level 0; low expression, expression level 1+ and 2+; high expression, expression level 3+. Immunostaining for extra-nuclear NCL was considered positive if it was observed in the membrane and/or cytoplasm in >10% of tumor cells.
Statistical Analysis
Statistical analyses were performed using GraphPad 8 and IBM SPSS 21.0 software. Between-group comparisons were performed with the Chi-squared or Fisher’s exact test for categorical data and the t-test, one-way ANOVA or nonparametric Kruskal–Wallis test for continuous data. Overall survival (OS) was measured from the date of diagnosis to death or last follow-up. Disease-free survival (DFS) was determined from the time of diagnosis to recurrence, death or last follow-up. OS and DFS were estimated with the Kaplan–Meier method and analyzed with the Log-rank Mantel-Cox test. Multivariate Cox models were applied to parameters that differed significantly in univariate analyses to determine if the expression level of NCL was an independent marker of EC survival. Statistical significance was defined as P<0.05.
Results
Association of NCL mRNA Levels with Clinicopathologic Characteristics in TCGA
RNA-seq and clinical data of 494 patients with endometrial carcinoma were downloaded from the official website for the Uterine Corpus Endometrial Carcinoma (UCEC) project of TCGA and analyzed. To define true “high levels” and “low levels”, the EC patients in the dataset were divided equally into three groups based on the expression of low (n=165), intermediate (n=165), or high levels (n=164) of NCL.
The clinicopathologic characteristics of the 494 patients with EC are summarized in Table 1. Endometrioid endometrial adenocarcinoma was most common (76.3%), followed by endometrial adenocarcinoma (19.4%) and mixed EC (4.3%). The age range of the patients was 31–90 (median 63). Grade 3 EC (56.3%) and stage I EC (62.6%) were the most common, and most patients underwent open surgery (57.5%). Other clinical characteristics are detailed in Table 1.
 |
Table 1 Patients' Information in the TCGA Cohorts |
As shown in Table 2, compared with low or medium group, high NCL expression was significantly related to serous endometrial adenocarcinoma (P<0.001), higher stage (P=0.029), and grade 3 (P<0.001). Moreover, when analyzed as a continuous variable, patients diagnosed with serous endometrial carcinoma (P<0.001), higher stage (P<0.01), or grade 3 (P<0.001) had higher expression of NCL (Figure 1A–C).
 |
Table 2 Associations of NCL Expression with Clinicopathologic Characteristics |
Association of NCL mRNA Levels with Overall and Disease-Free Survival in EC
Evaluation of the prognostic value of NCL in EC by Kaplan–Meier survival analysis revealed an obvious relationship between NCL mRNA expression levels and patient prognosis. Patients with tumors expressing high levels of NCL mRNA had poorer overall survival (OS) than patients with tumors expressing intermediate or low levels of NCL (P=0.001) (Figure 2A). A similar tendency was observed for DFS (P=0.006) (Figure 2B).
We further analyzed the expression of NCL in endometrioid endometrial adenocarcinoma, the most common pathology type. Compared with the high NCL expression group, the intermediate and low expression groups exhibited better OS (P=0.019) and DFS (P=0.010) in endometrioid endometrial adenocarcinoma (Figure 2C and D).
Furthermore, subgroup analyses based on pathologic grade and FIGO stage demonstrated that the high NCL group had poor OS (P=0.037) and DFS (P=0.013) for grade 1 or 2 tumors (Figure 3A and B). There was also a significant negative association of NCL expression levels with OS (P=0.037) in stage I or II tumors (P=0.012) (Figure 3C).
Prognostic Value of NCL mRNA Levels in EC
In univariate analysis, NCL expression levels corresponded to poor EC prognosis in terms of both OS (HR=1.717, 95% CI=1.250–2.359, P=0.001) and DFS (HR=1.404, 95% CI=1.134–1.739, P=0.002). Other clinicopathologic characteristics associated with poor survival included serous EC and high grade (Table 3). In multivariate COX regression, NCL was confirmed as an independent prognostic marker for DFS (HR=1.282, CI=1.027–1.601, P=0.028) (Table 4).
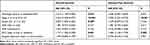 |
Table 3 Cox Regression Analysis of Associations of NCL Expression with Survival and Clinicopathologic Characteristics |
 |
Table 4 Multivariate Survival Model After Variable Selection |
As shown in Table 5, a similar correlation between high expression levels of NCL and unfavorable DFS was found for endometrioid endometrial carcinoma (HR=1.411, CI=1.083–1.840, P=0.011).
 |
Table 5 Univariate and Multivariate Analysis of Disease-Free Survival in Patients with Endometrioid Endometrial Adenocarcinoma |
Immunohistochemical Analysis of NCL Protein Levels and Clinicopathologic Characteristics
NCL is usually expressed in the nucleus. However, ectopic expression of NCL in the cytoplasm and cell membrane has been observed in some cancer cells. Heterotopic NCL may play a critical role in tumor development and progression. To clarify the expression level and localization of the NCL protein in EC, immunohistochemical analyses were performed.
We evaluated pathological sections from 82 patients with endometrioid endometrial adenocarcinoma; the clinicopathologic characteristics of the patients are detailed in Table 6. Patients with grade 1 tumors were most common (50%), and those with grade 3 tumors were least common (15.9%). Deep myometrial invasion was observed in 28%, and cervical invasion was noted in 9.8%. The age range of the 15 patients with non-malignant disease was 45–69 (median 59). Of these 15 patients, most were diagnosed with uterine myoma (46.7%) or adenomyosis (33.3%), and 3 were diagnosed with abnormal uterine bleeding (AUB) (20%). Most patients underwent open surgery (86.7%). Nuclear staining for NCL was observed in both non-malignant tissues and cancer tissues, and high expression of NCL in the nucleus was found in 55 (67.1%) of cancer tissues. Extra-nuclear NCL expression was absent in non-malignant tissues (Figure 4) but was positive in cancer tissues from 34 (41.5%) patients (Table 6).
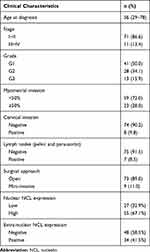 |
Table 6 Clinicopathologic Characteristics |
Prognostic Value of NCL Protein Level and Localization in Endometrioid Endometrial Adenocarcinoma
Kaplan–Meier survival analysis was performed to evaluate the prognostic value of the expression level and localization of the NCL protein in endometrioid endometrial adenocarcinoma, as shown in Figure 5. Patients with tumors with low nuclear expression levels of NCL had poorer DFS than patients with high nuclear expression levels (P=0.001) (Figure 5A). We also found a correlation between positive extra-nuclear expression of NCL and unfavorable DFS (P=0.031) (Figure 5B).
As shown in Table 7, in univariate survival analysis, patients with positive extra-nuclear NCL expression had a higher risk of an unfavorable outcome (DFS: HR=3.398, 95% CI=1.046–11.038, P=0.042), and patients expressing high nuclear NCL exhibited better prognosis (DFS: HR=0.178, 95% CI=0.055–0.580, P=0.004). Multivariate analysis showed that positive extra-nuclear NCL expression (HR=3.377, 95% CI=1.029–11.186, P=0.046) and low nuclear NCL expression (HR=0.233, 95% CI=0.068–0.796, P=0.020) were independent prognostic factors for shorter DFS in endometrioid endometrial adenocarcinoma.
 |
Table 7 Predictors of Disease-Free Survival in Patients with Endometrioid Endometrial Adenocarcinoma |
Discussion
Nucleolin (NCL) is a multifunctional protein that was first discovered in the nucleus.12 NCL is ectopically expressed in the cytoplasm and cell membrane in several pathological conditions, especially tumorigenesis,7 giving rise to the idea that a drug targeting NCL could be useful for the treatment of cancer.31 The main functions of nuclear NCL are controlling DNA and RNA metabolism, ribosome biogenesis and chromatin organization and stability,32 but NCL is also involved in cell growth and proliferation, cytokinesis, cell apoptosis and cytokinesis.33
Our analysis of TCGA data revealed that overexpression of NCL mRNA in EC is related to clinicopathologic features, including pathologic type, stage and histologic grade, and multivariate analysis further suggested that NCL mRNA overexpression is an independent poor prognostic factor. Disordered accumulation of NCL has also been related to poor survival in other human cancers, including hepatocellular carcinoma, acute myeloid leukemia, and pancreatic ductal adenocarcinoma.15,16,18 Given that endometrioid endometrial adenocarcinoma represents 75–80% of all ECs, we next focused on NCL protein expression in endometrioid endometrial adenocarcinoma. We found that high expression of NCL indicated poor OS and DFS in grade 1 or 2 tumors and early stages (stage I and II). As certain early-stage patients have a high risk of recurrence, NCL expression could be used to guide adjuvant therapy in low-risk endometrial cancer for those patients with early stage or low grade.
Immunohistochemical analysis showed that endometrioid endometrial cancer cells expressed NCL in both the cytoplasm and nucleus, whereas normal endometrial cells lacked NCL expression in the cytoplasm. Thus, heterotopic NCL may promote the development and progression of tumors. Moreover, positive extra-nuclear NCL expression and lower nuclear NCL expression were related to poor prognosis. These observations are consistent with previous findings that high nucleolar expression of NCL is an independent prognostic marker for better survival in patients with gastric cancer, while high cytoplasmic staining is closely associated with worse prognosis.14 Similar results have also been observed in patients with non-small cell lung cancer.11
In general, these findings indicate that the function of NCL is tightly dependent on its subcellular location. Cytoplasmic NCL plays important roles in the assembly of ribosomal subunits by directly interacting with a subset of ribosomal proteins and RNA.34 Moreover, NCL functions as a shuttling protein that mediates the nucleolar-cytoplasmic crosstalk of ribosomal proteins and subunits.7 NCL binds specifically to the ARE-1 instability element in the 3′-UTR of bcl2 mRNA to protect bcl2 mRNA from ribonuclease degradation, thereby inhibiting apoptosis and promoting the proliferation of tumor cells.35–37 NCL also binds to the 5ʹ-3ʹ-UTR base-pairing region of p53 mRNA, which is critical for the recruitment of RPL26 and the suppression of protein translation under DNA damage conditions.38 Conversely, NCL decreases the expression of AKT1 by binding to both the 5′- and 3′-UTRs of AKT1 mRNA. Activation of AKT 1 promotes glioma invasiveness, angiogenesis and migration.39
In addition to its utility as a biomarker in tumor diagnosis, the potential role of NCL in tumor treatment has received extensive attention in recent years. AS1411, the first DNA aptamer designed to bind NCL, has reached Phase I and II clinical trials for cancer treatment. AS1411 interacts with NCL on the cell surface, induces cell apoptosis and cycle arrest, and inhibits cell viability by up-regulating p53 and down-regulating Bcl-2 and Akt1 in various cancer cells.8,9 Moreover, AS1411 can be conjugated with cytotoxic agents to enable selective delivery to targeted cancer cells. The NCL-targeting peptide N6l has dual anticancer effects by inhibiting lymph angiogenesis and tumor development,28 and the antinucleotide immune agent 4LB5-HP-RNase reduces both the growth rate and survival rate of triple-negative breast cancer cells.29,30 We anticipate that adopting multiple approaches to target NCL would improve prognosis or enhance the effects of chemotherapy for EC.
Taken together, the findings of the present study support NCL as a potential prognostic biomarker for EC. Inhibiting the distribution of NCL to the cytoplasm and membrane may represent a new therapeutic opportunity for EC.
Conclusion
Positive expression of NCL in the cytoplasm and cell membrane is associated with poor outcomes in endometrioid endometrial adenocarcinoma, while low nuclear NCL levels are associated with unfavorable prognosis. Moreover, the prognostic value of NCL expression is more pronounced in patients with early-stage or low-grade EC. Our study suggests that heterotopic NCL is a potential prognostic biomarker for EC.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Qilu Hospital of Shandong University. All patients provided informed consent before data collection. We ensured that the data were anonymized before analysis to maintain confidentiality.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Qianhan Lin and Xiaoxue Ma contributed equally to the manuscript.
Funding
This work was supported by the National Nature Science Foundation of China (81702559 and 81902657), the China Postdoctoral Science Fund (21510077311145 and 21300076311047), and the Science Foundation of Qilu Hospital of Shandong Province.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Di Tucci C, Capone C, Galati G, et al. Immunotherapy in endometrial cancer: new scenarios on the horizon. J Gynecol Oncol. 2019;30(3):e46. doi:10.3802/jgo.2019.30.e46
3. Oaknin A, Leon-Castillo A, Lorusso D. Progress in the management of endometrial cancer (subtypes, immunotherapy, alterations in PIK3CA pathway): data and perspectives. Curr Opin Oncol. 2020;32(5):471–480. doi:10.1097/CCO.0000000000000658
4. Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:2017. doi:10.1200/PO.17.00073
5. Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978–2013. J Natl Cancer Inst. 2018;110(4):354–361. doi:10.1093/jnci/djx214
6. Banno K, Kisu I, Yanokura M, et al. Epigenetics and genetics in endometrial cancer: new carcinogenic mechanisms and relationship with clinical practice. Epigenomics. 2012;4(2):147–162. doi:10.2217/epi.12.13
7. Berger CM, Gaume X, Bouvet P. The roles of nucleolin subcellular localization in cancer. Biochimie. 2015;113:78–85. doi:10.1016/j.biochi.2015.03.023
8. Cheng Y, Zhao G, Zhang S, et al. AS1411-induced growth inhibition of glioma cells by up-regulation of p53 and down-regulation of Bcl-2 and Akt1 via nucleolin. PLoS One. 2016;11(12):e0167094. doi:10.1371/journal.pone.0167094
9. Subramanian N, Srimany A, Kanwar JR, et al. Nucleolin-aptamer therapy in retinoblastoma: molecular changes and mass spectrometry-based imaging. Mol Ther Nucleic Acids. 2016;5(8):e358. doi:10.1038/mtna.2016.70
10. Bose S, Tholanikunnel TE, Reuben A, Tholanikunnel BG, Spicer EK. Regulation of nucleolin expression by miR-194, miR-206, and HuR. Mol Cell Biochem. 2016;417(1–2):141–153. doi:10.1007/s11010-016-2721-2
11. Xu J-Y, Lu S, Xu X-Y, et al. Prognostic significance of nuclear or cytoplasmic nucleolin expression in human non-small cell lung cancer and its relationship with DNA-PKcs. Tumour Biol. 2016;37(8):10349–10356. doi:10.1007/s13277-016-4920-6
12. Orrick LR, Olson MO, Busch H. Comparison of nucleolar proteins of normal rat liver and Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1973;70(5):1316–1320. doi:10.1073/pnas.70.5.1316
13. Nguyen Van Long F, Lardy-Cleaud A, Bray S. Druggable nucleolin identifies breast tumours associated with poor prognosis that exhibit different biological processes. Cancers (Basel). 2018;10(10):390. doi:10.3390/cancers10100390
14. Qiu W, Zhou F, Zhang Q, et al. Overexpression of nucleolin and different expression sites both related to the prognosis of gastric cancer. APMIS. 2013;121(10):919–925. doi:10.1111/apm.12131
15. Guo X, Xiong L, Yu L, et al. Increased level of nucleolin confers to aggressive tumor progression and poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Diagn Pathol. 2014;9(1):175. doi:10.1186/s13000-014-0175-y
16. Marcel V, Catez F, Berger CM, et al. Expression profiling of ribosome biogenesis factors reveals nucleolin as a novel potential marker to predict outcome in AML patients. PLoS One. 2017;12(1):e0170160. doi:10.1371/journal.pone.0170160
17. Zhao H, Huang Y, Xue C, et al. Prognostic significance of the combined score of endothelial expression of nucleolin and CD31 in surgically resected non-small cell lung cancer. PLoS One. 2013;8(1):e54674. doi:10.1371/journal.pone.0054674
18. Gilles ME, Maione F, Cossutta M, et al. Nucleolin targeting impairs the progression of pancreatic cancer and promotes the normalization of tumor vasculature. Cancer Res. 2016;76(24):7181–7193. doi:10.1158/0008-5472.CAN-16-0300
19. Ugrinova I, Monier K, Ivaldi C, et al. Inactivation of nucleolin leads to nucleolar disruption, cell cycle arrest and defects in centrosome duplication. BMC Mol Biol. 2007;8(1):66. doi:10.1186/1471-2199-8-66
20. Xu JY, Lu S, Xu XY, et al. Knocking down nucleolin expression enhances the radiosensitivity of non-small cell lung cancer by influencing DNA-PKcs activity. Asian Pac J Cancer Prev. 2015;16(8):3301–3306. doi:10.7314/APJCP.2015.16.8.3301
21. Benedetti E, Antonosante A, d’Angelo M, et al. Nucleolin antagonist triggers autophagic cell death in human glioblastoma primary cells and decreased in vivo tumor growth in orthotopic brain tumor model. Oncotarget. 2015;6(39):42091–42104. doi:10.18632/oncotarget.5990
22. Qian B, Yao Y, Liu Y, Yan M, Huang Y, Chen Y. Nucleolin identified by comparative mass spectra analysis is a potential marker for invasive progression of hepatocellular carcinoma. Mol Med Rep. 2014;10(3):1489–1494. doi:10.3892/mmr.2014.2321
23. Li Y, Tang Y, Ye L, et al. Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis-related genes through cDNA microarray. J Cancer Res Clin Oncol. 2003;129(1):43–51. doi:10.1007/s00432-002-0396-4
24. Choi WT, Yang Y, Xu Y, An J. Targeting chemokine receptor CXCR4 for treatment of HIV-1 infection, tumor progression, and metastasis. Curr Top Med Chem. 2014;14(13):1574–1589. doi:10.2174/1568026614666140827143541
25. Niu H, Yang X, Xu Z, Du T, Wang R. Cell surface nucleolin interacts with CXCR4 receptor via the 212 c-terminal portion. Tumour Biol. 2015;36(2):1099–1104. doi:10.1007/s13277-014-2734-y
26. Destouches D, El Khoury D, Hamma-Kourbali Y, et al. Suppression of tumor growth and angiogenesis by a specific antagonist of the cell-surface expressed nucleolin. PLoS One. 2008;3(6):e2518. doi:10.1371/journal.pone.0002518
27. Krust B, El Khoury D, Soundaramourty C, Nondier I, Hovanessian AG. Suppression of tumorigenicity of rhabdoid tumor derived G401 cells by the multivalent HB-19 pseudopeptide that targets surface nucleolin. Biochimie. 2011;93(3):426–433. doi:10.1016/j.biochi.2010.10.015
28. Destouches D, Page N, Hamma-Kourbali Y, et al. A simple approach to cancer therapy afforded by multivalent pseudopeptides that target cell-surface nucleoproteins. Cancer Res. 2011;71(9):3296–3305. doi:10.1158/0008-5472.CAN-10-3459
29. D’Avino C, Palmieri D, Braddom A, et al. A novel fully human anti-NCL immunoRNase for triple-negative breast cancer therapy. Oncotarget. 2016;7(52):87016–87030. doi:10.18632/oncotarget.13522
30. Palmieri D, Richmond T, Piovan C, et al. Human anti-nucleolin recombinant immunoagent for cancer therapy. Proc Natl Acad Sci U S A. 2015;112(30):9418–9423. doi:10.1073/pnas.1507087112
31. Koutsioumpa M, Papadimitriou E. Cell surface nucleolin as a target for anti-cancer therapies. Recent Pat Anticancer Drug Discov. 2014;9(2):137–152. doi:10.2174/1574892808666131119095953
32. Tajrishi MM, Tuteja R, Nucleolin: TN. The most abundant multifunctional phosphoprotein of nucleolus. Commun Integr Biol. 2011;4(3):267–275. doi:10.4161/cib.4.3.14884
33. Wise JF, Berkova Z, Mathur R, et al. Nucleolin inhibits Fas ligand binding and suppresses Fas-mediated apoptosis in vivo via a surface nucleolin-Fas complex. Blood. 2013;121(23):4729–4739. doi:10.1182/blood-2012-12-471094
34. Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56(3):379–390. doi:10.1016/0092-8674(89)90241-9
35. Sengupta TK, Bandyopadhyay S, Fernandes DJ, Spicer EK. Identification of nucleolin as an AU-rich element binding protein involved in bcl-2 mRNA stabilization. J Biol Chem. 2004;279(12):10855–10863. doi:10.1074/jbc.M309111200
36. Otake Y, Sengupta TK, Bandyopadhyay S, Spicer EK, Fernandes DJ. Retinoid-induced apoptosis in HL-60 cells is associated with nucleolin down-regulation and destabilization of Bcl-2 mRNA. Mol Pharmacol. 2005;67(1):319–326. doi:10.1124/mol.104.006080
37. Otake Y, Soundararajan S, Sengupta TK, et al. Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mRNA. Blood. 2007;109(7):3069–3075. doi:10.1182/blood-2006-08-043257
38. Chen J, Guo K, Kastan MB. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J Biol Chem. 2012;287(20):16467–16476. doi:10.1074/jbc.M112.349274
39. Abdelmohsen K, Tominaga K, Lee EK, et al. Enhanced translation by nucleolin via G-rich elements in coding and non-coding regions of target mRNAs. Nucleic Acids Res. 2011;39(19):8513–8530. doi:10.1093/nar/gkr488
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





