Back to Journals » OncoTargets and Therapy » Volume 11
Overexpression of CD300A inhibits progression of NSCLC through downregulating Wnt/β-catenin pathway
Authors Tang Z, Cai H, Wang R, Cui Y
Received 28 August 2018
Accepted for publication 12 November 2018
Published 7 December 2018 Volume 2018:11 Pages 8875—8883
DOI https://doi.org/10.2147/OTT.S185521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Ze Tang, Hongfei Cai, Rui Wang, Youbin Cui
Department of Thoracic Surgery, The First Hospital of Jilin University, Changchun 130021, People’s Republic of China
Background: CD300A, a type I transmembrane glycoprotein receptor, plays an important role in immune response. Recent studies have reported that CD300A is involved in the development of hematological malignancies.
Purpose: The objective of this study was to investigate the role of CD300A in the progression of non-small-cell lung cancer (NSCLC) and explore the associated mechanism.
Materials and methods: Gene Expression Profiling Interactive Analysis (GEPIA) was used to analyze the expression of CD300A in NSCLC and its prognostic value. NSCLC cell lines A549 and H1650 were transfected with siRNA-CD300A or pcDNA3.1-CD300A vector to down- or up-regulate the expression of CD300A. Cell Counting Kit 8, colony formation and Transwell assays were used to assess the effects of CD300A on cell proliferation and migration capacities. Flow cytometry was performed to examine rate of apoptosis, and the protein levels of associated proteins was detected using Western blot assay.
Results: From GEPIA analysis, we observed that expression of CD300A mRNA was downregulated in NSCLC and positively correlated with the overall survival of NSCLC patients. Overexpression of CD300A significantly suppressed cell growth and migration capacities of A549 and H1650 cells and induced cell apoptosis via regulating apoptosis-related proteins. Moreover, decreasing level of CD300A promoted cell growth and migration and blocked apoptosis of NSCLC cells. Furthermore, upregulation of CD300A led to significant decrease in expression level of Wnt3 and β-catenin, the pivotal components in Wnt/β-catenin signaling pathway, and an increase in expression of E-cad, a key protein in tumor metastasis, in A549 and H1650 cells; while depletion of CD300A up-regulated the Wnt/β-catenin signaling pathway. In conclusion, the present study highlighted an anti-oncogenic role of CD300A in the progression of NSCLC via inhibiting Wnt/β-catenin pathway, suggesting that CD300A might be a potential target for the treatment of NSCLC.
Conclusion: CD300A plays an anti-oncogenic role in the progression of NSCLC through inhibiting the Wnt/β-catenin pathway, suggesting that CD300A might be a potential target for the treatment of NSCLC.
Keywords: non-small-cell lung cancer, CD300A, prognosis, progression
Introduction
Non-small-cell lung cancer (NSCLC) is a major type of primary lung cancer with high mortality that causes 1.5 million deaths each year in the world.1,2 A majority of patients are diagnosed at moderate or advanced stages, and the overall 5-year survival rate is <5%.3,4 Even though there has been great progress in the treatment of NSCLC, the survival of patients has not improved significantly.5 Therefore, it is very important to further explore the relevant molecular mechanisms of the occurrence and development of NSCLC to find the potential targets for the establishment of a new therapeutic strategy.
CD300A, also known as IRp60, is a type I transmembrane glycoprotein receptor of the CD300 glycoprotein family. It is located on the surface of cell membranes.6 CD300A is differentially expressed on the surface of various blood cells, including T lymphocytes, B lymphocytes, natural killer (NK) cells, neutrophils, and monocytes, and is involved in the regulation of cell growth, proliferation, apoptosis, differentiation, and immune regulation.7,8 Increasing evidences show that CD300A also plays a role in the development of hematological malignancies. Jiang et al revealed that knockdown of CD300A by shRNA interference could inhibit cell growth and division in diffuse large B-cell lymphoma (DLBCL) cells, but has no effect on cell apoptosis.9 In acute myeloid leukemia (AML), knockdown of CD300A also decreases cell proliferation and migration and induces cell apoptosis.10 However, whether CD300A plays a role in the progression of solid tumors remains unclear.
In an analysis from Gene Expression Profiling Interactive Analysis (GEPIA), a public online database, the expression of CD300A mRNA was shown to be downregulated in NSCLC and positively correlated with the overall survival of NSCLC patients. Herein, the effect of CD300A on progression of NSCLC was further explored by overexpressing and knocking down CD300A. We found that overexpression of CD300A inhibited growth capacity and migration of A549 and H1650 cells and promoted cell apoptosis in vitro; while CD300A knockdown could increase cell migration and decrease apoptosis of NSCLC cells. Furthermore, overexpression of CD300A blocked the Wnt/β-catenin pathway in NSCLC cells. These data indicated that CD300A might function as an anti-oncogene in the progression of NSCLC.
Materials and methods
Cell culture and transfection
The NSCLC cell lines, A549 and H1650, were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China) and cultured in DMEM (Hyclone, Logan, UT, USA) medium containing 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C with 5% CO2. Cells were transfected with siRNA-CD300A (CD300A knockdown, KD) or pcDNA3.1-CD300A (CD300A overexpression, OV) by using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s protocol, scramble siRNA or pcDNA3.1 vector was used as negative control (NC). Cells that did not undergo any treatment served as a control (CON) group. The siRNA-CD300A sequence was synthesized from Oligobio (Beijing, People’s Republic of China). The primers for CD300A overexpression were synthesized from Genewiz (Suzhou, People’s Republic of China), as follows: CD300A-EcoRI-sense: 5′-CCGGAATTCATGTGGCTGCCTTGGGCTCTGTTG-3′, CD300A-XbaI-antisense: 5′-GCTCTAGACTAGGCGTAGTCGGGCACGTCGTAGGGGTATGTCTTCCTTATCACACTGTA-3′.
Quantitative Reverse-Transcription PCR (qRT-PCR). After transfection for 24 hours, total RNA was extracted using Ultrapure RNA kit (CWBIO, Beijing, People’s Republic of China) and reverse transcribed to cDNA using the HiFiScript cDNA Synthesis Kit (CWBIO). The expression of mRNAs was detected using UltraSYBR Mixture (CWBIO). Primers were as follows: CD300A sense: 5′-TGGCTGCCCACAAGATAATG-3′, antisense: 5′-TCAATGTCGGCGCCTATTTC-3′; β-actin sense: 5′-TGTATGCCTCTGGTCGTACCAC-3′, and antisense: 5′-ACAGAGTACTTGCGCTCAGGAG-3′. The relative expression of CD300A was analyzed by the comparative Ct value and normalized to β-actin.
Cell proliferation assay
For Cell Counting Kit-8 (CCK8) assay: after transfection for 12 hours, about 1×103 cells were seeded in each well of 96-well plates. Cell proliferation was detected by CCK8 every 24 hours according to the instructions. For colony formation assay: after transfection for 12 hours, about 200 cells were seeded in a 6-cm disk to culture at 37°C with 5% CO2 until cells formed sufficiently large clones. Cells were fixed with 1 mL 4% paraformaldehyde for 30 minutes followed by being stained with crystal violet for 30 minutes. The number of colonies was counted and photographed.
Cell migration assay
Transwell assay was performed to assess cell migration of A549 and H1650 cells. After transfection for 12 hours, cells were re-suspended in serum-free culture medium. About 1×104 cells were transferred to the upper chamber, and complete medium was added to the lower chamber and cultured for 24 hours. The remaining cells were subsequently removed, while the migrated cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The migrated cells were imaged and counted under the microscope.
Flow cytometry analysis
After transfection for 12 hours, cells were treated with serum-free medium for 12 hours for starvation. Then, cells were stained with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) using Annexin V-FITC-PI apoptosis detection kit (4A Biotech, Beijing, People’s Republic of China) according to the instructions. Flow cytometer (BD FACSC anto II, BD Biosciences, Bedford MA, USA) was used for apoptotic rate analysis, and BD FACSDiva software was used to calculate.
Western blot assay
After transfection for 24 hours, cells were collected and protein was extracted using RIPA buffer (CWBIO). Protein of each sample was separated by using 10% SDS-PAGE gel and transferred to a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). The membrane was sequentially incubated with specific primary and secondary antibodies, and chemiluminescence detection kit was used for signal development. The primary antibodies used in this study were as follows: anti-Bcl-2, anti-Bax, anti-Caspase3 P17, anti-Wnt3, anti-β-catenin, anti-E-cad, and anti-GAPDH were purchased from PTG Company (Houston, TX, USA). All secondary antibodies used were also purchased from PTG Company.
Statistical analyses
In this study, all data were represented from three independent experiments and presented as mean ± SD and analyzed by using SPSS 18.0 software. Comparisons between two groups were analyzed using Student’s t-test, and one-way ANOVA was performed to compare three or more groups, and P<0.05 was considered as statistically significant.
Results
CD300A is downregulated in NSCLC and positively correlated with prognosis of NSCLC patients
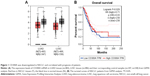
GEPIA is a useful online tool for transcriptomic analysis based on The Cancer Genome Atlas and The Genotype-Tissue Expression databases.11 In order to preliminarily investigate the expression of CD300A in NSCLC, GEPIA database analysis was performed. The results showed that the expression of CD300A mRNA was significantly downregulated in lung adenocarcinoma (LUAD) tissues (n=483) compared with the normal samples (n=347) (P<0.05, Figure 1A). Similarly, compared with the normal samples (n=338), the expression of CD300A mRNA was also observed to be decreased in lung squamous cell carcinoma (LUSC) tissues (n=486) (P<0.05, Figure 1A). Moreover, the survival curves from GEPIA analysis demonstrated that the expression levels of CD300A were significantly associated with prognosis of patient with NSCLC (P=0.029, Figure 1B) and low expression of CD300A predicted poor prognosis. These data suggested that the expression of CD300A is deregulated in NSCLC and might be associated with tumor progression.
Overexpression of CD300A inhibits growth capacity of NSCLC in vitro
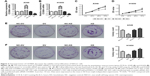
To assess the effect of CD300A on cellular biological behaviors of NSCLC, A549 and H1650 cells were transfected with pcDNA3.1-CD300A or siCD300A to upregulate or block the expression of CD300A, and scramble siRNA or pcDNA3.1 vector was used as NC. As RT-PCR indicated, compared with the NC group, the expression of CD300A mRNA was significantly upregulated in pcDNA3.1-CD300A transfected cells (OV) and downregulated in siRNA-CD300A transfected cells (KD; Figure 2A and B). As shown in Figure 2C and D, compared with the NC group, overexpression of CD300A resulted in a significant decrease in cell proliferation of A549 and H1650 cells (P<0.05), while CD300A knockdown did not have a significant effect on cell proliferation of NSCLC cells (P>0.05). In addition, the colony formation assay showed that the colony formation ability of A549 and H1650 cells was significantly decreased by overexpression of CD300A compared with the NC group (P<0.05, Figure 2E and F). However, CD300A knockdown did not significantly affect colony count of NSCLC cells (P>0.05), but the size of colony increased (Figure 2E and F). Taken together, overexpression of CD300A inhibits the growth capacity of NSCLC cells in vitro, suggesting that CD300A might play a role as a tumor suppressor in NSCLC.
Overexpression of CD300A inhibits migration of NSCLC in vitro
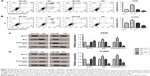
The effect of CD300A on migration of NSCLC cells was evaluated using Transwell migration assay. Compared with the control group, migration activity of A549 and H1650 cells was significantly decreased by upregulation of CD300A (P<0.05, Figure 3A and B), while it was significantly increased in CD300A knockdown cells (P<0.05). The results further substantiated that CD300A might play a role as a tumor suppressor in NSCLC by inhibiting cell migration activity.
Overexpression of CD300A enhances cell apoptosis of NSCLC in vitro
Dysregulation of apoptosis is an important hallmark of tumor cells, therefore, flow cytometry was performed to assess whether CD300A impaired cell apoptosis of NSCLC cells. As shown in Figure 4A, the apoptosis rate of A549 cells was clearly induced by overexpression of CD300A compared with the NC group (P<0.01) and significantly decreased in CD300A knockdown cells (P<0.05). Similar results were also observed in H1650 cells that overexpression of CD300A promoted apoptosis and CD300A knockdown decreased apoptosis (P<0.05, Figure 4B). To further confirm the relevant mechanism of apoptosis induced by CD300A in NSCLC cells, Western blot was carried out to detect the expression of apoptosis-associated proteins. The results demonstrated that the expression of Bcl-2, a pivotal antiapoptotic protein, was significantly downregulated in CD300A expression cells compared with the NC group, while it was upregulated in CD300A knockdown cells in both A549 and H1650 cells (P<0.05, Figure 4C and D). Meanwhile, the expression of Bax and Caspase3 p17, key pro-apoptotic proteins, was significantly increased when CD300A was upregulated in A549 or H1650 cells, and decreased accordingly when CD300A was knocked down (P<0.05, Figure 4C and D). These results suggested that CD300A could promote apoptosis of NSCLC cells via regulating Bcl-2/Bax and Caspase cascade.
CD300A suppresses the Wnt/β-catenin pathway in NSCLC cells
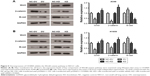
It is widely held that Wnt/β-catenin pathway is involved in cell proliferation, differentiation, migration, and apoptosis, which plays a pivotal role in tumorigenesis and progression, including NSCLC.12 To further investigate the relevant mechanism of how CD300A acts as a suppressor in progression of NSCLC cells, core proteins in Wnt/β-catenin pathway were detected by Western blot. We observed that the expression of Wnt3 and β-catenin significantly decreased in CD300A overexpression group compared with the NC group (P<0.05, Figure 5A and B), indicating that CD300A suppressed the Wnt/β-catenin pathway in A549 and H1650 cells. Moreover, the expression of downstream protein E-cad, a key cell adhesion-associated protein, increased accordingly when CD300A was upregulated in A549 and H1650 cells (P<0.05, Figure 5A and B). As shown in Figure 5A and B, deficiency of CD300A resulted in an increase in expression of Wnt3 and β-catenin and significant decrease in expression of E-cad in A549 and H1650 cells. These data confirmed that the Wnt/β-catenin pathway was involved in the suppression effect of CD300A on progression of NSCLC.
Discussion
CD300A is an inhibitory receptor with four immunoreceptor tyrosine-based activating motifs (ITAMs), its inhibitory signal initiation is dependent on the phosphorylation of tyrosine 267 located in the third immunoreceptor tyrosine-based activating motifs (ITAMs).13 Previous studies report that CD300A is involved in inflammation, autoimmunity, viral infections, etc., indicating that CD300A plays an important role in immune response.14–17 CD300A binds to phosphatidylserine (PS) on the surface of apoptotic cells and inhibits the phagocytosis by phagocyte. More importantly, recent study finds that CD300A can also bind to tumor cells by interacting with PS on the surface of tumor cells to inhibit the recognition and killing of tumor cells by NK cells, while the killing effect of NK cells for tumor cell will be promoted when the interaction is blocked.18 It has been confirmed that CD300A has abnormally high expression in hematological malignancies, such as acute lymphoblastic leukemia, AML, and DLBCL, and is closely correlated with prognosis.9,10,15 In the present study, we observed that CD300A mRNA was downregulated in both LUAD and LUSC, which was inconsistent with the pattern of CD300A in hematological tumors. In addition, the expression of CD300A was positively correlated with the prognosis of NSCLC patients, suggesting a potential clinical significance of CD300A for NSCLC. These results drive us to further investigate the effect of CD300A on the biological behaviors of NSCLC cells. Bioinformatics analysis in human protein atlas reveals that CD300A is not expressed in NSCLC cells at protein level but expressed in tumor macrophages. In order to understand why a gene with no protein level expression is associated with prognosis, we initially investigated whether upregulation or downregulation of CD300A may affect NSCLC cell behaviors in vitro. In the previous studies, CD300A was reported to act as an oncogene in DLBCL and AML. In contrast, our data revealed that CD300A played a role as tumor suppressor in NSCLC cells in which overexpression of CD300A inhibited cell growth capacity and migration of A549 and H1650 cells and promoted cell apoptosis. At the same time, CD300A knockdown was observed to possess an activating role as the promotion on cell migration and suspension on cell apoptosis. As mentioned previously, our data indicated an anti-oncogenic role of CD300A in the progression of NSCLC, which was contrary to the function in hematological malignancy.
Further study was performed to explore the relevant molecular mechanism underlying the inhibition on cell growth and migration of NSCLC cells by CD300A. Flow cytometry assay revealed a notable elevation on cell apoptosis of NSCLC cells by upregulation of CD300A. Therefore, apoptosis-related proteins were examined in CD300A overexpressed cells and CD300A knocked down cells. The cell apoptotic process involves a series of proteolytic events mediated mainly by an important cysteine protease family (Caspase), including Caspase3, a key executioner that triggers apoptosis.19 Another important family involved in apoptosis, Bcl-2 family, plays a pivotal role in the induction of apoptosis via triggering the mitochondrial pathway.20,21 It was shown that the ratio of Bcl-2/Bax was decreased by upregulation of CD300A and increased by downregulation of CD300A in A549 and H1650 cells (Figure 4C and D). In addition, the level of pro-apoptotic protein Caspase3 p17 was significantly enhanced by CD300A overexpression. Based on these results, CD300A inhibits the growth of NSCLC cells by promoting apoptosis through triggering mitochondrial pathway.
Previous studies demonstrate that the oncogene role of CD300A displayed in DLBCL and AML cells is derived through the regulation of PI3K/Akt pathway, indicating that CD300A may be a regulator upstream of the PI3K/Akt pathway.9,10 Because the cellular signaling pathways are not independent in cell, we initially explored the impact of CD300A on other signaling pathways involved in cell migration. It is widely held that Wnt/β-catenin pathway is critical to tumorigenesis and progression by affecting cellular processes, including proliferation, migration, and apoptosis.22 Wnt pathway is frequently deregulated in NSCLC,23 and the overactive Wnt/β-catenin pathway is associated with tumorigenesis and metastasis.24–26 β-catenin, a key downstream molecule of the Wnt pathway, is mainly combined with E-cad to form E-cad/β-catenin complex to maintain cell polarity and adhesion.27 E-cad, a downstream molecule of Wnt/β-catenin pathway, is involved in cell–cell adhesion and downregulation of E-cad is associated with tumor metastasis.28,29 Our data showed a significant suppression on Wnt/β-catenin pathway by CD300A by decreasing the expression levels of Wnt3 and β-catenin in NSCLC cells. Meanwhile, the expression of E-cad was significantly up-regulated with CD300A overexpression in NSCLC cells and downregulated after CD300A was knocked down. Taken together, these results suggested that CD300A suppressed cell growth and migration of NSCLC cells by inhibiting the Wnt/β-catenin pathway. Further research is needed to gain insight into the molecular mechanisms by which CD300A affects the growth and migration of NSCLC cells through its mRNA levels.
Conclusion
The present study highlights the anti-oncogenic role of CD300A in the progression of NSCLC via inhibiting Wnt/β-catenin pathway, suggesting that CD300A may be a potential target for the treatment of NSCLC.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Ni L, Zhu X, Gong C, et al. Trichosanthes kirilowii fruits inhibit non-small cell lung cancer cell growth through mitotic cell-cycle arrest. Am J Chin Med. 2015;43(2):349–364. | ||
Fang Y, Guan X, Cai T, et al. Knockdown of ANXA1 suppresses the biological behavior of human NSCLC cells in vitro. Mol Med Rep. 2016;13(5):3858–3866. | ||
Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin 2016. 2016;66(1):7–30. | ||
Borrego F. The CD300 molecules: an emerging family of regulators of the immune system. Blood. 2013;121(11):1951–1960. | ||
Alvarez Y, Tang X, Coligan JE, Borrego F. The CD300a (IRp60) inhibitory receptor is rapidly up-regulated on human neutrophils in response to inflammatory stimuli and modulates CD32a (FcgammaRIIa) mediated signaling. Mol Immunol. 2008;45(1):253–258. | ||
Lopez-Sejas N, Campos C, Hassouneh F, et al. Effect of CMV and aging on the differential expression of CD300a, CD161, T-bet, and Eomes on NK cell subsets. Front Immunol. 2016;7(5):476. | ||
Jiang L, Xu Y, Zeng X, Fang J, Morse HC 3rd, Zhou JX. Suppression of CD300A inhibits the growth of diffuse large B-cell lymphoma. Oncotarget. 2015;6(31):31191–31202. | ||
Sun X, Huang S, Wang X, Zhang X, Wang X. CD300A promotes tumor progression by PECAM1, ADCY7 and AKT pathway in acute myeloid leukemia. Oncotarget. 2018;9(44):27574–27584. | ||
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. | ||
Zhang X, Ke X, Pu Q, et al. MicroRNA-410 acts as oncogene in NSCLC through downregulating SLC34A2 via activating Wnt/β-catenin pathway. Oncotarget. 2016;7(12):14569–14585. | ||
Lankry D, Simic H, Klieger Y, Levi-Schaffer F, Jonjic S, Mandelboim O. Expression and function of CD300 in NK cells. J Immunol. 2010;185(5):2877–2886. | ||
Carnec X, Meertens L, Dejarnac O, et al. The phosphatidylserine and phosphatidylethanolamine receptor CD300a binds dengue virus and enhances infection. J Virol. 2016;90(1):92–102. | ||
Chen Z, Shojaee S, Buchner M, et al. Signalling thresholds and negative B-cell selection in acute lymphoblastic leukaemia. Nature. 2015;521(7552):357–361. | ||
Nakahashi-Oda C, Tahara-Hanaoka S, Shoji M, et al. Apoptotic cells suppress mast cell inflammatory responses via the CD300a immunoreceptor. J Exp Med. 2012;209(8):1493–1503. | ||
Zenarruzabeitia O, Vitallé J, García-Obregón S, et al. The expression and function of human CD300 receptors on blood circulating mononuclear cells are distinct in neonates and adults. Sci Rep. 2016;6:32693. | ||
Lankry D, Rovis TL, Jonjic S, Mandelboim O. The interaction between CD300a and phosphatidylserine inhibits tumor cell killing by NK cells. Eur J Immunol. 2013;43(8):2151–2161. | ||
Gui D, Guo Y, Wang F, et al. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS One. 2012;7(6):e39824. | ||
Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21(1):92–101. | ||
Wu R, Tang S, Wang M, Xu X, Yao C, Wang S. MicroRNA-497 induces apoptosis and suppresses proliferation via the Bcl-2/Bax-Caspase9-Caspase3 pathway and Cyclin D2 protein in HUVECs. PLoS One. 2016;11(12):e0167052. | ||
Hayward P, Kalmar T, Arias AM. Wnt/Notch signalling and information processing during development. Development. 2008;135(3):411–424. | ||
Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106(1):djt356. | ||
Taketo MM. Wnt signaling and gastrointestinal tumorigenesis in mouse models. Oncogene. 2006;25(57):7522–7530. | ||
Kim M, Suh YA, Oh JH, Lee BR, Kim J, Jang SJ. KIF3A binds to β-arrestin for suppressing Wnt/β-catenin signalling independently of primary cilia in lung cancer. Sci Rep. 2016;6:32770. | ||
Liu Q-Q, Chen K, Ye Q, Jiang X-H, Sun Y-W. Oridonin inhibits pancreatic cancer cell migration and epithelial-mesenchymal transition by suppressing Wnt/β-catenin signaling pathway. Cancer Cell Int. 2016;16(1):1–8. | ||
Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1–2):15–33. | ||
Yang YL, Chen MW, Xian L. Prognostic and clinicopathological significance of downregulated E-cadherin expression in patients with non-small cell lung cancer (NSCLC): a meta-analysis. PLoS One. 2014;9(6):e99763. | ||
Han T, Jiao F, Hu H, et al. EZH2 promotes cell migration and invasion but not alters cell proliferation by suppressing E-cadherin, partly through association with MALAT-1 in pancreatic cancer. Oncotarget. 2016;7(10):11194–11207. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.