Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 12
Overexpression of Adiponectin Receptors in Opium Users with and without Cancer
Authors Firouzabadi N , Haghnegahdar M, Khalvati B , Dehshahri A, Bahramali E
Received 1 April 2020
Accepted for publication 5 June 2020
Published 15 June 2020 Volume 2020:12 Pages 59—65
DOI https://doi.org/10.2147/CPAA.S256289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Negar Firouzabadi,1– 3 Maral Haghnegahdar,1 Bahman Khalvati,4 Ali Dehshahri,5 Ehsan Bahramali6
1Department of Pharmacology & Toxicology, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran; 2Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran; 3Non-Communicable Diseases Research Center, Fasa University of Medical Sciences, Fasa, Iran; 4Medicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran; 5Department of Pharmaceutical Biotechnology, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran; 6Digestive Disease Research Center, Digestive Disease Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
Correspondence: Negar Firouzabadi
Department of Pharmacology & Toxicology, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
Tel +98-917-314-5303
Fax +98-713-2424128
Email [email protected]
Aim: Opium addiction is a serious public health concern in the Middle East countries causing various illnesses. Opium use is associated with an increased risk of several cancers; however, the underlying mechanisms are not yet fully elucidated. Altered levels of adiponectin and its related main receptors, Adiponectin receptor 1 and 2 (AdipoR1 and AdipoR2) have been associated with several malignancies. Opium users are at risk of various cancers. All together let us to the hypothesis that probable overexpression of AdipoRs in opium users might be linked to the occurrence of cancer in this population.
Methods: One hundred opium users along with 100 healthy non-opium users were enrolled in the study. Opium users were followed up for 5 years (2014– 2019) to evaluate the occurrence of malignancies. AdipoR1 and AdipoR2 expressions were measured using a flow cytometry method.
Results: Expression of AdipoR1 and AdipoR2 was significantly higher in opium users compared with the healthy control group (P=0.0001 and 0.0001, respectively). Eight opium users developed cancer during the follow-up period. Subjects abusing opium developed cancer by 8.6 folds comparing to non-opium users (P=0.034; OR=8.6; 95% CI (1.06– 70.1)). Expression of these two receptors was significantly higher in opium users developing cancer compared with cancer-free opium (P=0.001).
Conclusion: Considering the significant overexpression of AdipoR1 and AdipoR2 in opium users and in opium users who developed malignancies and the association between upregulation of these receptors in most cancers affecting opium users and assessment of AdipoRs may serve as an early detection tool of cancer in this population.
Keywords: opium, addiction, adiponectin, adiponectin receptors, cancer
Introduction
Opium addiction is a serious public health threat in the Middle East countries. While heroin is the most widely used opiate in western countries, opium is vastly used in eastern regions.1 According to World Drug Report in 2015, 0.4% of the world’s adult population, abuse heroin and opium.2 Substance abuse and addiction regardless of the type of substance have major impacts on one’s health as well as on national healthcare systems.3,4 It is evidently associated with increased morbidity and mortality, as well as shortening the life span.5 According to the national household survey in Iran, prevalence of illegal drug use among which opioids were the most prevalent drug was estimated to be 2.5%, accounting for roughly 1.12 million Iranians.6
Opioid compounds affect a variety of hormones and their functions termed as opioid endocrinopathy. There are many investigations suggestive of modulation of the endocrine system by opioids.7 Opioids cause reduction in the plasma levels of many hormones such as TSH, prolactin, growth hormone and insulin8 by binding to opioid receptors in the hypothalamus, pituitary and testis.9,10
Adipose tissue as an important endocrine organ secretes multiple metabolically active proteins termed as adipokines which regulate pathophysiological processes, including insulin sensitivity and resistance, appetite, inflammation, hematopoiesis, and angiogenesis.11 Some well-known adipokines include leptin, tumor necrosis factor (TNF)-a, interleukin (IL)-6 and adiponectin.12,13 Adiponectin, a novel collagen-like protein synthesized by white adipose tissue circulates at relatively high (2–20 mg/mL) serum concentrations and is an adipose-secreted 244-amino-acid protein.14–16 This protein exerts its effect by binding to two distinct but structurally related receptors, adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2).17–19 Several studies reported association between circulating levels of adiponectin and increased risk of different malignancies.20–22
During the past decade, the carcinogenic properties of opium have taken much attention. The association between opium use and several malignancies has been frequently reported.23–28
A link between expression of AdipoRs and several cancers has also been reported.22,29–31 These reports are suggestive of a direct effect of adiponectin and its related receptors on tumor development and progression.
Although many studies have examined the role of adiponectin and its related receptors as mediators in various metabolic and hormonal disorders, no research has been conducted in relation to opium addiction and the possible contribution of AdipoRs in the occurrence of malignancies in opium users. Considering the link between adiponectin levels and its associated receptors with various cancers and higher risk of certain malignancies in opium users, on the other hand, led us to the hypothesis that in case of variation in expression of AdipoRs (AdipoR1 and AdipoR2) in opium users, these receptors can serve as prognostic biomarkers of cancer in opium users.
Materials and Methods
Blood Samples
In a nested case-control design, 100 regular opium users (age: 35–70 years) were enrolled in our study. Whole blood samples were obtained from of Fasa Cohort Study (FACS) and kept at −70°C in the refrigerators. FACS is a population-based cohort study that has enrolled 10,157 participants of 35–70-year-old in a region in southern Iran with high rates of opium use (22.1%). Opium users abused no other substances other than opium. Daily need of opium was provided to the enrolled opium users by FACS. Inclusion criteria were as follows: Individuals with more than 5 years of history of opium addiction were enrolled in our study and followed up for 5 years (2014–2019). Opium users abused no other substance other than opium. Opium users with a history of opium abuse less than 5 years and individuals with history of any malignancies and substance abuse other than opium were excluded from our study.
Daily need of opium was provided to the enrolled subjects by FACS. In accordance with FACS data acquisition protocol, a valid questionnaire was used to record the dose of opium consumption in all participants.32 FACS actively registers every outcome of enrolled participants including malignancies. Every patient’s files are reviewed by five specialists to reach the final diagnosis which is then registered as an ICD10 code (International Statistical Classification of Diseases and Related Health problems 10th Revision).
One hundred controls (age: 35–70 years) were enrolled by adjusting for the demographic (age, sex, place of residence), anthropometric (body mass index, waist and hip circumferences) and diseases history (diabetes mellitus, heart diseases, stroke, malignancies and endocrine diseases) variables. Blood samples were extracted from the biobank and defrosted for biochemical analyses.
The protocol of this study was reviewed and approved by the Ethics committee of Shiraz University of Medical Sciences with the ethical approval code of 11,347. The study was conducted in accordance with the Declaration of Helsinki. Each patient signed an informed consent form approved by his or her local institutional Review Board.
Isolation of Mononuclear Cells Ficoll-Paque Density Gradient Separation
The isolation of mononuclear cells from the whole blood samples was carried out using Ficoll-Paque density gradient separation method as described in the literature.33 Briefly, Ficoll-Paque media was added to the anticoagulant treated bloods. These tubes were centrifuged at 1500 rpm for 25 min at 4°C. Following the centrifuge, the upper layer containing plasma and platelets was separated using sterile pipette. The layer of mononuclear cells was transferred to another sterile centrifuge tube and re-suspended by balanced salt solution following the centrifuge at the same condition as described above. The washing and centrifuge step was repeated at least three times to get rid of platelets as well as erythrocytes. The cells were re-suspended in PBS media for further steps.
Evaluation of AdipoRs Expression by Flow Cytometry
The mononuclear cells obtained from the whole blood samples of the patients and control groups were used for the measuring of AdipoRs expression using indirect flow cytometry method according to the manufacturer’s instructions. The cells were re-suspended in ice-cold PBS, 10% FCS and 1% sodium azide at the final cell density of around 1–5 x 106 cells/mL. Then, 100 μL of the suspended cells were added to each tube and treated by 0.1–10 μg/mL of the primary antibody (Abcam 126,611, Abcam189446) followed by the at 4℃ in the dark for around 30 min. Then, the cells were washed 3 times by centrifugation at 400 g for 5 min and then suspended in ice-cold PBS. The prepared cells were suspended in 3% BSA/PBS containing fluorochrome-labeled secondary antibody (Goat Anti-Rabbit IgG H&L (FITC) (ab6717)) followed by the incubation at room temperature in dark for 20–30 mins. Finally, the cells were washed 3 times by centrifugation at 400 g for 5 min, suspended in ice-cold PBS, 3% BSA, 1% sodium azide and analyzed by flow cytometry (FACS Calibur, Becton Dickinson, Mountain View, CA) using FlowJo software (TreeStar Inc., San Carlos, CA).
Statistical Analysis
SPSS® 21.0 for windows® was used for data analysis (SPSS Inc., Chicago, Illinois). Continuous variables are reported as mean ± SD. Kolmogorov–Smirnov test was used to test the normal distribution of continuous variables. Comparison of continuous variables between two groups was performed using Student’s t-test for normally distributed variables and Mann–Whitney test for non-normal variables. AdipoRs expressions were analyzed using Flowjo® software version 7.6.
Result
Demographic Data
Table 1 represents descriptive characteristics of opium users and healthy individuals. As demonstrated no significant difference was observed between controls and opium users (P>0.05). Parameters affecting the expression of AdipRs such as alcohol consumption, endocrine diseases, diabetes mellitus, history of stroke and cigarette smoking were also not significantly different between two study groups (P>0.05). Among 200 enrolled subjects (one-hundred healthy non-opium users and one-hundred opium users), eight opium users developed cancer during a 5-year follow-up and only one non-opium user was diagnosed with cancer. Opium users developed cancer 8.6 folds more comparing with non-opium users (P=0.018; OR=8.6; 95% CI=1.01.05–70.1). Among the opium users who were diagnosed with malignancies, 3 individuals developed bladder cancer, 2 were diagnosed with laryngeal cancer, one diagnosed with esophageal cancer, one diagnosed with lung cancer and one with gastric cancer. One non-opium user developed Hodgkin’s lymphoma. Table 2 demonstrates demographic data of opium users diagnosed with cancer and cancer-free opium users.
 |
Table 1 Demographic Characteristics of Opium Users (Case) and Non-Opium Users (Controls) |
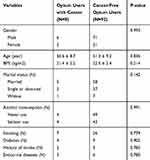 |
Table 2 Demographic Characteristics of Opium Users Diagnosed with Cancer and Cancer-Free Opium Users |
Expression of AdipoRs by Flow Cytometry
As demonstrated in Table 3, AdipoR1 and AdipoR2 levels significantly increased (P=0.001) in opium users (AdipoR1: 76.76±15.30; AdipoR2: 78.74±16.25) compared with controls (AdipoR1: 22.10±2.01; AdipoR2: 25.50±2.38). Opium users, who were diagnosed with cancer showed significantly higher expression of AdipoR1 (P=0.001) comparing with opium users not diagnosed with cancer (97.1±2.2 and 75.5±14.7, respectively). Regarding AdipoR2 (Table 4), opium users with cancer exhibited significantly higher expression levels (P=0.001) compared to opium users not diagnosed with cancer (95.1±3.8 and 77.2±16.1, respectively). Additionally, the level of AdipoR1 and AdipoR2 in the non-opium user who was diagnosed with cancer were 21.2 and 23.8, respectively, which was not significantly (P>0.05) different compared with non-opium users (AdipoR1:22.1±2.01 and AdipoR2: 25.5± 2.38).
 |
Table 3 Expression of AdipoR1 and AdipoR2 in Opium Users (Case) and Non-Opium Users (Controls) |
 |
Table 4 Expression of AdipoR1 and AdipoR2 in Opium Users Diagnosed with Cancer and Cancer-Free Opium Users Not Developing Malignancies |
Figure S1 represents AdipoR1 expression level measured by flow cytometry obtained from non-opium users A) and opium users with cancer B) as well as AdipoR2 expression level obtained from non-opium users C) and opium users with cancer D).
Discussion
Although many studies have examined the role of adiponectin and its related receptors as mediators in various metabolic and hormonal disorders, no research has been conducted in relation to opium addiction. Adiponectin levels and its associated receptors have been linked to various cancers. Opium users on the other hand are at risk of certain malignancies. The hypothesis of this study was that in case of variation in expression of AdipoRs (AdipoR1 and AdipoR2) in opium users, these receptors can serve as prognostic biomarkers of cancer in opium users.
In the present study, we evaluated the expression of AdipoR1 and AdipoR2 in opium users and their association with the occurrence of cancer in this population. Our results showed that opium users developed cancer 8.6 folds more than subjects not using opium. Levels of AdipoR1 & AdipoR2 in opium users were significantly higher than normal subjects (P<0.0001). Likewise, opium users diagnosed with different types of cancer exhibited significantly higher expression of both receptors compared with opium users who did not develop cancer in the period of follow-up (P=0.001).
According to the UN office of Drugs and Crime, an estimated 4 million people worldwide, mainly in central and west Asia, use opium illegally.34 Recently, many studies have focused on the carcinogenic consequences of opium use suggestive of positive associations with several malignancies such as lung, larynx, esophagus, stomach and pancreatic cancer.24,25,35–37
Adiponectin, an adipocyte-derived cytokine, is proposed to be involved in the pathogenesis of malignancies as well as affecting the progression of cancer.38–40 Adiponectin induces its actions in cancerous cells via two main receptors of AdipoR1 and AdipoR2.41–43 The expression of AdipoRs in various tumorous tissues30 suggests that adiponectin presumably exerts its biological effects in cancerous cells by means of signaling through these receptors. AdipoRon, an AdipoR1 and AdipoR2 agonist, has shown to have anti-proliferative and anti-apoptotic properties suggestive of its potential in activating the same signaling pathways as exogenous adiponectin via its two main receptors mainly overexpressed in cancerous tissues.44 As it is known, tumor growth relies pretty much on new blood vessel formation. In pathological states, tumor angiogenesis is influenced by the action of adiponectin and by acting on its main receptors which is indicative of the role of adiponectin in new blood vessels formation.41
Chronic opium abuse induces the risk of several malignancies including bladder, laryngeal, lung, gastric, esophageal and pancreatic cancers.25 Adiponectin stimulates insulin sensitivity in the liver and muscle and thus low levels of adiponectin may cause insulin resistance. Recent studies have demonstrated that the use of oral antidiabetics, thiazolidinediones (TZDs), acting as insulin sensitizers, has been associated with reduced risk of developing lung cancer,45 a cancer frequently reported in opium users.46–48 These agents alter insulin sensitivity mainly through the elevation of adiponectin levels.49,50 It is assumed that these agents reduce the risk of lung cancer by binding to AdipoRs overexpressed in cancerous cells.22
The relative risk of esophageal cancer and opium use is reported to be near two.25 A study conducted in human esophageal tumors disclosed the overexpression of AdipoR1 and AdipoR2 in cancerous cells. Taking into account that angiogenesis is a key regulator in malignancy51 and that adiponectin has strong proangiogenic actions, it is plausible that high AdipoRs expression may boost greater angiogenesis inside these tumors, leading to a rise in tumor invasion and metastasis.52 This hypothesis is further supported in adiponectin knockout mice, in which a delay in the onset of tumor, its rate of growth and angiogenesis was observed compared with the wild-type model.53
Another malignancy frequently reported in opium users is the gastric cancer. Results of case-control and cohort studies are suggestive of approximately three times higher risk of gastric cancer in opium users than in non-users.25 It is reported that both AdipoR1 and AdipoR2 are vastly expressed at substantial levels in gastric tumors and that growth inhibition induced by adiponectin was significantly abolished by down-regulation of these receptors by specific siRNAs.29
AdipoRs are believed to have a fundamental role in the physiological regulation of the antiapoptotic sphingosine 1 phosphate (S1P) and ceramide balance. AdipoR1and AdipoR2 augment ceramidase activity.54 Upon activation, both AdipoR1 and AdipoR2 catabolize ceramides to downstream degradation of sphingosines and S1P. Ceramide and S1P are considered as strategic mediators in inflammatory processes, cell growth, and cell survival.55 Extreme accumulation of ceramide is linked to the incidence of some metabolic processes such as insulin resistance and heart failure.55 In addition, S1P is known as a potent inhibitor of apoptosis and a powerful inducer of cell proliferation.56 Increased levels of S1P are associated with enhanced cell survival and greater local proangiogenic properties observed in animal tumor models.53,55,57 Accordingly, the mentioned metabolic pathway which is critical in different processes such as inflammation, cell survival and cell growth might explain the role of AdipoRs in the signaling processes involved in malignancies.58 Therefore, these data suggest that measuring levels of adiponectin and its related receptors may serve as a useful prognostic screening tool for early detection of cancers.38 As observed in our study, significant rise in expression of AdipoRs in opium users comparing to healthy individuals, on one hand, and significant increase in expression of these receptors in opium users who developed cancer may suggest the role of these receptors as prognostic tools of malignancies.
Conclusion
Considering the significant overexpression of AdipoR1 and AdipoR2 in opium users and the association between upregulation of these receptors in cancers mostly affecting opium users, assessment of adiponectin and its associated receptors may be used as an early detection tool of cancer in this population. In addition, utilizing agents affecting AdipoRs may assist in preventing an individual from encountering malignancies.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgment
This work was financially supported by a grant from Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran (Grant NO. 95-01-36-11347). The project was a part of a Pharm.D. thesis by Maral Haghnegahdar, Department of Pharmacology & Toxicology, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran.
Author Contributions
Negar Firouzabadi: Supervision, conceptualization, methodology, formal analysis, data Curation, writing original draft, writing/review & editing, resources, project administration, funding acquisition;
Maral Haghnegahdar: Methodology, investigation
Bahman Khalvati: Methodology, investigation
Ali Dehshahri: Methodology, review & editing, project administration
Ehsan Bahramali: Conceptualization, methodology, writing/review & editing
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors of the manuscript have no conflicts of interest to declare.
References
1. Mejía D. The war on drugs under plan Colombia. Rethinking “War on Drugs” Through US-Mexico Prism. 2012;19.
2. First MB, Gibbon M, Spitzer RL, Williams JB. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders Research Version. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996.
3. Chehrazi E, editor Epidemiology of addiction in Iran.
4. Alemi A, Naraghi M. The iceberg of opium addiction an epidemiological survey of opium addiction in a rural community. Drug Alcohol Depend. 1978;3(2):107–112. doi:10.1016/0376-8716(78)90023-6
5. Mee-Lee D. ASAM (American Society of Addiction Medicine) Patient Placement Criteria for the Treatment of Substance-Related Disorders. American Society of Addiction Medicine; 1996.
6. Narenjiha H, Rafiey H, Jahani MR, Assari S, Moharamzad Y, Roshanpazooh M. Substance-dependent professional drivers in Iran: a descriptive study. Traffic Inj Prev. 2009;10(3):227–230. doi:10.1080/15389580902849017
7. Barber A, Gottschlich R. Opioid agonists and antagonists: an evaluation of their peripheral actions in inflammation. Med Res Rev. 1992;12(5):525–562. doi:10.1002/med.2610120505
8. Vuong C, Van Uum SH, O’dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2009;31(1):98–132.
9. Frenk H. Pro-and anticonvulsant actions of morphine and the endogenous opioids: involvement and interactions of multiple opiate and non-opiate systems. Brain Res Rev. 1983;6(2):197–210. doi:10.1016/0165-0173(83)90039-5
10. Dhawan B, Cesselin F, Raghubir R, et al. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48(4):567–592.
11. La Cava A. Leptin in inflammation and autoimmunity. Cytokine. 2017;98:51–58. doi:10.1016/j.cyto.2016.10.011
12. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi:10.1210/jc.2004-0395
13. Flier JS, Cook KS, Usher P, Spiegelman BM. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237:405–409. doi:10.1126/science.3299706
14. Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPoseMost abundant Gene transcript 1). Biochem Biophys Res Commun. 1996;221(2):286–289. doi:10.1006/bbrc.1996.0587
15. Funahashi T, Nakamura T, Shimomura I, et al. 3. Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Internal Med. 1999;38(2):202–206. doi:10.2169/internalmedicine.38.202
16. Saito K, Tobe T, Minoshima S, et al. Organization of the gene for gelatin-binding protein (GBP28). Gene. 1999;229(1):67–73. doi:10.1016/S0378-1119(99)00041-4
17. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–451. doi:10.1210/er.2005-0005
18. Takahashi M, Arita Y, Yamagata K, et al. Genomic structure and mutations in adipose-specific gene, adiponectin. Int J Obes. 2000;24(7):861. doi:10.1038/sj.ijo.0801244
19. Hug C, Wang J, Ahmad NS, Bogan JS, Tsao T-S, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101(28):10308–10313. doi:10.1073/pnas.0403382101
20. Petridou E, Mantzoros C, Dessypris N, et al. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003;88(3):993–997. doi:10.1210/jc.2002-021209
21. Soliman PT, Wu D, Tortolero‐Luna G, et al. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106(11):2376–2381.
22. Petridou ET, Mitsiades N, Gialamas S, et al. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: two case-control studies. Oncology. 2007;73(3–4):261–269. doi:10.1159/000127424
23. Hewer T, Rose E, Ghadirian P, et al. Ingested mutagens from opium and tobacco pyrolysis products and cancer of the oesophagus. Lancet. 1978;312(8088):494–496. doi:10.1016/S0140-6736(78)92218-3
24. Moossavi S, Mohamadnejad M, Pourshams A, et al. Opium use and risk of pancreatic cancer: a prospective cohort study. Cancer Epidemiol Prev Biomarkers. 2018;27(3):268–273. doi:10.1158/1055-9965.EPI-17-0592
25. Kamangar F, Shakeri R, Malekzadeh R, Islami F. Opium use: an emerging risk factor for cancer? Lancet Oncol. 2014;15(2):e69–e77. doi:10.1016/S1470-2045(13)70550-3
26. Mahmoodpoor A, Golzari SE. Epigenetics, opium, and cancer. Lancet Oncol. 2014;15(4):e153. doi:10.1016/S1470-2045(14)70077-4
27. Alizadeh H, Naghibzadeh Tahami A, Khanjani N, et al. Opium use and head and neck cancers: a matched case-control study in Iran. Asian Pacific J Cancer Prev. 2020;21(3):783–790. doi:10.31557/APJCP.2020.21.3.783
28. Szczepaniak A, Fichna J, Zielińska M. Opioids in cancer development, progression and metastasis: focus on colorectal cancer. Curr Treat Options Oncol. 2020;21(1):6. doi:10.1007/s11864-019-0699-1
29. Ishikawa M, Kitayama J, Yamauchi T, et al. Adiponectin inhibits the growth and peritoneal metastasis of gastric cancer through its specific membrane receptors AdipoR1 and AdipoR2. Cancer Sci. 2007;98(7):1120–1127. doi:10.1111/j.1349-7006.2007.00486.x
30. Hiyoshi M, Tsuno NH, Otani K, et al. Adiponectin receptor 2 is negatively associated with lymph node metastasis of colorectal cancer. Oncol Lett. 2012;3(4):756–760. doi:10.3892/ol.2012.583
31. Dalamaga M, Migdalis I, Fargnoli JL, et al. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case–control study. Cancer Causes Control. 2009;20(5):625–633. doi:10.1007/s10552-008-9273-z
32. Poustchi H, Eghtesad S, Kamangar F, et al. Prospective epidemiological research studies in Iran (the PERSIAN Cohort Study): rationale, objectives, and design. Am J Epidemiol. 2018;187(4):647–655. doi:10.1093/aje/kwx314
33. Yeo C, Saunders N, Locca D, et al. Ficoll-Paque™ versus Lymphoprep™: a comparative study of two density gradient media for therapeutic bone marrow mononuclear cell preparations. Regen Med. 2009;4(5):689–696. doi:10.2217/rme.09.44
34. UNODC W. Opioid Overdose: Preventing and Reducing Opioid Overdose Mortality. Vienna: United Nations Office of Drugs and Crime, World Health Organization; 2013.
35. Afshari M, Janbabaei G, Bahrami MA, Moosazadeh M. Opium and bladder cancer: A systematic review and meta-analysis of the odds ratios for opium use and the risk of bladder cancer. PLoS One. 2017;12(6):e0178527. doi:10.1371/journal.pone.0178527
36. Bakhshaee M, Raziee HR, Afshari R, Amali A, Roopoosh M, Lotfizadeh A. Opium addiction and risk of laryngeal and esophageal carcinoma. Iranian j Otorhinolaryngol. 2017;29(90):19.
37. Shakeri R, Kamangar F, Mohamadnejad M, et al. Opium use, cigarette smoking, and alcohol consumption in relation to pancreatic cancer. Medicine. 2016;95:28. doi:10.1097/MD.0000000000003922
38. Guan G, Zhang D, Zheng Y, et al. microRNA-423-3p promotes tumor progression via modulation of AdipoR2 in laryngeal carcinoma. Int J Clin Exp Pathol. 2014;7(9):5683–5691.
39. Christodoulatos GS, Spyrou N, Kadillari J, Psallida S, Dalamaga M. The role of adipokines in breast cancer: current evidence and perspectives. Curr Obes Rep. 2019;8(4):413–433. doi:10.1007/s13679-019-00364-y
40. Gelsomino L, Naimo GD, Catalano S, Mauro L, Andò S. The emerging role of adiponectin in female malignancies. Int J Mol Sci. 2019;20(9):2127. doi:10.3390/ijms20092127
41. Bråkenhielm E, Veitonmäki N, Cao R, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101(8):2476–2481. doi:10.1073/pnas.0308671100
42. Sun G. Adiponectin-AdipoR1 axis in renal cell carcinoma plays a pivotal role in tumor progression and drug resistance. Am Soc Clin Oncol. 2019;37:634. doi:10.1200/JCO.2019.37.7_suppl.634
43. Babińska A, Pęksa R, Wiśniewski P, Sworczak K. Expression of adiponectin receptors 1 and 2 and the leptin receptor in human adrenal tumors. Arch Med Sci. 2019;15(5):1254. doi:10.5114/aoms.2018.76142
44. Ramzan AA, Bitler BG, Hicks D, et al. Adiponectin receptor agonist AdipoRon induces apoptotic cell death and suppresses proliferation in human ovarian cancer cells. Mol Cell Biochem. 2019;461(1–2):37–46. doi:10.1007/s11010-019-03586-9
45. Govindarajan R, Ratnasinghe L, Simmons DL, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25(12):1476. doi:10.1200/JCO.2006.07.2777
46. MacLennan R, Da Costa J, Day N, Law CH, Ng Y, Shanmugaratnam K. Risk factors for lung cancer in Singapore Chinese, a population with high female incidence rates. Int J cancer. 1977;20(6):854–860. doi:10.1002/ijc.2910200606
47. Masjedi MR, Naghan PA, Taslimi S, et al. Opium could be considered an independent risk factor for lung cancer: a case-control study. Respiration. 2013;85(2):112–118. doi:10.1159/000338559
48. Khademi H, Malekzadeh R, Pourshams A, et al. Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50 000 adults in Iran. BMJ. 2012;344:e2502. doi:10.1136/bmj.e2502
49. Kubota N, Terauchi Y, Kubota T, et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and-independent pathways. J Biol Chem. 2006;281(13):8748–8755. doi:10.1074/jbc.M505649200
50. Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to PPARgamma-agonists. J Biol Chem. 2005.
51. Folkman J. Tumor angiogenesis. In: Advances in Cancer Research. 43. Elsevier; 1985:175–203.
52. Howard J, Beddy P, Ennis D, Keogan M, Pidgeon G, Reynolds J. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. British J Surgery. 2010;97(7):1020–1027. doi:10.1002/bjs.7072
53. Denzel MS, Hebbard LW, Shostak G, Shapiro L, Cardiff RD, Ranscht B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin Cancer Res. 2009;15(10):3256–3264. doi:10.1158/1078-0432.CCR-08-2661
54. Villa NY, Kupchak BR, Garitaonandia I, et al. Sphingolipids function as downstream effectors of a fungal PAQR receptor. Mol Pharmacol. 2008.
55. Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17(1):55. doi:10.1038/nm.2277
56. Takabe K, Paugh SW, Milstien S, Spiegel S. Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi:10.1124/pr.107.07113
57. Landskroner-Eiger S, Qian B, Muise ES, et al. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009;15(10):3265–3276. doi:10.1158/1078-0432.CCR-08-2649
58. Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33(4):547–594. doi:10.1210/er.2011-1015
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
