Back to Journals » International Journal of Women's Health » Volume 14
Ovarian Mature Cystic Teratoma is an Independent Risk Factor for the Premature Rupture of Membranes in Pregnancy: A Single-Center Retrospective Study
Authors Sheng Y, Yuan J, Wang J, Wang L, Li Y, Wang Y
Received 8 July 2022
Accepted for publication 5 October 2022
Published 17 October 2022 Volume 2022:14 Pages 1477—1487
DOI https://doi.org/10.2147/IJWH.S381297
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Yaru Sheng,1 Jiangjing Yuan,1 Jing Wang,1 Liya Wang,1 Yuhong Li,1 Yudong Wang1– 3
1Department of Gynecologic Oncology, International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, People’s Republic of China; 2Shanghai Municipal Key Clinical Specialty, Female Tumor Reproductive Specialty, Shanghai, People’s Republic of China; 3Shanghai Key Laboratory of Embryo Original Disease, Shanghai, People’s Republic of China
Correspondence: Yudong Wang; Yuhong Li, Department of Gynecologic Oncology, International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University, 910 Hengshan Road, Shanghai, People’s Republic of China, Tel +86-21-64070434-18602 ; +86-21-64070434-25517, Email [email protected]; [email protected]
Background: Ovarian mature cystic teratomas (MCTs) are the most common tumors in pregnant women. The premature rupture of membranes (PROM) is a typical complication of pregnancy; however, the relationship between MCT and PROM is unknown. Therefore, we aimed to determine whether MCT is associated with the occurrence of PROM during pregnancy.
Methods: The data of patients with adnexal masses during pregnancy between January 2017 and August 2021 were retrospectively analyzed. Ovarian cystectomy was performed during cesarean delivery or after vaginal delivery. Univariate and multivariate logistic regression models were used for statistical analysis.
Results: A total of 234 patients with histopathological results were included. Among these patients, 21 occurred PROM during pregnancy, of which 11 were diagnosed with MCT. Compared with other subtypes, MCT (p=0.025) showed a stronger correlation with PROM and was an independent risk factor for PROM (odds ratio [OR], 2.811; 95% confidence interval [CI], 1.096– 7.215; p=0.032). Furthermore, we found that MCT with a diameter > 5 cm (p=0.0037) was more likely to promote the development of PROM than those that with a diameter < 5 cm.
Conclusion: MCT was an independent risk factor for PROM during pregnancy. Positive actions and preventative clinical treatments should be fully taken into consideration by clinicians for pregnant women with MCTs, especially those ≥ 5 cm in diameter, to reduce the clinical complications related to MCT-associated PROM.
Keywords: benign ovarian tumor, mature cystic teratoma, premature rupture of membranes, pregnancy
Introduction
Non-epithelial ovarian cancers comprise germ-cell and sex-cord stromal tumors, with each cancer type being subdivided into several histological sub-types, of which ovarian small cell cancers and sarcomas are extremely rare and miscellaneous, having unknown histogenesis.1 Ovarian mature cystic teratoma (MCT), also known as dermoid cyst, is an ovarian germ-cell tumor and the most common benign ovarian neoplasm diagnosed in pregnancy, comprising 24–40% of all cases of ovarian tumors.2 In clinical practice, ultrasound examination can detect it with an accuracy of 97.5%–100%.3 Previous studies have reported multiple complications related to MCT, including torsion (16%), rupture (1–4%), malignant transformation (1–2%), infection (1%), and obstructed labor.4–7
There is still controversy regarding whether surgical intervention and general anesthesia may increase the risk of complications in pregnant women, as an increased incidence rate of spontaneous abortion, pre-term labor, fetal growth restriction, and neonatal death due to surgery during pregnancy have been reported in previous studies.8,9 Therefore, a conservative approach is performed for most ultrasonographically diagnosed MCTs during pregnancy, especially those that are small or nongrowing, in order to justify these potential adverse effects during surgery and anesthesia, and an urgent operative procedure is performed for large tumors because of cyst-related complications–typically, torsion or rupture.10
However, pregnancy is a special state in which the uterus is increasing in size and hormonal levels are changing. It is becoming increasingly important and urgent for clinicians to assess the adverse effects of MCT on pregnancy complications.
Membrane rupture before the onset of labor is defined as a premature rupture of membranes (PROM), and that before 37 weeks of gestation is defined as pre-term PROM (PPROM). PROM occurs in approximately 6–10% of all deliveries, whereas PPROM has a rate of 1–2%. PROM could cause adverse perinatal morbidity and mortality, as well as maternal-fetal perinatal infection, abruptio placentae, umbilical cord accident, and neonatal prematurity.11,12 Previous studies have reported several risk factors for PROM, including maternal infections, such as vaginitis, urinary tract infection, and periodontal infection; incompetent cervix; prenatal diagnostic procedures; cigarette smoking; pre-term delivery; vaginal bleeding; and placental pathology.13,14 Thus far, the etiology of PROM has not been thoroughly clarified. Therefore, it is critical for gynecologists and obstetricians to identify the associated clinical risk factors for PROM.
MCT has been linked to adverse pregnant outcomes, including intrauterine growth restriction, pre-term deliveries, and complicated delivery, in previous studies;4–7,15 however, none of these studies focused on the relationship between MCT and PROM. The inflammatory process involving increased levels of cytokines, prostaglandins, and matrix-degrading enzymes has been reported to promote cervical dilatation, uterine contraction, and PROM.16 Accordingly, given that inflammation may occur from MCT, this study aimed to evaluate the clinical outcomes of patients with adnexal masses during pregnancy to clarify whether MCT is associated with PROM. We analyzed whether MCT is an independent risk factor for PROM and explored the association between MCT diameter and the development of the aforementioned complication, which could help clinicians establish an early surveillance strategy for PROM and provide timely and adequate treatment, consequently reducing the PROM-associated adverse maternal-fetal outcomes.
Materials and Methods
Study Population
This single-center retrospective study included all consecutive patients diagnosed with adnexal masses during pregnancy at the International Peace Maternity and Child Health Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China) from January 2017 to August 2021. All eligible patients underwent cystectomy during cesarean or after vaginal delivery. The baseline characteristics of patients, including their medical history, examination data, and surgical information, were retrospectively collected from the medical records of hospital and outpatient examinations. This study was approved by the Ethics Committee of the International Peace Maternity and Child Health Hospital (approval number: [GKLW] 2021-34) in accordance with the principles of the Declaration of Helsinki.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) diagnosis of adnexal masses during pregnancy; (2) cystectomy during cesarean section or after vaginal delivery; and (3) complete clinical data.
The exclusion criteria were as follows: (1) underlying malignant tumors; (2) opting in for elective abortion for personal reasons; (3) short cervical length or incompetent cervix, uterine anomalies, and trauma; and (4) incomplete clinical data.
Data Collection and Clinical Evaluation
Clinical data, including maternal demographics, pregnancy health, pregnancy characteristics, and neonatal characteristics, were collected. Adnexal masses were detected during routine pre-pregnancy or prenatal examination by ultrasonography, measured during regular obstetric examination, and finally detected before cesarean section or vaginal delivery. The masses were measured in three right-angled planes. The mean diameter was defined as the sum of the three diameters divided by 3. Cystectomy was performed during cesarean section or within three months after vaginal delivery. Histopathological evaluation was conducted by independent pathology specialists who were blinded to the study. Membrane rupture was determined by sterile speculum examination showing pooling of fluid with alkaline pH and ferning on a slide.
A body-mass index (BMI) <18.5 kg/m2 before pregnancy was defined as pre-pregnancy low BMI. Fetal macrosomia refers to a birth weight of ≥4000 g. Delivery at a gestational age of <37 weeks was defined as pre-term delivery. Hypertension (preeclampsia, pregnancy-induced, and preexisting) and diabetes (pregnancy-induced and preexisting) were diagnosed and classified according to the technical bulletin of the American College of Obstetricians and Gynecologists.17,18 Intrahepatic cholestasis of pregnancy (ICP) was suspected when pregnant women complained of pruritus and was further confirmed by elevated serum bile acid concentrations (>10 mmol/L). Reproductive tract infection was diagnosed by laboratory examination of the genital tract secretions, urinary tract infection was diagnosed based on a combination of clinical symptoms and positive urine analysis or culture, and periodontal infection was diagnosed based on clinical symptoms and laboratory examinations by periodontal specialists. Polyhydramnios was defined as an amniotic fluid index ≥25 cm or a vertical pocket of at least 8 cm. Abruptio placentae was defined by ultrasonography or by obvious blood clots between the placenta and uterine wall during cesarean section after 20 weeks of gestation. An abnormal fetal position was diagnosed through transvaginal ultrasonography in the third trimester. Newborns whose 5’ Apgar scores were <7 were diagnosed with neonatal asphyxia.
Statistical Analysis
Unpaired Student’s t-test, Chi-squared test, and Fisher’s exact test were used to compare different variables. Values and percentages, mean and standard deviations, and median and interquartile range (IQR) were used to describe the different varieties of data. Multivariate logistic regression analyses were performed to determine risk factors for PROM. IBM SPSS version 23.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism version 5 (San Diego, CA, USA) were used for statistical analysis. In this study, all hypothesis tests were two-sided with a confidence interval (CI) of 95%, and a p-value <0.05 was considered statistically significant.
Results
Main Characteristics of Patients
As shown in the flowchart of this study (Figure 1), 237 patients diagnosed with adnexal masses during pregnancy who underwent adnexal cyst resection were evaluated at our hospital from January 2017 to August 2021. Among these patients, clinical data from three patients were incomplete. Thus, 234 eligible patients were finally included in the current study. In the cohort, 223 (95.3%) patients underwent cyst resection during cesarean delivery, and the remaining 11 (4.7%) underwent surgery within three months after vaginal delivery.
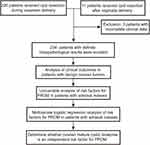 |
Figure 1 Flow chart of the study methodology. |
Patients had a median age at diagnosis of 32 years (IQR, 29.0–35.0]) and a mean pre-pregnancy BMI of 21.0 ± 2.9 kg/m2. Of the tumors included, 203 (86.8%) tumors were unilateral, and 31 (13.2%) were bilateral. In the whole cohort, 21 (9.0%) pregnant women had PROM, of which 4 (19.0%) were PPROM. Moreover, 144 (61.5%) patients presented with tumors <5 cm in diameter, and 90 (38.5%) had tumors ≥5 cm. Additionally, 227 (97.0%) patients were asymptomatic, and seven patients underwent cesarean section due to cyst-related complications, of which five (2.1%) patients had rupture and two (0.9%) experienced torsion. The detailed clinical data are shown in Table 1.
 |
Table 1 Main Baseline Characteristics of Patients |
Histological Subtypes Analysis
We first analyzed the histological type of adnexal masses. As shown in Table 2, 29 (12.4%) patients were diagnosed with functional cysts; there were 11 (4.7%) corpus luteum cysts, 17 (7.3%) theca lutein cysts, and 1 (0.4%) ovarian hyperstimulation syndrome. We found that 205 (87.6%) patients were diagnosed with pathological cysts, including 72 (30.8%) MCTs, 26 (11.1%) serous cystadenomas, 18 (7.7%) mucinous cystadenomas, and 70 (29.9%) endometriomas. The remaining 19 (8.1%) patients had uncommon pathological types, including simple cysts (N=7), mesosalpinx cysts (N=6), ovarian white matter hyperplasia (N=2), ovarian abscess (N=2), ovarian Brenner tumor (N=1), and follicular membrane fibromas (N=1), demonstrating that ovarian MCT was the most common histological subtype of adnexal masses during pregnancy.
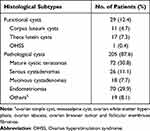 |
Table 2 Histological Subtypes Analysis of Adnexal Masses |
Comparison of the Clinical Outcomes in Benign Ovarian Tumors with Different Histopathological Types
To determine whether histopathological types correlated with clinical outcomes, common ovarian histopathological types, including MCT, serous cystadenoma, mucinous cystadenoma, and endometrioma, were compared between the non-PROM and PROM groups and between the non-pre-term birth and pre-term birth groups. As shown in Table 3, 21 patients with benign ovarian tumors developed PROM during pregnancy; the proportions of PROM among patients with MCT, serous cystadenoma, mucinous cystadenoma, and endometrioma was 11 (52.4%), 2 (9.5%), 0 (0.0%), and 5 (23.8%), respectively. We found that PROM was more common in patients with MCT than in those with other histological types (p=0.025). There were no statistically significant differences in serous cystadenomas, mucinous cystadenomas, and endometriomas between groups. Twenty-four patients had pre-term births, and the proportion of pre-term births in patients with MCT, serous cystadenomas, mucinous cystadenomas, and endometriomas was 7 (29.2%), 2 (8.3%), 2 (8.3%), and 6 (25%), respectively. Moreover, there were no differences in the correlation between pathological subtypes and pre-term birth between groups. Therefore, we speculate that ovarian MCT is closely associated with PROM during pregnancy.
 |
Table 3 The Analysis of Clinical Outcomes (PROM and Pre-Term Birth) in Patients with Benign Ovarian Tumors |
MCT is an Independent Risk Factor for PROM in Pregnancy
Based on the above results, we speculate that ovarian MCT is a risk factor for PROM. Univariate analysis revealed that ovarian MCT (p=0.025), reproductive tract infection (p=0.044), periodontal infection (p=0.034), and a history of PROM (p=0.026) were significantly associated with PROM. There were no significant differences between the non-PROM and PROM groups in terms of urinary tract infection, twin pregnancy, abnormal fetal position, fetal macrosomia, and low pre-pregnancy BMI (Table 4). Multivariate logistic regression further demonstrated that MCT (odds ratio [OR], 2.857; 95% CI, 1.056–7.728; p=0.039) was an independent risk factor for PROM in pregnancy (Table 5).
 |
Table 4 Univariable Analysis of Risk Factors for PROM in Patients with Adnexal Masses |
 |
Table 5 Multivariate Logistic Regression Analysis of Risk Factors for PROM in Patients with Adnexal Masses |
Furthermore, we explored the relationship between MCT diameter and PROM occurrence. As shown in Figure 2, we found that the MCTs associated with PROM had a significantly larger diameter than those that were not (5.773 cm vs 3.662 cm, p=0.0037), indicating that MCTs with a diameter >5 cm may be more likely to develop PROM.
 |
Figure 2 The diameter of mature cystic teratoma in non-PROM group vs PROM group. **Means p <0.01 (p=0.0037). |
Discussion
As a widespread of obstetric ultrasound examination, the incidence of adnexal masses during pregnancy has increased to 0.05–2.4% in recent years.2 Most masses are benign, and only 1–6% are malignant.19 As of 2018, 193 gestational patients have been diagnosed with non-epithelial ovarian cancers, of which 145 were germ cell tumors, 52 were endodermal sinus tumors, 45 were dysgerminomas, and 24 were immature teratomas. Mixed elements were revealed in 13 patients.20 Conservative treatment is predominantly considered for most patients with benign adnexal masses during pregnancy; emergent surgical intervention could be performed for symptomatic complications, including typically ovarian torsion or cyst rupture in large masses.21
MCT is the most common adnexal mass found in pregnancy, comprising 24–40% of all ovarian tumors.2,19 Many previous studies have reported that MCT could invade neighboring organs, including urinary bladder, small intestine, sigmoid colon, rectum, and transverse colon, leading to the formation of fistulas.22–34 Recent studies have even concluded that MCT could be strikingly related to Anti-N-Methyl-D-Aspartate Receptor Encephalitis, a rare and understudied paraneoplastic neurological syndrome in both pregnant and non-pregnant patients.35,36 It has been linked to adverse pregnancy outcomes, such as intrauterine growth restriction and pre-term deliveries, and could even complicate labor by obstructing the birth canal.4–7,15 However, current knowledge on the correlation between MCT and PROM is still scarce. Our study reported that MCT occurred in 52.4% of the included PROM cases with adnexal masses. Furthermore, this study is the first to indicate that MCT is an independent risk factor for PROM. Our findings further improved the knowledge of rare complications of MCT during pregnancy.
PROM complicates approximately 8% of all pregnancies, and PPROM occurs in approximately 1%–2% of all pregnancies, with serious maternal and neonatal consequences, including intrauterine infection, abruptio placentae, umbilical cord accident, and risks of pulmonary hypoplasia and fetal deformation abnormalities.11,12 Although multiple clinical risk factors have been proposed, including reproductive tract infection, short cervical length or incompetent cervix, uterine anomalies, low body mass index, low socioeconomic status, cigarette smoking, illicit drug use, multiple gestations, and trauma, the etiology of PROM appears quite varied, and it is not uncommon that some patients with PROM have no previous risk, indicating that the exact etiology has not yet been fully elucidated.13,14
Various mechanisms have been proposed for PROM, including mechanical and infectious or inflammatory processes.37,38 Herein, we indicated that teratoma-associated inflammation during pregnancy could promote the development of PROM. A previous study revealed that inflammatory pathways sequentially involving periodontal tissue, maternal serum, and finally the vaginal compartment could contribute to periodontitis-triggered PPROM.39 Moreover, teratoma is a germ cell tumor with bizarre cellular compositions. Multiple studies have proposed that the presence of an inflammatory response in patients with MCT leads to invasion into neighboring or distant organs.22–33,35,36 Collectively, inflammatory responses originating from ovarian MCT sequentially involve maternal serum and ultimately the intrauterine compartment, which could possibly lead to the development of PROM.
Additionally, we also found that the diameter of the MCT associated with PROM was significantly larger than that without PROM, indicating that MCT with a large diameter (>5 cm) was more likely to promote the development of PROM. Our results were, to some extent, consistent with those of a previous study, reporting that cyst-related complications, including torsion, rupture, and malignancy, all increased during pregnancy in most cases with a teratoma of >5 cm in diameter.40 Moriarty et al reported that inflammatory processes secondary to teratoma rupture in pregnancy could promote PPROM by stimulating uterine contraction.41 Based on previous researches, we speculated that the pressure effect and inflammation culminated in large teratomas, which are more likely to trigger PROM during pregnancy by directly stimulating uterine contraction or initiating the maternal inflammatory cascades derived from MCT, maternal blood, and ultimately the vaginal compartment.
Predicting and identifying patients at risk of PROM or PPROM is important and challenging for clinicians. The results of our study provide new insights into MCT-associated complications during pregnancy and contribute to a novel understanding of clinical risk factors for PROM. Therefore, it is important for clinicians to take appropriate intervention measures before pregnancy or adequate prenatal care for MCTs with a diameter >5 cm to reduce the risk of perinatal morbidity.
The limitations of our study include incomplete data, and the sample size was relatively small in this retrospective study. Further multicenter studies with larger sample sizes are needed to confirm our results.
Conclusion
In the present study, we found that MCT was the most common benign ovarian tumor in patients with adnexal masses during pregnancy and that patients with MCT were more likely to develop PROM. We first demonstrated that MCT was an independent risk factor for PROM, and patients with an MCT ≥5 cm in diameter were more likely to develop PROM than those with smaller-diameter MCTs. Therefore, early surveillance of the occurrence of PROM and positive interventions should be considered by clinicians in patients with MCT, especially those >5 cm in diameter, to lower the risk of unfavorable maternal and neonatal outcomes due to PROM.
Abbreviations
MCT, Ovarian mature cystic teratoma; PROM, Premature Rupture of Membranes; PPROM, Preterm premature Rupture of Membranes; BMI, Body mass index; ICP, Intrahepatic cholestasis of pregnancy; AFI, amniotic fluid index; UO, Unilateral ovary; BO, Bilateral ovaries; IVF, In vitro fertilization; OHSS, Ovarian hyperstimulation syndrome.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of IPMCH ((GKLW)2021-34). All participants provided written informed consent.
Data Sharing Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Author Contributions
Yudong Wang and Yuhong Li conceived the study. Yaru Sheng analyzed the data and wrote the manuscript. Jiangjing Yuan assisted in some analysis and substantial revision of the article. Jing Wang and Liya Wang contributed to the acquisition of data. All authors contributed to data analysis, data drafting and revision of the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by Shanghai Municipal Key Clinical Specialty (No. shslczdzk06302), “Science and Technology Innovation Action Plan” International Science and Technology Cooperation Project (No. 20550760600), Clinical Technology Innovation Project of Shanghai Shenkang Hospital Development Center (No. SHDC12020130), Joint research projects of Xuhui Health Commission for critical diseases (XHLHGG202111), Shanghai Jiao Tong University Medicine-Engineering Fund (No. YG2022ZD027), National Natural Science Foundation of China (No. 81172477, 81402135), Shanghai Jiao Tong University School of Medicine, multi-center clinical research project (No. DLY201827).
Disclosure
The authors declare that they have no competing interests.
References
1. Boussios S, Karathanasi A, Zakynthinakis-Kyriakou N, et al. Ovarian carcinosarcoma: current developments and future perspectives. Crit Rev Oncol Hematol. 2019;134:46–55. doi:10.1016/j.critrevonc.2018.12.006
2. Grigoriadis C, Eleftheriades M, Panoskaltsis T, et al. Ovarian cancer diagnosed during pregnancy: clinicopathological characteristics and management. G Chir. 2014;35(3–4):69–72.
3. Caspi B, Appelman Z, Rabinerson D, Elchalal U, Zalel Y, Katz Z. Pathognomonic echo patterns of benign cystic teratomas of the ovary: classification, incidence and accuracy rate of sonographic diagnosis. Ultrasound Obstet Gynecol. 1996;7(4):275–279.
4. Chen L-H, Chang S-D, Huang H-Y, Wang H-S, Soong Y-K, Wu H-M. Repeated pregnancy with concomitant presence of ovarian teratoma: a case report and literature review. Taiwan J Obstet Gynecol. 2017;56(5):694–696.
5. Katz L, Levy A, Wiznitzer A, Sheiner E. Pregnancy outcome of patients with dermoid and other benign ovarian cysts. Arch Gynecol Obstet. 2010;281(5):811–815.
6. Turkcuoglu I, Meydanli MM, Engin-Ustun Y, Ustun Y, Kafkasli A. Evaluation of histopathological features and pregnancy outcomes of pregnancy associated adnexal masses. J Obstet Gynaecol. 2009;29(2):107–109.
7. Apostolakis-Kyrus K, Indermaur MD, Prieto J. Teratoma in pregnancy: a case report. J Reprod Med. 2013;58(9–10):458–460.
8. Duncan PG, Pope WD, Cohen MM, Greer N. Fetal risk of anesthesia and surgery during pregnancy. Anesthesiology. 1986;64(6):790–794.
9. Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Am J Obstet Gynecol. 1989;161(5):1178–1185.
10. Cavaco-Gomes J, Jorge Moreira C, Rocha A, Mota R, Paiva V, Costa A. Investigation and management of adnexal masses in pregnancy. Scientifica. 2016;2016:3012802. doi:10.1155/2016/3012802
11. Major CA, de Veciana M, Lewis DF, Morgan MA. Preterm premature rupture of membranes and abruptio placentae: is there an association between these pregnancy complications? Am J Obstet Gynecol. 1995;172(2 Pt 1):672–676. doi:10.1016/0002-9378(95)90591-X
12. Naef RW 3rd, Allbert JR, Ross EL, Weber BM, Martin RW, Morrison JC. Premature rupture of membranes at 34 to 37 weeks’ gestation: aggressive versus conservative management. Am J Obstet Gynecol. 1998;178(1 Pt 1):126–130. doi:10.1016/S0002-9378(98)70638-6
13. Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101(1):178–193. doi:10.1016/s0029-7844(02)02366-9
14. Mercer BM, Goldenberg RL, Meis PJ, et al. The Preterm Prediction Study: prediction of preterm premature rupture of membranes through clinical findings and ancillary testing. The National Institute of Child Health and Human Development maternal-fetal medicine units network. Am J Obstet Gynecol. 2000;183(3):738–745. doi:10.1067/mob.2000.106766
15. Caspi B, Levi R, Appelman Z, Rabinerson D, Goldman G, Hagay Z. Conservative management of ovarian cystic teratoma during pregnancy and labor. Am J Obstet Gynecol. 2000;182(3):503–505. doi:10.1067/mob.2000.103768
16. Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition–a review. Placenta. 2003;24(Suppl):
17. American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237–e260. doi:10.1097/AOG.0000000000003891
18. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 190: gestational Diabetes Mellitus. Obstet Gynecol. 2018;131(2):e49–e64. doi:10.1097/AOG.0000000000002501
19. Baser E, Erkilinc S, Esin S, et al. Adnexal masses encountered during cesarean delivery. Int J Gynaecol Obstet. 2013;123(2):124–126. doi:10.1016/j.ijgo.2013.06.015
20. Boussios S, Moschetta M, Tatsi K, Tsiouris AK, Pavlidis N. A review on pregnancy complicated by ovarian epithelial and non-epithelial malignant tumors: diagnostic and therapeutic perspectives. J Adv Res. 2018;12:1–9.
21. Sherard GB 3rd, Hodson CA, Williams HJ, Semer DA, Hadi HA, Tait DL. Adnexal masses and pregnancy: a 12-year experience. Am J Obstet Gynecol. 2003;189(2):
22. Suzuki M, Fukasawa M, Hara T. Dermoid cyst with thyroid follicle perforated into bladder and ileum: a case report. Nihon Hinyokika Gakkai Zasshi. 1999;90(5):586–589.
23. Tabata K, Nagakura K, Hirano I, Baba S. A case of fistula between the urinary bladder and an ovarian dermoid cyst. Hinyokika Kiyo. 2004;50(3):219–221.
24. Kizaki Y, Nagai T, Ohara K, et al. Ovarian mature cystic teratoma with fistula formation into the rectum: a case report. Springerplus. 2016;5(1):1700.
25. Conway AZ, Hanna T. A dermoid cyst fistulating with the transverse colon. Ann R Coll Surg Engl. 2012;94(7):e230–231.
26. von-Walter AR, Nelken RS. Benign cystic ovarian teratoma with a fistula into the small and large bowel. Obstet Gynecol. 2012;119(2 Pt 2):434–436.
27. Salame G, Sherer DM, Shah T, et al. Mature cystic teratoma of the sigmoid colon. Ultrasound Obstet Gynecol. 2011;37(6):739–740.
28. Rajaganeshan R, Wang H, Abouleid A, Scott G, Selvasekar CR. Conservative surgery in the management of a benign ovarian cystic teratoma presenting as a rectal mass: a case report. Ann R Coll Surg Engl. 2011;93(5):e46–48.
29. Tandon A, Gulleria K, Gupta S, Goel S, Bhargava SK, Vaid NB. Mature ovarian dermoid cyst invading the urinary bladder. Ultrasound Obstet Gynecol. 2010;35(6):751–753.
30. Cebesoy FB, Baskonus I, Mete A, Kutlar I, Aybasti N. Benign ovarian dermoid cyst complicated with rectal fistula formation: an unusual case. Arch Gynecol Obstet. 2009;279(2):179–181.
31. Landmann DD, Lewis RW. Benign cystic ovarian teratoma with colorectal involvement. Report of a case and review of the literature. Dis Colon Rectum. 1988;31(10):808–813.
32. Ulstein M, Baydia JL, Pradhan U. Fistula between the urinary bladder and an ovarian dermoid tumor. Acta Obstet Gynecol Scand. 1987;66(8):723–724.
33. Shiels WE, Dueno F, Hernandez E. Ovarian dermoid cyst complicated by an entero-ovarian fistula. Radiology. 1986;160(2):443–444.
34. Robinson RH. Ovarian Teratoma, invading the Bladder. Proc R Soc Med. 1938;31(9):1076–1077.
35. Wu CY, Wu JD, Chen CC. The association of ovarian teratoma and Anti-N-Methyl-D-Aspartate receptor encephalitis: an updated integrative review. Int J Mol Sci. 2021;22:20.
36. Gu J, Chen Q, Gu H, Duan R. Research progress in teratoma-associated anti-N-methyl-D-aspartate receptor encephalitis: the gynecological perspective. J Obstet Gynaecol Res. 2021;47(11):3749–3757.
37. Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27(11–12):1037–1051.
38. Zhu J, Ma C, Luan X, Li J, Peng F, Huang L. Inflammasome components and ADAMTS4 in premature rupture of membranes. Mol Med Rep. 2021;23:2.
39. Mohr S, Amylidi-Mohr SK, Stadelmann P, et al. Systemic inflammation in pregnant women with periodontitis and preterm prelabor rupture of membranes: a prospective case-control study. Front Immunol. 2019;10:2624.
40. Peterson WF, Prevost EC, Edmunds FT, Hundley JM
41. Moriarty KT, Wahab L, Vittay G, Neales K. Rupture of dermoid cyst presenting with recurrent abdominal pain, preterm prelabour rupture of membranes and sterile chorioamnionitis. J Obstet Gynaecol. 2005;25(5):514.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
