Back to Journals » Clinical Ophthalmology » Volume 16
Outcomes of Severe Fungal Keratitis Using in vivo Confocal Microscopy and Early Therapeutic Penetrating Keratoplasty
Authors Sourlis C, Seitz B , Roth M, Hamon L , Daas L
Received 27 January 2022
Accepted for publication 25 May 2022
Published 12 July 2022 Volume 2022:16 Pages 2245—2254
DOI https://doi.org/10.2147/OPTH.S358709
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Chrysovalantis Sourlis,1 Berthold Seitz,1 Mathias Roth,2 Loïc Hamon,1 Loay Daas1
1Department of Ophthalmology, Saarland University Medical Center, UKS, Homburg/Saar, Germany; 2Department of Ophthalmology, Heinrich-Heine University Düsseldorf, Düsseldorf, Germany
Correspondence: Loïc Hamon, Department of Ophthalmology, Saarland University Medical Center, UKS, Homburg/Saar, Germany, Tel +49-6841-1622387, Fax +49-6841-1622400, Email [email protected]
Purpose: The purpose of this study was to assess the impact of early diagnosis using in vivo confocal microscopy and early therapeutic penetrating keratoplasty (TPK) on the outcomes of severe cases of fungal keratitis.
Methods: This retrospective single-center study included 38 patients (40 eyes) with fungal keratitis who presented between December 2013 and February 2020. Preoperative, intraoperative, and postoperative parameters were recorded to assess the role of early correct diagnosis and early surgical therapy on visual acuity outcome and enucleation rate during follow-up.
Results: The mean patient age was 51 years (71% females). The initial external diagnosis was correct in 20 cases (50%). The mean time from symptom onset until admission to our department was 46.8 ± 68.0 (median 28.5) days. The mean time to correct diagnosis after admission to our department was 1 day with in vivo confocal microscopy (IVCM). IVCM was performed in 38 cases, of which 36 (sensitivity: 94.7%) were positive for fungal infection. Twenty-seven out of 40 (67.5%) eyes received a TPK 4.2 ± 3.9 days after admission, with a mean graft size of 8.9 ± 1.9 mm. Three eyes (7.5%) were enucleated. The corrected distance visual acuity of the entire study population increased from 2.0 ± 1.2 LogMAR to 0.96 ± 1.17 LogMAR.
Conclusion: In vivo confocal microscopy is a powerful tool for the early detection of fungal organisms in infectious keratitis. An early TPK with a large graft helps to eradicate the infection timely and results in a favorable visual acuity outcome and lower enucleation rate, especially when treating filamentous fungi.
Keywords: fungal keratitis, in vivo confocal microscopy, penetrating keratoplasty
Introduction
Fungal keratitis is a severe and potentially devastating eye disease. The global incidence is estimated at a minimum of 1 million cases annually, and probably about 100000 eyes are removed annually because of late diagnosis and poor therapeutic outcome.1 Although the infection mostly affects male agricultural workers in developing countries following eye trauma with organic material,2 in recent years, there has been a significant rise in fungal keratitis also in western countries such as Germany, which is mostly attributed to an increase in soft contact lens (CL) wear.3,4
The large international outbreak of contact lens-associated fungal keratitis in 2005–2006 in developed countries and recent results from the German Fungal Keratitis Registry showed how certain filamentous fungal species, such as Fusarium sp., can lead to devastating disease, up to eye loss in otherwise young and healthy patients.4–7
The clinical picture of fungal keratitis varies and cannot be clearly differentiated in the early phase from other types of CL-associated keratitis. The lapse of time until the correct organism is identified and the delay in administering the proper therapy often results in a poor long-term outcome and a higher enucleation rate in fungal keratitis than in other cases of microbial keratitis.8 In addition, a contributing factor to severe outcomes of Fusarium sp. keratitis is the poor response to antifungal medication.9,10
The purpose of this study was to analyze the impact of early diagnosis using immediate in vivo confocal microscopy and early therapeutic penetrating keratoplasty (TPK) on the outcomes of severe cases of fungal keratitis (presenting intraocular involvement) with regard to long-term eradication of fungal organisms, enucleation rate, and long-term visual acuity.
Materials and Methods
This retrospective single-center study included 40 eyes of 38 patients with confirmed fungal keratitis that were treated between December 2013 and February 2020 at our Department. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethical committee (Ethikkommission der Ärztekammer des Saarlandes) with the nr. 128/21.
On the day of admission, all patients underwent slit lamp examination and in vivo confocal microscopy (IVCM) (Heidelberg Retina Tomograph III with Rostock Cornea Module, Heidelberg Engineering GmbH, Dossenheim, Germany). In addition, an A and B-scan ultrasonography was routinely performed to assess potential endophthalmitis and the necessity of further surgical treatment with pars plana vitrectomy and intravitreal antibiotic/antifungal injections.
Microbiological confirmation of the fungal organism was performed by means of polymerase chain reaction (PCR), microbiological growth culture, and histopathological examination (from the recipient’s corneal button, if penetrating keratoplasty). Only patients with at least one positive confirmation of fungal organisms with one of the aforementioned diagnostic methods or a high suspicion of fungal organisms in the in vivo confocal microscopy (IVCM) were included in this study.
Initial treatment of fungal keratitis required the use of topical voriconazole 2% after admission if the infectious pathogen was unknown. The most commonly used antifungals included topical and systemic voriconazole, topical amphotericin B 0.5%, and topical natamycin 5%. For Fusarium sp. keratitis, topical natamycin 5% was the drug of choice.11 In addition, a combination with polyhexamethylene biguanide was used in selected cases. In severe cases of fungal keratitis (presenting intraocular involvement), systemic allylamines such as terbinafine hydrochloride 250 mg and systemic voriconazole 2×200 mg were added.
Multiple anterior chamber irrigations with antifungal medication were performed every two days in cases of anterior chamber involvement (in the sense of therapy-refractory hypopyon).
If necessary, TPK was performed under general anesthesia. The donor and host trephination was performed mechanically or by utilization of a 193-nm excimer laser.12,13 A large-size host excision with a safe distance to the corneal stromal infiltrate was preferably used if feasible. The graft (oversize 0.1 to 0.5 mm) was fixed using 24–32 deep single sutures. Postoperative therapy included topical antifungal and antimicrobial agents, and regular checkups twice daily. Topical and systemic corticosteroids were started 1–2 weeks after the TPK, when the initial infection had subsided, in order to prevent immunological graft reactions. As an interim solution until the use of corticosteroids, cyclosporin A was alternatively applied systemically for immunosuppression.
Patients with endophthalmitis underwent pars plana vitrectomy with or without intravitreal injections of antifungal agents.
Routine follow-ups were performed to assess the long-term corrected distance visual acuity (CDVA) and successful eradication of the fungal organism. In the absence of improvement after multiple TPKs with persistence of the fungal organism, the patients` eyes were enucleated.
Preoperative patient data such as demographics, onset of symptoms until the first surgery, medical history, and CDVA were collected, analyzed, and correlated with the follow-up data. CDVA was tested with spectacles, counting fingers were registered as LogMAR 2, hand motion was counted as LogMAR 3, and light perception as LogMAR 4.
Statistical evaluation was performed using SPSS version 27 (SPSS Inc., Chicago, IL, USA). Values were expressed as mean ± standard deviation (SD) (minimum – maximum). Wilcoxon signed-rank test was used to compare CDVA at admission and at the last follow-up (14.9 ± 17.6 months). Statistical significance was set at P < 0.05.
Results
Twenty-seven out of 38 patients were female (71%) and the mean age of the patients was 50.6 ± 17.9 (16–88) years. Twenty-seven patients (71%) wore contact lenses with a female-to-male ratio of 5.75:1. Seven patients (18.4%) had a history of ocular trauma. Farm or garden work preceding ocular symptoms was reported in three patients (7.9%). The mean time interval between symptom onset and first presentation at our department was 46.8 ± 68.0 (1–410, median 28.5) days. The demographic characteristics and preoperative data are summarized in Table 1.
 |
Table 1 Patient Demographics, Symptom Onset, Time Before TPK After Admission, Pre- and Postoperative Outcome |
Fungal keratitis was diagnosed by external physicians prior to admission in 20 eyes (50%). The most common misdiagnoses were bacterial keratitis in 8 cases (20%), herpetic keratitis in 8 cases (20%), and acanthamoeba keratitis in 4 cases (10%).
IVCM was performed in 38 cases, of which 36 (sensitivity: 94.7%) were positive for fungal infection. PCR was performed in 33 cases, of which 25 (75.7%) were positive for fungal organisms. Microbiological culture was performed in 38 cases, of which 26 (68.4%) were positive for fungal growth. Histopathological examination of the cornea was performed in 29 cases, of which 18 (62.1%) showed fungal organisms. The absolute numbers of examinations performed and positive results are displayed in Figure 1.
Coinfection with two fungal species was present in seven (17.5%) cases. The fungal species identification obtained by either microbiological culture or PCR revealed in 14 out of 47 (29.8%) cases Fusarium sp., in 9 out of 47 (19.1%) cases Aspergillus sp., in 7 out of 47 (14.9%) cases Candida sp. and in 17 out of 47 (36.2%) cases “others”. The subgroup “others” included the fungal species Malassezia sp., Pseudallescheria boydii, Sarocladium kiliense, Purpureocillium lilacinum and fungal species without further classification, only identified by IVCM or histopathological examination. In total, 7 cases (17.5%) could only be identified by fungal hyphae present in IVCM or histopathology without further classification. After admission to our Department, it took on average 0.6 ± 1.0 (0–4) days to confirm the diagnosis of fungal keratitis with IVCM, 7.6 ± 12.6 (1–61) days with PCR, 15.0 ± 14.4 (1–57) days with microbial culture growth and 8.2 ± 5.1 (3–21) days with histopathological examination.
At the time of admission to our department, the mean CDVA was 2.0 ± 1.2 LogMAR and improved highly significantly to 0.96 ± 1.17 LogMAR (p < 0.001) at the end of the follow-up. In 16 patients, the initial CDVA was particularly poor, with 12 cases (30%) presenting hand movements and 4 cases (10%) presenting light perception. The cases that did not need further surgical treatment had a favourable outcome with a mean initial CDVA of 0.9 LogMAR and 0.25 LogMAR visual acuity at the end of follow-up.
Prior to presentation at our department, 8 patients were surgically treated externally. Three eyes had already received a photo-activated chromophore for Keratitis-Corneal Cross-linking (PACK-CXL) and another 3 eyes had a small-size TPK, without success in eradicating the fungal organisms. Of the three PACK-CXL cases, two eyes could be durably treated with one TPK after PACK-CXL. The last eye presented a severe reinfection after the PACK-CXL, needed two TPKs and was enucleated in the course.
Twenty-seven out of 40 cases (67.5%) underwent anterior chamber irrigation, including sample aspiration for PCR testing on the day of admission. The mean number of all anterior chamber irrigations was 4.8 ± 6.6 (1–29), whereas 6 cases received more than 10 anterior chamber irrigations.
In total, 27 (67.5%) of all cases received a TPK 4.2 ± 3.9 (1–21) days after admission, including the 3 eyes that had previously received a small-size TPK externally. A large graft with at least 24–32 single sutures was performed, when feasible, because of the risk of early suture loosening and the subsequent need for resuturing. In 9 of the 27 TPKs (33.3%), we carried out re-TPK due to the recurrence of fungal keratitis in 7 cases and due to graft failure in 2 cases. The mean time interval between the first and second TPK was 147.1 ± 152.3 (6–422) days. Nine patients received two, 5 patients received three and 2 patients received four TPKs, respectively. The fungal species present in the eyes that received multiple TPKs were Fusarium sp. in 6/9 cases, Aspergillus sp. in 2/9 cases and Pseudallescheria boydii in 1/9 cases.
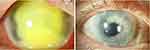
The mean size of the grafts used at our Department was 8.9 ± 1.9 (7.75–13.0) mm for the first TPK. The indication for re-TPKs was a recurrence of the fungal keratitis in 8 cases, with a mean graft size of 8.4 ± 0.7 (7.5–10.0) mm, and graft failure in 1 case, with a graft size of 10.5 mm. One patient received a 13.0 mm graft (case no. 15) in the first TPK, without recurrence of the fungal keratitis and received a small-sized 7.6 mm optical re-keratoplasty after 317 days due to secondary endothelial graft failure (Figure 2).
Severe cases of fungal infection with intraocular involvement (endophthalmitis) underwent further surgical treatment. Seven out of the 40 (17.5%) eyes needed at least one pars plana vitrectomy (PPV), including the 3 patients who had received TPK prior to admission to our department. Six patients required more than 3 PPVs. The mean time interval from admission to the first PPV was 31.3 ± 31.6 (5–81) days. In addition, 7 patients received a mean of 8.2 ± 7.1 (1–21) intravitreal antifungal injections.
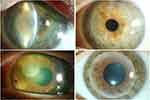
Three patients (7.5%) with an unfavorable course before admission to our department (58.3 ± 43.1 days) showed a destructive course despite therapy and had to be enucleated. They all presented with Fusarium sp. keratitis, one with coinfection with Aspergillus sp. All three patients were treated for a prolonged time at an external hospital before admission to our department. Patients who were directly referred to our department for first-line treatment did not require enucleation in any of the cases. The 15 cases that were referred to our department in the first two weeks from symptom onset showed a favorable outcome with the aforementioned combined conservative and surgical treatment. Those cases showed a mean CDVA of 1.2 ± 0.85 LogMAR at admission, which improved to 0.41 ± 0.82 LogMAR at the last follow-up (Figure 3).
Discussion
In developed countries, fungal organisms are rare agents of keratitis and are frequently associated with poor prognosis. In particular, contact lens wearers have a higher risk of developing a highly resistant strain of Fusarium sp. This underlines the role of contact lenses as a substantial risk factor for fungal keratitis in developed countries such as Germany.4 Our epidemiologic data are consistent with similar comparable studies conducted in Europe (Table 2).14–16
 |
Table 2 Comparison of the Data of European Studies with Comparable Climate and Study Design |
The type of fungal species seems to be an important factor in determining the long-term outcome of fungal keratitis. All three enucleated eyes in our study showed infection with Fusarium sp. This fungal organism belongs to the family of filamentous fungi, which is reported to cause severe outcomes of fungal keratitis with a higher enucleation rate than other species.2 The main reason seems to be the high intrinsic resistance of Fusarium sp. to antifungal medication or disinfection solutions,3 which enables the organism to survive for a prolonged time and progress from superficial keratitis to endophthalmitis.17 Our study showed that those organisms can nonetheless be eradicated by applying polyenes intracamerally multiple times early on, in order to reach a high antifungal concentration locally while diminishing the systemic toxicity in humans. A favorable effect in the reduction of hypopyon and fungi with intracameral polyene drugs has been reported previously.18
The decisive factor for a poor outcome of fungal keratitis is the long delay from clinical manifestation to the establishment of the correct diagnosis. Since the clinical picture of CL-associated fungal keratitis can be similar to that of other microbial pathogens in the early stages, this circumstance leads to a delayed onset of the correct therapy.
By using IVCM, a faster diagnosis of fungal keratitis can be made in the hospital at the time of admission, even in the case of atypical clinical manifestations, thus before microbiological detection with PCR or culture. In vivo confocal microscopy helps to visualize fungal hyphae instantaneously, nonetheless depending on the examiner`s expertise.19 This allows a fast adjustment of the therapy to the fungal species.20,21
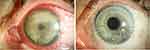
Case no. 21 in our cohort is a perfect example of the role of IVCM as a diagnostic tool. This patient underwent multiple PCR, microbial cultures, and histopathological examinations of corneal scrapings externally, and all were negative for any microbial or fungal infection. The IVCM examination showed fungal hyphae with a typical parallel “railway tracks” pattern22 at a depth of over 200 µm in the cornea (Figure 4, right). At this depth, most corneal scrapings for PCR would be negative because of insufficient depth. The sensitivity of confocal microscopy has repeatedly been reported in the range of 80–94%,23–25 which coincides with our findings.
 |
Figure 4 In vivo confocal microscopy findings. Many hyphae in the form of bright branched hyperreflective lines of varying width (left, middle, right) some of which appeared parallel (“railways tracks” - white arrow) in the corneal stroma, in the sense of fungal hyphae in context of fungal keratitis; (left) 44-year-old female patient as described in Figure 3 (case no. 3); (middle) 52-year-old female patient as described in Figure 2 (case no. 15); (right) 33-year-old female patient (case no. 21) with fungal hyphae at a depth over 200 µm in the cornea identified with in vivo confocal microscopy. |
In previous studies, some authors advised performing a TPK mostly in cases of very severe fungal keratitis with pending corneal perforation or in case of therapy-resistant infections after prolonged use of topical and systemic antifungal medication, mostly presenting hypopyon.26,27 Gao et al reported the treatment of fungal keratitis performing a deep anterior lamellar keratoplasty (DALK) with a recurrence rate of 9% due to fungal hyphae persistence in the deep stroma.28,29 In our study, no DALK was performed.
Other recent studies have reported favorable results after early TPK in fungal keratitis. Xi et al showed that early TPK only 2 days after admission, in combination with anterior chamber irrigation, was very effective in treating fungal keratitis, with a low rate of recurrence while improving the CDVA. In more than half of the patients, the graft size was larger than 8.5 mm, similar to our approach.30
Another study included 28 patients with very severe fungal keratitis who received TPK with an oversized graft (mean 10.5 mm) within the first week of admission.31 In 80% of these cases, the anatomic integrity of the eye was reduced, the infection was completely eradicated, and vision was saved or improved. The enucleation rate was 14%. Similar findings were reported by Rangel et al in a study assessing the outcome of large-diameter keratoplasty in severe cases of infectious keratitis.8 In which the enucleation rate was 17%.
Koçluk et al compared two groups of cases of fungal/bacterial keratitis. One group received an early TPK within 15 days of symptom onset, and the second group received a TPK much later. The first group had a 100% therapeutic success with clear grafts, improved vision, and no recurrence, whereas the second group had only 83.3% therapeutic success, with 16.7% recurrence and one eviscerated eye.32 Laurik et al reported similar findings comparing two groups of patients with therapy-resistant acanthamoeba keratitis, where one group receiving early TPK showed better visual rehabilitation and graft survival than the second group receiving a TPK later on with an enucleation rate of 4.3%.33
Chatterjee et al showed that the ability of fungi to infiltrate usually inaccessible tissue is a major factor for the recurrence of fungal keratitis. Since filamentous fungi may easily penetrate Descemet’s membrane, they spread quickly into the anterior chamber angle and even into the retroiridal space.34 The authors reported that a misplaced optimism regarding the success of conservative therapy associated with the limited availability of donor cornea delayed the decision for a TPK. For this reason, early TPK should be considered and remain an effective treatment for filamentous fungal keratitis.
To allow early detection of fungal keratitis, we proposed the use of IVCM on the day of admission or the first examination. This should always be performed by an experienced examiner prior to corneal scrapping. In vivo confocal microscopy can be used as a recurrence marker in unclear situations, also after TPK.22
Regarding the TPK, a large graft is favorable with a safe distance from the infectious infiltrate and multiple (24–32) single sutures (Figure 5). After completion of the suture placement, a circular intrastromal voriconazole injection using a 30 gauge needle at the excision site of the host stroma may add to the safety of the procedure in terms of preventing recurrence of the infection.35,36
The main limitation of this study is that the patient population was referred to a tertiary cornea-specialized referral center. The most severe cases at a late stage of the infection were referred to our department. For this reason, it is difficult to generalize our results to an “average population” with fungal keratitis.
In conclusion, fungal keratitis is a severe and difficult to treat infectious disease. This outcome is influenced by multiple factors. The delayed correct diagnosis enables the pathogen to spread deep into the eye, resulting in a poor visual outcome. Our results underline the following: (1) the important role of in vivo confocal microscopy for the immediate diagnosis of fungal keratitis and (2) the need for early TPK with a sufficiently large graft diameter and safety distance. These considerations could improve the outcomes of patients with fungal keratitis.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the local ethical committee (Ethikkommission der Ärztekammer des Saarlandes with the registration number 128/21).
Informed Consent
According to the guidelines of the local ethical committee (Ethikkommission der Ärztekammer des Saarlandes), formal consent was not required for this study. Patient data confidentiality was guaranteed at all time.
Acknowledgments
We thank Mrs. Christina Turner for her valuable linguistic additions and corrections in this manuscript and Mr. Cristian Munteanu for his valuable help with the statistical analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosures
Chrysovalantis Sourlis, Berthold Seitz, Mathias Roth, Loïc Hamon and Loay Daas declare that they do not have conflicts of interest in this work.
Funding
This study did not receive any grants or funding.
References
1. Brown L, Leck AK, Gichangi M, Burton MJ, Denning DW. The global incidence and diagnosis of fungal keratitis. Lancet Infect Dis. 2021;21:e49–e57. doi:10.1016/S1473-3099(20)30448-5
2. Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009;57:273–279. doi:10.4103/0301-4738.53051
3. Khor W-B, Aung T, Saw S-M, et al. An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. JAMA. 2006;295:2867–2873. doi:10.1001/jama.295.24.2867
4. Roth M, Holtmann C, Daas L, et al. Results From the German Fungal Keratitis Registry. Cornea. 2021;40:1453–1461. doi:10.1097/ICO.0000000000002705
5. Chang DC, Grant GB, O’Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296:953–963. doi:10.1001/jama.296.8.953
6. Gaujoux T, Chatel MA, Chaumeil C, Laroche L, Borderie VM. Outbreak of contact lens-related Fusarium keratitis in France. Cornea. 2008;27(9):1018–1021. doi:10.1097/ICO.0b013e318173144d
7. Morgan PB, Efron N. A decade of contact lens prescribing trends in the United Kingdom (1996–2005). Contact Lens Anterior Eye. 2006;29:59–68. doi:10.1016/j.clae.2006.02.008
8. Alfaro Rangel R, Szentmáry N, Lepper S, et al. Perforierende Keratoplastiken mit großem Durchmesser sind meist auf eine sehr schwere infektiöse Keratitis zurückzuführen und können eine sekundäre Enukleation nicht immer verhindern. [Large-diameter penetrating keratoplasties are mostly due to very severe infectious keratitis and cannot always prevent secondary enucleation]. Klin Monbl Augenheilkd. 2021. German. doi:10.1055/a-1396-4787
9. Ams A-H, Bonifaz A, Ranque S, Sybren de Hoog G, Verweij PE, Meis JF. Current antifungal treatment of fusariosis. Int J Antimicrob Agents. 2018;51:326–332. doi:10.1016/j.ijantimicag.2017.06.017
10. Prajna NV, Krishnan T, Mascarenhas J, et al. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131:422–429. doi:10.1001/jamaophthalmol.2013.1497
11. Behrens-Baumann W, Finis D, MacKenzie C, Roth M, Geerling G. Keratomykose - Therapiestandards und aktuelle Entwicklungen. [Keratomycosis - therapy standards and new developments]. Klin Monbl Augenheilkd. 2015;232:754–764. German. doi:10.1055/s-0035-1546032
12. Alfaro Rangel R, Szentmáry N, Lepper S, Daas L, Langenbucher A, Seitz B. 8.5/8.6-mm excimer laser-assisted penetrating keratoplasties in a tertiary corneal subspecialty referral center: indications and outcomes in 107 eyes. Cornea. 2020;39(806–811):806–811. doi:10.1097/ICO.0000000000002327
13. Küchle M, Seitz B, Langenbucher A, Naumann GO. Nonmechanical excimer laser penetrating keratoplasty for perforated or predescemetal corneal ulcers. Ophthalmology. 1999;106:2203–2209.
14. Nielsen SE, Nielsen E, Julian HO, et al. Incidence and clinical characteristics of fungal keratitis in a Danish population from 2000 to 2013. Acta Ophthalmol. 2015;93:54–58. doi:10.1111/aos.12440
15. Roth M, Daas L, Renner-Wilde A, et al. Das Deutsche Pilz-Keratitis-Register : Erste Ergebnisse einer multizentrischen Erhebung. [The German keratomycosis registry: initial results of a multicenter survey]. Ophthalmologe. 2019;116:957–966. German. doi:10.1007/s00347-019-0871-9
16. Tuft SJ, Tullo AB. Fungal keratitis in the United Kingdom 2003–2005. Eye. 2009;23:1308–1313. doi:10.1038/eye.2008.298
17. Walther G, Stasch S, Kaerger K, et al. Fusarium keratitis in Germany. J Clin Microbiol. 2017;55:2983–2995. doi:10.1128/JCM.00649-17
18. Hu J, Zhang J, Li Y, et al. A combination of intrastromal and intracameral injections of amphotericin b in the treatment of severe fungal keratitis. J Ophthalmol. 2016;2016:3436415. doi:10.1155/2016/3436415
19. Roth M, Daas L, MacKenzie CR, et al. Development and assessment of a simulator for in vivo confocal microscopy in fungal and acanthamoeba keratitis. Curr Eye Res. 2020;45:1484–1489. doi:10.1080/02713683.2020.1772830
20. Farah CJ, Seitz B, Hamon L, Sourlis C, Daas L. Mischinfektionen bei kontaktlinsenassoziierter mykotischer Keratitis mit Pseudomonas oder Akanthamöben. [Coinfections in contact lens-associated mycotic keratitis with pseudomonas or acanthamoeba]. Ophthalmologe. 2020;118:940–943. German. doi:10.1007/s00347-020-01207-1
21. Hamon L, El Halabi M, Flockerzi FA, Seitz B, Daas L. Purpureocillium lilacinum : Atypischer Erreger einer mykotischen Keratitis bei einer immunkompetenten Patientin. [Purpureocillium lilacinum: atypical pathogen of mycotic keratitis in an immunocompetent patient]. Ophthalmologe. 2021. German. doi:10.1007/s00347-021-01325-4
22. Daas L, Bischoff-Jung M, Viestenz A, Seitz B, Viestenz A. Konfokale Mikroskopie als früher Rezidivmarker nach Keratoplastik infolge einer Fusarium-solani-Keratitis. [Confocal microscopy as an early relapse marker after keratoplasty due to Fusarium solani keratitis]. Ophthalmologe. 2017;114:66–69. German. doi:10.1007/s00347-016-0270-4
23. Das S, Samant M, Garg P, Vaddavalli PK, Vemuganti GK. Role of confocal microscopy in deep fungal keratitis. Cornea. 2009;28:11–13. doi:10.1097/ICO.0b013e318181cff7
24. Nielsen E, Heegaard S, Prause JU, Ivarsen A, Mortensen KL, Hjortdal J. Fungal keratitis-improving diagnostics by confocal microscopy. Case Rep Ophthalmol. 2013;4:303–310. doi:10.1159/000357558
25. Vaddavalli PK, Garg P, Sharma S, Sangwan VS, Rao GN, Thomas R. Role of confocal microscopy in the diagnosis of fungal and acanthamoeba keratitis. Ophthalmology. 2011;118:29–35. doi:10.1016/j.ophtha.2010.05.018
26. Said DG, Otri M, Miri A, Kailasanathan A, Khatib T, Dua HS. The challenge of fungal keratitis. Br J Ophthalmol. 2011;95:1623–1624. doi:10.1136/bjophthalmol-2011-301148
27. Yang JW, Lin HC, Hsiao CH, Chen PY. Therapeutic penetrating keratoplasty in severe infective keratitis using glycerol-preserved donor corneas. Cornea. 2012;31:1103–1106. doi:10.1097/ICO.0b013e31821c9ba2
28. Gao H, Song P, Echegaray JJ, et al. Big bubble deep anterior lamellar keratoplasty for management of deep fungal keratitis. J Ophthalmol. 2014;2014:1–8. doi:10.1155/2014/209759
29. Xie L, Hu J, Shi W. Treatment failure after lamellar keratoplasty for fungal keratitis. Ophthalmology. 2008;115:33–36. doi:10.1016/j.ophtha.2007.03.072
30. Xie L, Zhai H, Shi W. Penetrating keratoplasty for corneal perforations in fungal keratitis. Cornea. 2007;26:158–162. doi:10.1097/01.ico.0000248381.24519.0d
31. Jain R, Bhutia KL, Mohan N, Gupta CKC, Ghai A. Outcome of therapeutic keratoplasty in hopeless microbial keratitis cases otherwise advised evisceration. Cornea. 2018;37:151–155. doi:10.1097/ICO.0000000000001432
32. Koçluk Y, Sukgen EA. Results of therapeutic penetrating keratoplasty for bacterial and fungal keratitis. Int Ophthalmol. 2017;37:1085–1093. doi:10.1007/s10792-016-0372-7
33. Laurik KL, Szentmáry N, Daas L, Langenbucher A, Seitz B. Early penetrating keratoplasty à chaud may improve outcome in therapy-resistant acanthamoeba keratitis. Adv Ther. 2019;36:2528–2540. doi:10.1007/s12325-019-01031-3
34. Chatterjee S, Agrawal D. Recurrence of infection in corneal grafts after therapeutic penetrating keratoplasty for microbial keratitis. Cornea. 2020;39:39–44. doi:10.1097/ICO.0000000000002044
35. Sharma N, Agarwal P, Sinha R, Titiyal JS, Velpandian T, Vajpayee RB. Evaluation of intrastromal voriconazole injection in recalcitrant deep fungal keratitis: case series. Br J Ophthalmol. 2011;95:1735–1737. doi:10.1136/bjo.2010.192815
36. Prakash G, Sharma N, Goel M, Titiyal JS, Vajpayee RB. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am J Ophthalmol. 2008;146:56–59. doi:10.1016/j.ajo.2008.02.023
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.