Back to Journals » Infection and Drug Resistance » Volume 14
Outcomes of Adjunctive Therapy with Intravenous Cefoperazone-Sulbactam for Ventilator-Associated Pneumonia Due to Carbapenem-Resistant Acinetobacter baumannii
Authors Kanchanasuwan S , Kositpantawong N, Singkhamanan K, Hortiwakul T, Charoenmak B, Ozioma F N , Doi Y, Chusri S
Received 8 February 2021
Accepted for publication 18 March 2021
Published 29 March 2021 Volume 2021:14 Pages 1255—1264
DOI https://doi.org/10.2147/IDR.S305819
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Siripen Kanchanasuwan,1 Narongdet Kositpantawong,1 Kamonnut Singkhamanan,2 Thanaporn Hortiwakul,1 Boonsri Charoenmak,1 Nwabor Ozioma F,1,3 Yohei Doi,4,5 Sarunyou Chusri1,2
1Department of Internal Medicine, Faculty of Medicine, Prince of Songkla University, Songkhla, 90110, Thailand; 2Department of Biomedical Sciences, Faculty of Medicine, Prince of Songkla University, Songkhla, 90110, Thailand; 3Excellence Research Laboratory on Natural Products, Division of Biological Science, Faculty of Science and Natural Product Research Center of Excellence, Prince of Songkla University, Hat Yai, Songkhla, 90112, Thailand; 4Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; 5Department of Microbiology, Fujita Health University, Aichi, Japan
Correspondence: Sarunyou Chusri Tel +66-89-7340446
Fax +66-74-281457
Email [email protected]
Introduction: The efficacy of adjunctive therapy with cefoperazone-sulbactam (CEP-SUL) for ventilator-associated pneumonia (VAP) due to carbapenem-resistant A. baumannii (CRAB) is unclear.
Methods: We retrospectively analyzed the therapeutic effect of adding CEP-SUL to standard regimens for VAP due to CRAB. Patients with VAP due to CRAB strains that were susceptible to CEP-SUL were enrolled into the study. The patients were divided into two groups: those who receive cefoperazone-sulbactam (CEP-SUL+), and those who did not receive cefoperazone-sulbactam (CEP-SUL). Mortality rates and resource utilization of these two groups were compared. Factors associated with mortality were explored.
Results: Eighty patients were enrolled into the study, 52 CEP-SUL+ and 28 CEP-SUL–. The baseline characteristics of the two groups were comparable, except for median Acute Physiology and Chronic Health Evaluation (APACHE) II score which was significantly higher for CEP-SUL+. Thirty-day, and in-hospital mortality rates for CEP-SUL+ were significantly lower than CEP-SUL– with values of 35%, 39% and 61%, 68%, for CEP-SUL+ and CEP-SUL–, respectively. The survival rate for CEP-SUL+ was significantly higher compared with CEP-SUL– (P < 0.001). The number of hospital days, ventilator days since diagnosis of VAP and hospital costs were lower for CEP-SUL+.
Conclusion: Overall results suggested that patients with VAP due to CRAB strains who received adjunctive therapy with CEP-SUL had lower mortality rates and resource utilization compared with CEP-SUL–.
Keywords: outcome, cefoperazone-sulbactam, ventilator-associated pneumonia, carbapenem-resistant Acinetobacter baumannii
Introduction
Ventilator-associated pneumonia (VAP) is a frequently encountered hospital-acquired infection (HAI) that causes substantial morbidity and mortality to the patients and financial burden to hospitals.1 Outcomes of this infection are unfavorable due to vulnerable baseline conditions patients and antimicrobial resistance of the causative pathogens.2 Acinetobacter baumannii has increasingly been recognized as a troublesome causative organism in healthcare setting, particularly as a culprit of VAP.3 Its remarkable ability to develop antimicrobial resistance limits treatment options.4 Carbapenems, including imipenem, meropenem and doripenem, are broad-spectrum antibacterial agents effective against a range of pathogenic bacteria and show clinical efficacy for the treatment of several healthcare-associated infections.5 However, infections due to carbapenem-resistant A. baumannii (CRAB) are very difficult to treat due to multidrug resistance and are associated with high mortality rates.6 Although colistin has been employed as an active therapeutic agent for infections due to carbapenem-resistant gram-negative bacilli, the efficacy of this agent for pneumonia is limited perhaps due to the inadequate exposure of colistin in the epithelial lining fluid and lung tissue.7 These concerns underscore the need for additional therapeutic options for CRAB-mediated VAP.
Cefoperazone, a third-generation cephalosporin with broad-spectrum antibacterial activity, is generally active against non-fermentative gram-negative bacilli including A. baumannii.8 Cefoperazone is typically administered with β-lactamase inhibitor sulbactam, to prevent hydrolysis of cefoperazone by several types of β-lactamase enzymes produced by multidrug-resistant gram-negative bacteria.9 Additionally, studies have demonstrated in vitro activities of sulbactam alone and its combinations with existing antimicrobial agents including colistin against several CRAB strains.10 Furthermore, previous studies showed sufficient concentration of sulbactam in the lung tissues of animals and healthy human subjects to achieve therapeutic levels for treatment of pneumonia due to A. baumannii.11–14 Hence, adjunctive therapy with intravenous cefoperazone-sulbactam (CEP-SUL) has been employed in addition to colistin and other agents for the treatment of CRAB mediated VAP in our hospital. In this retrospective analysis, the clinical outcome, characteristics, and factors associated with mortality of VAP patients due to CRAB were explored and the therapeutic effects of CEP-SUL adjunctive therapy were analyzed.
Patients and Methods
Patients and Setting
This study was conducted as a retrospective review of clinical and microbiological information of patients admitted at Songklanagarind Hospital, which is an 800-bed university hospital and referral medical center located in southern Thailand between 1 January 2014 and 31 December 2019. Adult patients (age ≥18 years) diagnosed with VAP due to CRAB were initially included into the study. VAP was defined as new and persistent infiltrate on chest radiograph plus two or more of the three criteria namely fever of >38.3°C, leukocytosis of >12 × 109/mL, and/or purulent tracheobronchial secretions after 48 h of intubation and mechanical ventilation.12 Clinical data of patients were reviewed from electronic medical record and their microbiological data was extracted from hospital microbiology database. To avoid case duplication, only the first episodes of VAP were included in the analysis.
Species Identification and Antimicrobial Susceptibility
A. baumannii was identified in the clinical microbiology laboratory. Routine identification of A. baumannii for medical service in Songklanagarind Hospital is performed by standard microbiological and biochemical reaction techniques as gram-negative, oxidase-negative, non-motile, lactose-non-fermenting coccobacilli. All archived isolates were confirmed as A. baumannii by polymerase chain reaction (PCR) detection of blaOXA-51-like genes, using primers F_oxa51_001 (5ʹ-TAA TGC TTT GAT CGG CCT TG-3ʹ) and R_oxa51_001 (5ʹ-TGGATT GCA CTT CAT CTT GG-3ʹ).15 Isolates with a positive result for blaOXA-51-like genes were assigned as A. baumannii and those with a negative result were subjected to rpoB gene sequencing, using primers rpoB-F (5ʹ-TAY CGY AAA GAY TTG AAA GAA G-3ʹ) and rpoB-R (5ʹ-CMA CAC CYT TGT TMC CRT GA-3ʹ).16 The rpoB gene sequences were queried against Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/BLAST). Assigned species were further confirmed using Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF/MS) (Bruker Daltonics, Bremen, Germany) with ClinProTools software (version 2.2; Bruker Daltonics). Carbapenem susceptibility was initially tested by the disk diffusion technique, followed by the agar dilution method to determine the minimum inhibitory concentrations (MICs) of meropenem. Resistance breakpoint for meropenem was set at ≥16 μg/mL, as MIC.17 Colistin susceptibility was determined with disk diffusion technique and Etest (AB BIODISK, Solna, Sweden) according to the manufacturer’s instructions. An Etest colistin strip (range, 0.06–1024 μg/mL) was placed on each plate and was incubated at 35°C for 20 h. Breakpoints of susceptibility were defined as an inhibition zone of >11 mm and an MIC of <2 μg/mL. All CEP-SUL-susceptible CRAB isolates which were included in the final analysis were confirmed to be susceptible to colistin with broth microdilution test. Tigecycline susceptibility was determined with the disk diffusion method and interpreted using the US Food and Drug Administration (FDA) breakpoints for Enterobacteriaceae.18 Susceptibility of CEP-SUL was determined initially with the disk diffusion method followed by the agar dilution method to determine MICs. Cefoperazone concentrations ranged from 0.25 to 64 µg/mL with combination of sulbactam in a 1:1 ratio. Resistance of CEP-SUL was classified according to the MIC of cefoperazone ≥16 µg/mL.19
Study Design and Data Collection
The study design was based on comparison of outcomes of two groups of patients with VAP due to CRAB: The first group of patients received intravenous (IV) CEP-SUL (CEP-SUL+) as adjunctive therapy to IV colistin alone or combinations of IV colistin and other antimicrobial agents and the second group of patients received only IV colistin or combinations of IV colistin and other antimicrobial agents without CEP-SUL (CEP-SUL). Thirty-day mortality rate was obtained as the primary outcome. Secondary outcomes were 14-day mortality rate, in-hospital mortality rate, length of hospital stay, number of ventilator days and hospital costs including antimicrobial and non-antimicrobial costs as well as complications of treatments. Potential complication of treatment included bone marrow suppression, bleeding disorder and renal complication. Renal complication was defined with Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) classification as follows: risk for renal dysfunction (rise of serum creatinine [sCr] by ≥1.5 times or decrease in glomerular filtration rate [GFR] ≥25%, or urine output <0.5 mL/kg/hour in 6 h), renal injury (rise of sCr 2 times or decrease in GFR ≥50% or urine output <0.5 mL/kg/h in 12 h), renal failure (rise of sCr by ≥3 times, or decrease in GFR ≥75% or urine output <0.3 mL/kg/h in 24 h or anuria in 12 h), loss of renal function (complete loss of renal function >4 weeks) and end-stage renal disease (complete loss of renal function >3 months). GFR was determined as creatinine clearance estimated by Cockcroft-Gault Equation with ideal body weight.20 Bone marrow suppression was defined by depletion of hemoglobin <9 gm/dl, depletion of white blood cell <4000/mm3 and depletion of platelet <100,000/mm3 with evidence of decrease of all cell lineage from bone marrow biopsy. Bleeding disorder was defined by any minor bleeding without requirement of intervention and blood transfusion, including gum bleeding, anterior nasal epistaxis as well as major bleeding including internal organ bleeding or bleeding with requirement of intervention and blood transfusion, intracranial bleeding, gastrointestinal bleeding, and pulmonary hemorrhage.
During the study period, no documented standard guideline was available for treatment of CRAB mediated VAP. The common regimens for IV colistin were based on the recommended dosage of colistimethate sodium 1–2 million IU every 8 h (q8h) with a loading dose of 1–2 million IU.21,22 Carbapenems including meropenem and imipenem were adjunctively added to IV colistin based on clinical judgement of the treating physicians. Adjunctive therapy with CEP-SUL was considered as either empirical or definitive regimens. According to the antimicrobial stewardship policy in our hospital as well as the relatively low susceptibility rate of CEP-SUL among several nosocomial organisms, this agent was rarely used for empirical treatment. The definitive regimens were based on the initial disk diffusion susceptibility testing results and the continuation or modification of the regimen was based on the MIC results as they became available. The only CEP-SUL formulation available at the hospital was a combination of cefoperazone and sulbactam compounded at a 1:1 ratio. The first 24-h dosage of CEP-SUL ranged from 2 g q8h to 2 g q4h followed by renally adjusted dosages after 24 h of initial treatment.
Data Retrieval
The electronic medical records of patients were reviewed, and demographic data and clinical characteristics were extracted including age, sex, comorbidities, and immunological status. The comorbidities included diabetes mellitus, cardiovascular diseases, cerebrovascular diseases, chronic kidney diseases and HIV infection.
Immunocompromised patients were defined as patients with prolonged neutropenia (absolute neutrophil count of <0.5 × 109 neutrophils/L for ≥2 weeks) or receiving immunosuppressive agents (chemotherapy within 6 weeks or corticosteroids at a dosage equivalent to or higher than 15 mg of prednisolone daily for >14 days within 4 weeks prior to the onset of infection). Clinical characteristics included type of admission, initial intensive care (ICU) admission, Acute Physiology and Chronic Health Evaluation (APACHE) II score at the time of VAP diagnosis, bacteremia, and concomitant infection. Microbiological data included MIC of meropenem and CEP-SUL. The treatment data included appropriateness of empirical antimicrobial agents, adjunctive therapy with carbapenems, tigecycline and CEP-SUL as well as the duration of treatment for VAP.
Statistical Analysis
Variables of survivors and non-survivors were compared by tabulation, followed by χ2 test or Fisher’s exact test as appropriate for categorical variables, and by Student’s t-test or Rank sum test as appropriate for continuous variables. Differences were expressed by the crude odds ratio (OR) and 95% confidence interval (CI). Potential mortality-associated variables and variables with P values of <0.2 were included in a multivariate logistic regression model. These models were fitted to evaluate the effect of each characteristic, expressed as adjusted ORs. The association of each variable with the outcome was expressed with adjusted OR and 95% CI. Survival analysis with Cox proportional hazard regression was used to assess the differences in the durations of survival after developing VAP. The time started was defined as the day that VAP was diagnosed. The time ended was defined as the date that the patient outcome was documented, or the patient was excluded from the observation frame.
Ethical Statement
This retrospective study was approved by the Institutional Review Board of the Faculty of Medicine, Prince of Songkla University with EC:58–059-14-1 for clinical data from medical record review and EC:54–080-14-1-2 for microbiological data extraction. The researchers were granted permission to extract the data from the database with waiver of consent. All data were fully anonymized before the researcher accessed and analyzed them. Medical records of patients admitted between 1 January 2014 and 31 December 2019 were used in the study. The author also confirmed that this current study was this study was conducted in accordance with the Declaration of Helsinki.
Results
Between 1 January 2014 and 31 December 2019, 80 patients were diagnosed with VAP mediated by CEP/SUL-susceptible CRAB strains which accounted for 24% total CRAB mediated VAP (340 patients). Figure 1 shows patient enrollment into this study, with a total of 80 patients infected with CEP-SUL susceptible CRAB, 52 patients received IV CEP-SUL as adjunctive therapy to treatment including IV colistin (100%), IV imipenem/meropenem (38%) and tigecycline (18%).
 |
Figure 1 Flowchart of study enrollment. |
Clinical Features and Characteristics
The baseline characteristics of patients with VAP due to CRAB are shown in Table 1. The APACHE II scores of CEP-SUL+ patients were significantly higher than those of CEP-SUL– patients and those with VAP due to CEP-SUL-resistant CRAB. Other baseline characteristics including demographic data, clinical characteristics and treatment were not different between CEP-SUL+ patients and CEP-SUL− patients. IV vitamin K supplement was administrated weekly to both CEP-SUL– and CEP-SUL+ patients, to prevent coagulopathy from hypoprothrombinemia. Among the 52 CEP-SUL+ patients, CEP-SUL dosages for the first 24 h were 3 g q4h (33%), 2 g q4h (31%), 2 g q6h (24%) and 2 g q8h (11%). In addition, 13 (25%) of the 52 CEP-SUL+ patients had renal impairment and received renally adjusted dosages of CEP-SUL after initial 24 h dosages. Ten patients (19%) received CEP-SUL for empiric therapy in combination with IV colistin and/or imipenem/meropenem. All 80 isolates used in this study were susceptible to colistin, tigecycline and CEP-SUL but were resistant to meropenem/imipenem. The MICs of meropenem ranged from 8 to 512 µg/mL with MIC50 of 32 µg/mL. The MICs of CEP-SUL ranged from 0.25 to 8 µg/mL with MIC50 and MIC90 of 4 and 8 µg/mL, respectively. The MICs of SUL ranged from 0.25 to 4 µg/mL with MIC50 and MIC90 of 2 and 4 µg/mL, respectively. The MIC50 and MIC90 among CEP-SUL+ patients and CEP-SUL− patients were not significantly different.
 |
Table 1 Comparison of Clinical Features Between Patients with Carbapenem-Resistant Acinetobacter baumannii Ventilator-Associated Pneumonia |
Clinical Outcomes of Patients
The 14-day, 30-day, and in-hospital mortality rates among the 80 patients with VAP due to CEP-SUL-susceptible CRAB were 20%, 44%, and 49%, respectively. CEP-SUL+ patients had significantly lower 30-day, and in-hospital mortality rates than CEP-SUL– patients. When compared to those with VAP due to CEP-SUL-resistant CRAB, CEP-SUL+ patients had significantly favorable length of hospital days, length of ventilator days, total hospital costs and non-antimicrobial costs. Among the CEP-SUL+ patients, 3 developed renal complications; 2 had renal injury, 1 patient had renal failure in the first week of administration, and 2 patients developed bone marrow suppression in the first week of CEP-SUL administration but spontaneously recovered in 2 weeks after discontinuation of treatment. The 2 patients with bone marrow suppression also received intravenous meropenem 2 g q8h in the first week of treatment. All 3 patients with renal complications completely regained their renal function within 4 weeks. Among the CEP-SUL– patients, 2 patients developed renal complications in the first week of treatment, 1 patient developed bone marrow suppression in the first week. This patient also received intravenous imipenem-cilastatin 1 g q8h. No report of bleeding disorder was recorded among the two groups. Significantly shorter length of hospital days and length of ventilator days were observed for CEP-SUL+ patients since diagnosis of VAP than CEP-SUL– patients. Total hospital costs and non-antimicrobial costs were also significantly lower for CEP-SUL+ patients than for CEP-SUL– patients. The total antimicrobial cost for CEP-SUL– patients was higher than those of the CEP-SUL+ patients but were not statistically significant. The antimicrobial cost for treatment of VAP due to CRAB for CEP-SUL+ (median [interquartile] = 27,465[19,856–29,445]) was higher than those of CEP-SUL– patients (median [interquartile] = 26,889[19,441–29,001]). However, the difference was not statistically significant. Outcomes for CEP-SUL+ and CEP-SUL– are shown in Table 2.
 |
Table 2 Comparison of Outcomes Between Patients with Carbapenem-Resistant Acinetobacter baumannii Ventilator-Associated Pneumonia |
Factors Associated with the 30-Day Mortality Rate
Factors associated with the 30-day mortality rates of the patients with CRAB VAP are shown in Table 3. The results indicated that only the APACHE II score and adjunctive therapy with CEP-SUL were associated with 30-day mortality rates. Duration of antimicrobial therapy and underlying diseases showed association with 30-day mortality rates but were not of statistical significance. Among the patients receiving CEP-SUL, the median dosing among the survivors was 8 mg/day (interquartile [IQR] =8,12) and those of non-survivors was 8 mg/day (IQR=8,12) (P= 0.795).
 |
Table 3 Factors Influencing 30-Day Mortality Among 80 Patients with Ventilator-Associated Pneumonia Due to Carbapenem-Resistant and Cefoperazone-Sulbactam-Susceptible Acinetobacter baumannii |
Survival Rates of CEP-SUL+ and CEP-SUL– Patients
The 30 days Kaplan-Meier survival curves for VAP due to CEP-SUL-susceptible CRAB strains between CEP-SUL+ and CEP-SUL– patients demonstrated significant differences in mortality rates over time (P < 0.001, Log rank test) as shown in Figure 2. The survival rates of CEP-SUL+ patients were significantly higher than those of CEP-SUL– patients. The Cox proportional hazard model survival analysis showed that the factors associated with 30-day mortality rates were APACHE II score (hazard ratio [HR], 1.21; 95% confident interval [CI], 1.14 to 1.26; P = 0.032) and adjunctive therapy with CEP-SUL (HR = 0.41, CI = 0.20–0.84; P = 0.029).
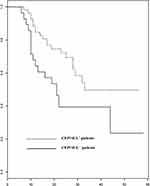 |
Figure 2 Survival of patients with VAP due to CRAB who received CEP-SUL and did not receive CEP-SUL. |
Discussion
In this retrospective observational study, the 14-day, 30-day, and in-hospital mortality rates of CEP-SUL+ patients were significantly lower than those of CEP-SUL– patients. The length of hospital stay, ventilator days and hospital costs were also more favorable among CEP-SUL+ patients. This was in spite of a significantly higher APACHE II score and nominally higher proportion of immunocompromised patients were observed among CEP-SUL+.
Similar to previous reports on VAP due to CRAB,23–25 this study demonstrated high mortality rates and economic burden including high hospital cost, prolonged hospital stay and lengthy duration of ventilator support. Approximately 50% of patients died within 30 days, underscoring the overall poor prognosis of this infection, which might be attributed to the underlying comorbidities of the patients as well as difficulty in administering appropriate antimicrobial regimens due to multiple drug resistance.26,27 Unlike previous reports of CRAB infection, the appropriateness of empirical antimicrobial regimens was not associated with mortality.28–30 This suggests that with the administration of IV colistin as empirical antibiotic regimen, the levels of colistin in lung tissue or epithelial lining fluid might be too low to achieve clinical benefit. Previous animal studies and a study in healthy human volunteers have demonstrated low levels of colistin in lung tissue and epithelial lining fluid after administration of IV colistin.11,13,14
This study was conducted between January 2014 to December 2019 with appropriate dosing of colistin as recommended.31 However, dosing of the antimicrobial agents including CEP-SUL was at the discretion of the treating physicians. The dosages of CEP-SUL in this study were heterogeneous and relatively high with initial dosage of 6 g to 18 g per day. Most patients (64%) received more than or equal to 12 g per day. These relatively high dosages of CEP-SUL reflected the population pharmacokinetics study of appropriate sulbactam dosage for VAP due to A. baumannii.32 Although several in vitro studies demonstrated activity of tigecycline against CRAB with relatively high levels of tigecycline in lung tissues, our study did not show corresponding clinical benefit of tigecycline.33–35 However, the number of patients administered tigecycline was relatively low, thus definitive conclusions cannot be drawn. Similarly, no survival benefit was recorded for additional treatment with meropenem/imipenem. One potential explanation is that the MIC of meropenem (median = 32 µg/mL) was too high to achieve synergistic effect with other antibacterial agents, making unclear the synergistic effect of colistin with carbapenem among the colistin-susceptible CRAB.
There are several limitations in this study which should be acknowledged. Firstly, due to the nature of retrospective study, the data on decision-making process, particular indications to use CEP-SUL, were insufficient thus indication bias cannot be excluded. This concern is at least partially mitigated by the observation of significantly higher APACHE II score among CEP-SUL+ patients compared with CEP-SUL– patients, which suggests that treating physicians were more likely to add CEP-SUL when the prognosis was felt to be less favorable. Secondly, with the restricted inclusion criteria of only infection with isolates susceptible to CEP-SUL, the generalizability of this study may be limited. The finding in this study indicated that only 24% of CRAB isolates causing VAP were susceptible to CEP-SUL. Thirdly, the number of patients in this study was relatively low. With the 30-day mortality rate of 31% in this study, enrollment of 80 patients achieved only 67% power of prediction. With the 30-day mortality rate among CEP-SUL+ patients of 35% and that among CEP-SUL– patients of 65%, the enrolled 80 patients achieved only 49% power of discrimination. Fourthly, there were no data on microbiological outcome of CRAB during treatment. It therefore is not known whether the causes of death among the patients during treatment were related to infection. Fifth, the results do not inform the appropriate dosing of CEP-SUL due to the observed variations. Lastly, the dosages of colistin ranging from 3 MIU to 6 MIU/day were possibly too low to achieve clinical benefit, even though there were no significant differences between the CEP-SUL+ patients and CEP-SUL– patients.
Conclusion
Adjunctive therapy with IV CEP-SUL for VAP due to CEP-SUL-susceptible CRAB was associated with significantly lower mortality rates and hospital resources compare with therapy without CEP-SUL in this retrospective cohort.
Acknowledgments
The authors appreciate manuscript preparation by Mr. Chakorn Leehem, Infectious disease Unit, department of Internal Medicine, Faculty of Medicine, Prince of Songkla University.
Disclosure
The authors declare that no competing interests exist.
References
1. Magill SS, Edwards JR, Bamberg W, et al. Emerging infections program healthcare-associated infections and antimicrobial use prevalence survey team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi:10.1056/NEJMoa1306801
2. Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis. 2010;51(S1):S120–S125. doi:10.1086/653060
3. Antunes L, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71(3):292–301. doi:10.1111/2049-632X.12125
4. Asif M, Alvi IA, Rehman SU. Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect Drug Resist. 2018;11:1249. doi:10.2147/IDR.S166750
5. Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011;55(11):4943–4960. doi:10.1128/AAC.00296-11
6. Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(Supplement_7):S521–S528. doi:10.1093/cid/ciz824
7. Kengkla K, Kongpakwattana K, Saokaew S, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Antimicrob Chemother. 2018;73(1):22–32. doi:10.1093/jac/dkx368
8. Jones RN, Barry AL. Cefoperazone: a review of its antimicrobial spectrum, β-lactamase stability, enzyme inhibition, and other in vitro characteristics. Rev Infect Dis. 1983;5(Supplement_1):S108–S126. doi:10.1093/clinids/5.Supplement_1.S108
9. Akova M. Sulbactam-containing β-lactamase inhibitor combinations. Clin Microbiol Infect. 2008;14:185–188. doi:10.1111/j.1469-0691.2007.01847.x
10. Leelasupasri S, Santimaleeworagun W, Jitwasinkul T. Antimicrobial susceptibility among colistin, sulbactam, and fosfomycin and a synergism study of colistin in combination with sulbactam or fosfomycin against clinical isolates of carbapenem-resistant Acinetobacter baumannii. J Pathog. 2018;2018:1–5. doi:10.1155/2018/3893492
11. Rodríguez-Hernández M-J, Cuberos L, Pichardo C, et al. Sulbactam efficacy in experimental models caused by susceptible and intermediate Acinetobacter baumannii strains. J Antimicrob Chemother. 2001;47(4):479–482. doi:10.1093/jac/47.4.479
12. Rodvold KA, Gotfried MH, Isaacs RD, O’Donnell JP, Stone E. Plasma and intrapulmonary concentrations of ETX2514 and sulbactam following intravenous administration of ETX2514SUL to healthy adult subjects. Antimicrob Agents Chemother. 2018;62(11). doi:10.1128/AAC.01089-18
13. Wildfeuer A, Rühle K, Bölcskei P, Springsklee M. Concentrations of ampicillin and sulbactam in serum and in various compartments of the respiratory tract of patients. Infection. 1994;22(2):149–151. doi:10.1007/BF01739027
14. Yokoyama Y, Matsumoto K, Ikawa K, et al. Pharmacokinetic/pharmacodynamic evaluation of sulbactam against Acinetobacter baumannii in in vitro and murine thigh and lung infection models. Int J Antimicrob Agents. 2014;43(6):547–552. doi:10.1016/j.ijantimicag.2014.02.012
15. Woodford N, Ellington MJ, Coelho JM, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27(4):351–353. doi:10.1016/j.ijantimicag.2006.01.004
16. La Scola B, Gundi VA, Khamis A, Raoult D. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol. 2006;44(3):827–832. doi:10.1128/JCM.44.3.827-832.2006
17. Wayne P. Clinical and Laboratory Standards Institute: performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI Document M100-S20. 2010.
18. Alsoud RA, Svensson RJ, Svensson EM, et al. Simultaneous assessment of time-to-positivity and colony-forming unit in tuberculosis patients under high-dose rifampicin therapy. PAGE2019 Poster Abstracts. 59.
19. Barry AL, Jones RN. Criteria for disk susceptibility tests and quality control guidelines for the cefoperazone-sulbactam combination. J Clin Microbiol. 1988;26(1):13–17. doi:10.1128/JCM.26.1.13-17.1988
20. Kellum JA, Bellomo R, Ronco C. Definition and classification of acute kidney injury. Nephron Clin Pract. 2008;109(4):c182–c187. doi:10.1159/000142926
21. Karvanen M, Plachouras D, Friberg LE, et al. Colistin methanesulfonate and colistin pharmacokinetics in critically ill patients receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother. 2013;57(1):668–671. doi:10.1128/AAC.00985-12
22. Vila J, Pachón J. Therapeutic options for Acinetobacter baumannii infections: an update. Expert Opin Pharmacother. 2012;13(16):2319–2336. doi:10.1517/14656566.2012.729820
23. Chaari A, Mnif B, Bahloul M, et al. Acinetobacter baumannii ventilator-associated pneumonia: epidemiology, clinical characteristics, and prognosis factors. Int J Infect Dis. 2013;17(12):e1225–e1228. doi:10.1016/j.ijid.2013.07.014
24. Hurley JC. World-wide variation in incidence of Acinetobacter associated ventilator associated pneumonia: a meta-regression. BMC Infect Dis. 2016;16(1):577. doi:10.1186/s12879-016-1921-4
25. Lim SMS, Abidin AZ, Liew S, Roberts J, Sime F. The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: a systematic review and meta-analysis. J Infect. 2019;79(6):593–600. doi:10.1016/j.jinf.2019.09.012
26. Harris BD, Thomas GA, Greene MH, Spires SS, Talbot TR. Ventilator bundle compliance and risk of ventilator-associated events. Infect Control Hosp Epidemiol. 2018;39(6):637–643. doi:10.1017/ice.2018.30
27. Liu J, Zhang S, Chen J, et al. Risk factors for ventilator-associated events: a prospective cohort study. Am J Infect Control. 2019;47(7):744–749. doi:10.1016/j.ajic.2018.09.032
28. Dickstein Y, Lellouche J, Ben Dalak Amar M, et al. Treatment outcomes of colistin-and carbapenem-resistant Acinetobacter baumannii infections: an exploratory subgroup analysis of a randomized clinical trial. Clin Infect Dis. 2019;69(5):769–776. doi:10.1093/cid/ciy988
29. Piperaki E-T, Tzouvelekis L, Miriagou V, Daikos G. Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clin Microbiol Infect. 2019;25(8):951–957. doi:10.1016/j.cmi.2019.03.014
30. Russo A, Bassetti M, Ceccarelli G, et al. Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: clinical features, therapy and outcome from a multicenter study. J Infect. 2019;79(2):130–138. doi:10.1016/j.jinf.2019.05.017
31. Nation RL, Garonzik SM, Li J, et al. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis. 2016;62(5):552–558. doi:10.1093/cid/civ964
32. Jaruratanasirikul S, Nitchot W, Wongpoowarak W, Samaeng M, Nawakitrangsan M. Population pharmacokinetics and Monte Carlo simulations of sulbactam to optimize dosage regimens in patients with ventilator-associated pneumonia caused by Acinetobacter baumannii. Eur J Pharm Sci. 2019;136:104940. doi:10.1016/j.ejps.2019.05.018
33. Li J, Yang X, Chen L, Duan X, Jiang Z. In Vitro activity of various antibiotics in combination with tigecycline against Acinetobacter baumannii: a systematic review and meta-analysis. Microb Drug Resist. 2017;23(8):982–993. doi:10.1089/mdr.2016.0279
34. Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. Pharmacokinetic/pharmacodynamic profile for tigecycline—a new glycylcycline antimicrobial agent. Diagn Microbiol Infect Dis. 2005;52(3):165–171. doi:10.1016/j.diagmicrobio.2005.05.006
35. Pournaras S, Koumaki V, Gennimata V, Kouskouni E, Tsakris A. In vitro activity of tigecycline against Acinetobacter baumannii: global epidemiology and resistance mechanisms. Adv Microbiol Infect Dis Public Health. 2015;1–14.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
