Back to Journals » Journal of Hepatocellular Carcinoma » Volume 8
Outcomes in the Era of Interferon-Free Direct-Acting Antiviral Therapy After Liver Transplantation in Patients with Hepatitis C Virus and Hepatocellular Carcinoma
Authors Ismail MS , Mohamed I , Polychronopoulou E, Goss JA, Kuo YF, Kanwal F, Jalal PK
Received 9 March 2021
Accepted for publication 2 June 2021
Published 29 June 2021 Volume 2021:8 Pages 701—711
DOI https://doi.org/10.2147/JHC.S309354
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Mohamed Saleh Ismail,1,2 Islam Mohamed,1,2 Efstathia Polychronopoulou,3 John A Goss,4 Yong-Fang Kuo,3 Fasiha Kanwal,1 Prasun K Jalal1,4
1Division of Gastroenterology, Baylor College of Medicine, Houston, TX, USA; 2Department of Internal Medicine, Gastroenterology & Hepatology, Ain Shams University, Cairo, Egypt; 3Department of Biostatistics, The University of Texas Medical Branch, Galveston, TX, USA; 4Michael E. DeBakey Department of Surgery, Division of Abdominal Transplantation, Baylor College of Medicine, Houston, TX, USA
Correspondence: Prasun K Jalal
Division of Gastroenterology, Baylor College of Medicine, Baylor Clinic 6620 Main Street, Suite 1450, Houston, TX, 77030, USA
Tel +1 832-355-1424
Fax +1 7136102479
Email [email protected]
Background/Aims: Several studies have shown improved outcome of liver transplant (LT) recipients with hepatitis C virus (HCV) since the widespread clinical use of interferon-free direct-acting antivirals (IFN-free DAAs). However, the association of IFN-free DAA therapy on tumor characteristics and on the outcome of LT in patients with hepatocellular carcinoma (HCC) has not been studied. We aimed to examine pre-transplant HCC characteristics and post-LT outcomes in the IFN-based DAA treatment and IFN-free DAA treatment eras.
Methods: Using the United Network for Organ Sharing/Organ Procurement and Transplantation Network database, we analyzed adults with a diagnosis of HCV and HCC who received LTs from deceased donors from 04/2012 to 12/2017. Cox regression models were used to identify the association between the IFN-based DAA treatment vs IFN-free DAA treatment era and study outcomes (mortality, graft failure, and HCC recurrence at 1 and 3 years).
Results: Complete tumor necrosis was significantly higher in the IFN-free DAA treatment era (22.73% vs 18.22%; P < 0.01). No other HCC tumor characteristics differed significantly between the two eras. HCC recurrence rates were similar between the two eras. On multivariate Cox regression analysis, patients who had transplants in the IFN-free DAA treatment era had lower risk of graft failure compared with the IFN-based DAA treatment group (hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.25– 0.77; P < 0.01). Patient mortality was lower in the IFN-free DAA treatment era although the difference was not statistically significant (HR, 0.82; 95% CI, 0.60– 1.13; P =0.22).
Conclusion: LT recipients in the IFN-free DAA treatment era had significantly higher complete tumor necrosis in explants. Other HCC tumor characteristics were similar between the two eras. Post-LT graft failure at 1 and 3 years significantly decreased in the IFN-free DAA treatment era among patients with HCV and HCC, although patient mortality was not statistically different.
Keywords: hepatocellular carcinoma, liver transplantation, hepatitis C virus, direct-acting antiviral
Hepatitis C virus (HCV) infection accounts for 50–60% of hepatocellular carcinoma (HCC) cases in the United States.1 Since the introduction of interferon-free direct-acting antiviral (IFN-free DAA) therapy, management of HCV has transformed with sustained virologic response (SVR) rates exceeding 90%.2 Achieving SVR has been shown to be associated with reduction of HCV-related HCC risk.3 However, the impact of IFN-free DAA therapy on HCC tumor characteristics is unknown.4 There is concern that IFN-free DAA therapy can lead to aggressive tumor characteristics that may affect liver transplant (LT) outcomes, including recurrence.
Both United States and European data demonstrated improved outcome of LT recipients with HCV in the IFN-free DAA treatment era.5,6 However, no similar data have been published in patients with HCV-related HCC after LT. A large-scale prospective study in the LT population would show the impact of IFN-free DAA therapy on the outcome in patients with HCV-related HCC, but randomization would be difficult to justify in this era. Retrospective analysis of the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN) Standard Transplant Analysis and Research (STAR) database gives us a unique opportunity to evaluate outcomes between the IFN-based DAA treatment and IFN-free DAA treatment eras. In addition, UNOS has been collecting explant pathology data since April 2012, which allows us to study detailed HCC characteristics in patients who have received transplants.
We undertook this study of patients with HCV-related HCC from the UNOS/OPTN database with three objectives: 1) to compare HCC histopathological characteristics in the IFN-free DAA vs IFN-based DAA treatment eras; 2) to analyze LT outcomes (patient mortality, graft failure, and HCC recurrence) after LT in the IFN-free DAA vs IFN-based DAA treatment eras; and 3) to compare rates of dropout among patients on the waiting list for LT between the two eras. We hypothesized that patients with HCV-related HCC would have improved outcomes in the IFN-free DAA treatment era.
Patients and Methods
Study Design and Patient Population
For our primary study cohort, we retrospectively analyzed data in the UNOS/OPTN STAR file for all adult patients who received deceased-donor liver-alone transplants between April 1, 2012, and December 31, 2017, with an HCC explant form generated and a diagnosis of HCV. We excluded patients without evidence of HCC on explant forms and those who had intrahepatic cholangiocarcinoma or mixed HCC/cholangiocarcinoma on explant. Also, patients with previous LT or multi-organ transplants were excluded.
We classified patients into 2 eras: IFN-based DAA treatment era (between April 1, 2012, and December 31, 2014) and IFN-free DAA treatment era (between January 1, 2015, and December 31, 2017). Since April 2012, UNOS/OPTN has collected explant pathology data in patients with HCC undergoing LT. The period of 2012–2014 was chosen because it corresponded to the era before the widespread use of highly effective interferon-free all-oral third-generation DAA therapy. Third-generation DAA was first approved by the US Food and Drug Administration in October 2014 and introduced in the American Association for the Study of Liver Diseases guidelines for treatment of HCV infection at its 65th annual meeting in November 2014.7 Before that, available first- and second-generation DAAs were used in combination with interferon ± ribavirin and had lower SVR rates and higher rates of adverse events, especially in patients with Child-Turcotte-Pugh (CTP) score B and C.8,9
In this study, we also analyzed a second cohort to examine the dropout from the wait-listed patients. We included all patients listed for LT between April 1, 2012, and December 31, 2017, with a diagnosis of HCV and HCC. We stratified patients on the waiting list in each era into: 1) patients transplanted regardless of whether an HCC explant form was generated, 2) active patients on the waiting list on the last day of each era who did not receive an organ, and 3) patients who dropped out. Dropout was defined as patient removal from the waiting list without receiving LT. Dropout included 1) patients who died while waiting for an organ, 2) patients who deteriorated or improved and transplant was no longer indicated, 3) patients lost to follow-up, and 4) patients removed from the list for other causes. This study received Institutional Review Board approval from Baylor College of Medicine, Houston, TX and guidelines outlined in the Declaration of Helsinki were followed. Data accessed through UNOS/OPTN STAR file complies with relevant data protection and privacy regulations. UNOS confirms that all organs were donated voluntarily with written informed consent, and that donations were conducted in accordance with the Declaration of Istanbul.
Study Variables
The following study variables from the UNOS/OPTN database were analyzed: 1) recipient characteristics [age, sex, race/ethnicity, body mass index (BMI), Model for End-stage Liver Disease (MELD) score, waiting time on transplant list, alpha fetoprotein (AFP) and other comorbidities]; 2) donor characteristics [age, sex, race/ethnicity, BMI, cold ischemia time, donor risk index (DRI), comorbidities, HCV antibody (HCV Ab) and hepatitis B core antibody (HBcAb) status]; and 3) explant pathology features. DRI was calculated from donor characteristics.10 These variables were compared between the IFN-based DAA treatment and IFN-free DAA treatment eras. HCV nucleic acid amplification data for the recipient and donor have been entered in the UNOS/OPTN database since February 28, 2018, and March 31, 2015, respectively, and were not analyzed.
Study Outcomes
The primary aim of the study was to compare histopathological HCC characteristics between the IFN-based DAA treatment and IFN-free DAA cohorts; secondary aims were to compare 1- and 3-year post-LT outcomes (patient mortality, graft failure, and HCC recurrence). We also aimed to estimate waiting-list dropouts (frequency and percentages) between the IFN-based DAA treatment and IFN-free DAA treatment eras.
Statistical Analysis
Clinical and tumor characteristics were described using frequencies and percentages or medians and interquartile ranges (IQRs). Patient and tumor characteristics were compared between the IFN-based DAA treatment and IFN-free DAA treatment eras with the use of chi-square, t-test, or non-parametric tests as appropriate to assess statistical differences. Unadjusted post-LT outcomes and HCC recurrence rates were estimated at 1 and 3 years by using the Kaplan–Meier method. Multivariate Cox proportional hazards models were used to assess the impact of DAA on the outcomes, while adjusting for patient, clinical, and tumor characteristics. All patients were censored at whichever of the following occurred first: loss of follow-up, the end of the follow-up period (1- and 3-year maximum follow-up), or the end of the study period (December 31, 2014, for the IFN-based DAA treatment era and December 31, 2017, for the IFN-free DAA treatment era) to allow for at least 1 year and a maximum of 3 years of follow-up. In the analysis of graft failure and HCC recurrence, patients were also censored at death if it occurred first. A full multivariate model was constructed with all significant variables from the bivariate analyses. Baseline demographics and clinical characteristics, including age, sex, race/ethnicity, education, history of diabetes, donor sex, DRI, donor HCV Ab–positive status, waiting time, and HCC characteristics, were added to the final model regardless of their significance in the bivariate analyses. We followed a backward elimination process in which we removed variables one by one and compared model estimates and standard errors of the final model. Any variable whose removal resulted in appreciable impact on the model estimates or standard error was reintroduced and kept in the final model. We reported hazard ratios before and after 4 months for mortality and graft failure, and before and after 8 months for HCC recurrence; as the proportional hazards assumption was violated for the first 4 (or 8) months after LT. The proportional hazards assumption was assessed by testing the interaction of survival time with DAA, and the cutoff follow-up time was selected by visual inspection of hazard plots. Statistical significance was reached with P <0.05. All analyses were conducted using SAS 9.4 (Cary, NC, USA).
Results
Primary Study Cohort
Population Characteristics (Recipient/Donor)
The IFN-based DAA treatment-era group (April 2012-December 2014) included 2497 LT recipients with an HCC explant form generated and a diagnosis of HCV in the database, while the IFN-free DAA-era group (January 2015-December 2017) included 2547 LT recipients. Patients with previous LT, multi-organ transplants, cholangiocarcinoma, incomplete explant data, or age less than 18 years at the time of transplant were excluded (Figure 1).
 |
Figure 1 Flowchart of primary study cohort selection. |
The baseline characteristics of the final LT recipient cohort are summarized in Table 1. The median age at transplant for the IFN-based DAA treatment era was 60 years (IQR 56–63), compared with 62 years (IQR 58–65) for the IFN-free DAA treatment era (P <0.01). At the time of transplant, the calculated median MELD score (without MELD exception points) was 11 (IQR 8–15) for the IFN-based DAA treatment era, compared with 10 (IQR 8–14) for the IFN-free DAA treatment era (P <0.01). The median waiting time from listing to LT was 226 days (IQR 104–431) in the IFN-based DAA treatment era, compared with 273 days (IQR 191–464) in the IFN-free DAA treatment era (P <0.01). The median AFP level at transplant was 12 ng/mL (IQR 6–32) in the IFN-based DAA treatment era, compared with 7 ng/mL (IQR 4–17) in the IFN-free DAA treatment era (P <0.01). Significantly higher proportions of patients in the IFN-free DAA treatment era had diabetes mellitus, history of malignancy, previous abdominal surgery, and previous transjugular intrahepatic portosystemic shunt (TIPS) procedures compared with patients in the IFN-based DAA treatment era (P <0.01). History of spontaneous bacterial peritonitis (SBP) at listing was significantly lower in the IFN-free DAA treatment era (P <0.01). Hepatitis B surface antigen (HBsAg) was positive in 1.92% of patients in the IFN-based DAA treatment era and 1.65% of patients in the IFN-free DAA treatment era (Table 1).
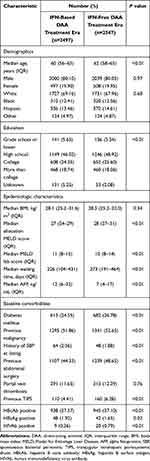 |
Table 1 Recipient Characteristics for the Primary Study Cohort |
Donor demographics were comparable between the IFN-based DAA treatment and IFN-free DAA treatment eras (Table 2). DRI scores were similar between the two groups (P=0.42). Transplantation of HCV antibody (HCV Ab)–positive liver allografts was performed in 10.21% of patients in the IFN-based DAA treatment era, compared with 15.63% in the IFN-free DAA treatment era (P <0.01) (Table 2).
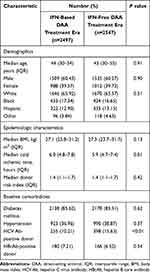 |
Table 2 Donor Characteristics for the Primary Study Cohort |
Tumor Characteristics at Transplantation
The median for the sum of viable tumors diameter in the IFN-based DAA treatment era was 3.5 cm (IQR 2.3–5.4), compared with 3.6 cm (IQR 2.2–5.7) in the IFN-free DAA treatment era (P=0.09; Table 3). Infiltrative HCC was found in 0.60% of liver explants in the IFN-based DAA treatment era, compared with 0.43% in the IFN-free DAA treatment era (P <0.01). Complete necrosis with no viable tumor as a result of locoregional therapy was found in 18.22% of patients in the IFN-based DAA treatment era, compared with 22.73% in the IFN-free DAA treatment era (P <0.01). Among patients with viable tumors in the explant, 29.58%, 60.19%, and 10.24% had well-differentiated, moderately differentiated, and poorly differentiated HCC, respectively, in the IFN-based DAA treatment era. In contrast, 29.47%, 61.53%, and 8.99% had well-differentiated, moderately differentiated, and poorly differentiated HCC in the IFN-free DAA treatment era (P=0.36; Table 3).
 |
Table 3 HCC Characteristics for the Primary Study Cohort |
Macro- and microvascular invasions were found in 1.92% and 15.06% of patients, respectively, in the IFN-based DAA treatment era, compared with 1.69% and 13.58% in the IFN-free DAA treatment era (P=0.25). While on the waiting list, 67.24% of patients in the IFN-based DAA treatment era received 1 locoregional therapy regimen and 15.42% received more than 1 locoregional therapy regimen, compared with 64.27% and 20.14% of patients, respectively, in the IFN-free DAA treatment era (P <0.01).
Post-Transplant Outcomes
The median post-LT follow-up till the end of the study period was 848 days (IQR 599–1107) in the IFN-based DAA treatment era, compared with 945 days (IQR 660–1242) in the IFN-free DAA treatment era (P <0.01). Overall 1- and 3-year post-LT mortality rates were 7.29% and 13.10%, respectively, in the IFN-based DAA treatment era, compared with 6.16% (P=0.11) and 11.35% (P=0.06) in the IFN-free DAA treatment era. One- and 3-year graft failure rates were 4.12% and 5.53%, respectively, in the IFN-based DAA treatment era, compared with 2.83% (P=0.01) and 3.88% (P <0.01) in the IFN-free DAA treatment era. Figure 2A and B show the probability of mortality and graft failure at each time point after LT and the associated log-rank scores. The graphs were similar for the first 4 months, suggesting similar cumulative patient mortality and graft failure in the first few months after LT, but started to diverge 4 months after transplant. The cumulative risk of graft failure was lower in the IFN-free DAA treatment era, and this difference became more prominent as time elapsed.
HCC recurrence at 1 and 3 years was found in 3.32% and 6.49% of patients, respectively, in the IFN-based DAA treatment era, compared with 3.18% (P=0.77) and 5.9% (P=0.41) of patients in the IFN-free DAA treatment era (Figure 2C). Median time to HCC recurrence was 350 days (IQR 185–570) in the IFN-based DAA treatment era vs 340 days (IQR 167–570) in the IFN-free DAA treatment era (P=0.99).
Risk Factors Associated with Post-Transplant Outcomes
On multivariate analysis, there was a trend of lower mortality in the IFN-free DAA treatment era, with 18% (HR 0.82; 95% CI 0.60–1.13; P=0.22) reduction in patient mortality at 1 year after LT and 16% (HR 0.84; 95% CI 0.69–1.03, P=0.10) reduction at 3 years, although it did not reach statistical significance (Supplementary Table 1). Other risk factors of patient mortality were comparable at 1 and 3 years after LT.
On multivariate analysis, the IFN-free DAA treatment era was associated with 56% (HR 0.44; 95% CI 0.25–0.77; P <0.01) reduction in graft failure at 1 year after LT and 41% (HR 0.59; 95% CI 0.40–0.87; P <0.01) reduction at 3 years (Supplementary Table 2). Other risk factors of graft failure at 1 and 3 years after LT were black race, recipient history of diabetes, higher DRI, donor history of hypertension, moderately and poorly differentiated HCC, and history of acute rejection episode. Female donors were associated with 40% and 32% reduction in graft failure at 1 and 3 years after LT, respectively.
There was no significant difference in the risk of HCC recurrence between the IFN-based DAA treatment and IFN-free DAA treatment eras (Supplementary Table 3). Risk factors of HCC recurrence at 1 and 3 years after LT included AFP at LT (at the following cutoffs: 100–999 and ≥1000 ng/mL), ≥5 HCC tumors, infiltrative HCC, moderately and poorly differentiated HCC, macro- and microvascular invasion, and lymph node metastasis.
Second Study Cohort
Dropout While on the Waiting List
We identified a total of 12,519 patients with HCV-related HCC who were on the LT waiting list at any time during the study period. Table 4 shows that a significantly higher percentage of patients received transplants in the IFN-free DAA treatment era compared with the IFN-based DAA treatment era (50.94% vs 47.99%, respectively; P <0.01). However, patients within the IFN-free DAA treatment era had a higher dropout rate (n=1685, 26.78%) than those in the IFN-based DAA treatment era (n=1461, 23.47%) from the waiting list (P <0.01). Reasons for dropout included death, deterioration in patient condition, improvement in patient condition, and loss to follow-up. When stratified by reason for removal from the waiting list, 56.40% of dropouts in the IFN-based DAA treatment era, compared with 43.74% of dropouts in the IFN-free DAA treatment era, were due to death (P=0.15). Patient deterioration was found in 12.32% of dropouts in the IFN-based DAA treatment era, compared with 20.71% in the IFN-free DAA treatment era (P <0.01). Improvement of patients’ condition was found in 1.57% of dropouts in the IFN-based DAA treatment era, compared with 2.61% in the IFN-free DAA treatment era (P=0.17).
 |
Table 4 Patients Listed for Transplant with HCV and HCC (Second Study Cohort) and Their Waiting List Outcomes |
Discussion
Our retrospective study of 5044 patients with HCV-related HCC provided better understanding of pre-LT HCC characteristics and yielded mixed results with regard to our hypothesis that these patients would have improved outcomes in the IFN-free DAA treatment era. LT recipients in the IFN-free DAA treatment era had significantly higher complete tumor necrosis. For post-LT outcomes, we observed lower trends of 1- and 3-year patient mortality and graft failure in the IFN-free DAA treatment era, although only graft failure reached statistical significance. The recurrence rate was similar between the two eras. Significantly higher proportion of patients received transplants in the IFN-free DAA treatment era, with an increase in dropouts during that period.
Our results are similar to those of a published Italian cohort of 23 patients treated with DAA before receiving LT for HCV-induced HCC.11 However, the study had a small sample size and it was underpowered to detect a true difference between the two groups.
Patients in the IFN-free DAA treatment era were on the waiting list for a longer period of time than patients in the IFN-based DAA treatment era. This may have resulted from improvement in MELD score with DAA treatment, which moved the patients down the waiting list.5 It is also possible that longer waiting time and MELD score improvement may have allowed patients to receive more than 1 locoregional treatment before transplant, resulting in a significantly higher number of patients achieving complete tumor necrosis and lower AFP level in the DAA group.11,12 Another explanation for longer waiting-list time in the IFN-free DAA treatment era is that the 6-month waiting time rule for assigning HCC exception points was implemented by UNOS in October 2015.13 This policy allowed for observation of HCC tumor biology with avoidance of transplant in patients with aggressive tumors that continued to grow while the patient was on the waiting list and that are likely to recur after transplant.14 Nonetheless, a higher percentages of complete tumor necrosis in the IFN-free DAA treatment era and similar incidence of HCC recurrence between the two eras point towards a favorable outcome.
Our study showed that patients in the IFN-free DAA treatment era were significantly older, with more comorbidities compared with the IFN-based DAA treatment group. Patients with HCV are getting older, and elderly patients have certainly benefited from the use of DAAs, with comparable SVR rates to those of the general population reported.15 Interferon-based regimens have been shown to be poorly tolerated in older patients, with high adverse event rates and low SVR, and are not used in patients with advanced liver disease.16 We also demonstrated increased utilization of HCV Ab–positive liver allografts in the IFN-free DAA treatment era. This may be attributed to changes in discard rates of HCV-positive allografts owing to emerging data of similar long-term outcomes in recipients of HCV-negative and HCV-positive donors.17,18 Less than 2% of patients in both the IFN-based DAA treatment and IFN-free DAA treatment eras had positive HBsAg which could have played a role in HCC development in these patients.
We found a lower but statistically insignificant trend in patient mortality after LT in the DAA group. We assume that a prominent difference was not observed because of the relatively short follow-up period (3 years) and that a longer follow-up period may show a difference in the future. We observed a significant difference between the IFN-based DAA treatment and IFN-free DAA groups regarding graft failure. This observation is similar to previously published data.5 DAA therapy has been shown to prevent complications of recurrent HCV infection after LT.19 The incidence of graft failure was divergent between the IFN-based DAA treatment and IFN-free DAA treatment eras after 4 months from transplant. This impact may be the result of peri-transplant DAA therapeutic options that can prevent and treat fibrosing cholestatic hepatitis and other complications related to recurrent hepatitis.20 Fibrosing cholestatic hepatitis usually occur 2–3 months after LT, resulting in considerable graft loss.19,21 Furthermore, IFN-free DAA therapy was not found to be a risk factor for HCC recurrence after adjustment in the multivariate Cox regression analysis. HCC recurrence rates at 1 and 3 years after LT were remarkably lower than those in the published literature in both eras.22,23
A significantly higher percentage of patients received transplants in the IFN-free DAA treatment era. The IFN-free DAA treatment era was also associated with improvement in a small group of patients on the waiting list in whom LT was no longer indicated. These observations align with previous data showing improvement of MELD and CTP scores, as well as lower rates of hepatic decompensation, after DAA therapy.24,25 On the other hand, we observed a significantly higher rate of dropout due to deterioration on the waiting list in the IFN-free DAA treatment era. Unfortunately, no available data regarding the precise definition of deterioration on the waiting list have been outlined in the UNOS database. This observation may be attributed to a longer waiting time. The implementation of the 6-month waiting-time rule, as mentioned earlier, may also have contributed with exclusion of any patient who suffered progression of HCC within the first 6 months of listing, rather than being a consequence of DAA therapy. We did not observe any difference in HCC characteristics in the explanted liver between the IFN-based DAA treatment and IFN-free DAA treatment eras, suggesting that IFN-free DAA therapy does not affect HCC aggressiveness.
This is the first study using a large national database of explanted livers to examine HCC characteristics in the IFN-free DAA treatment era and the association of IFN-free DAAs on post-LT outcome. Our study has limitations associated with the retrospective nature, reflecting indirect effects of IFN-free DAA therapy. The UNOS/OPTN database did not record patients who achieved SVR with IFN-free DAAs in the peri-transplant period. Furthermore, there were no data available regarding the IFN-free DAA regimen used in these patients. We were not able to study data before April 2012 as the explant pathology information was not entered in the UNOS/OPTN database before that time. Potential bias may be present in our study as LT centers do not list patients with vascular invasion, extrahepatic metastasis, or multinodular or large HCC that exceeds the transplant criteria. The UNOS STAR file has no data regarding dropout specifically due to HCC progression while on the waiting list. Given the huge resource of UNOS database, we suggest change of data collection forms either retrospective or prospective to include DAA treatment protocols, duration of treatment and SVR rates.
Conclusions
In conclusion, HCC characteristics were similar between the IFN-based DAA treatment and IFN-free DAA treatment eras, and HCC recurrence rates were low. Post-LT graft survival significantly improved in the IFN-free DAA treatment era among patients with HCV and HCC, and there was a trend of improved patient survival at 1 and 3 years, though it was not statistically significant. Longer-term follow-up after LT is warranted to determine the impact of DAA therapy on patient survival.
Abbreviations
AASLD, American Association for the Study of Liver Diseases; AFP, alpha fetoprotein; BMI, body mass index; CI, confidence interval; CTP, Child-Turcotte-Pugh; DAA, direct-acting antiviral; DRI, donor risk index; FDA, Food and Drug Administration; HBcAb, hepatitis B core antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HCV Ab, hepatitis C virus antibody; HIVAb, human immunodeficiency virus antibody; HR, hazard ratio; IQR, interquartile ranges; LT, liver transplant; MELD, Model for End-Stage Liver Disease; OPTN, Organ Procurement and Transplantation Network; SBP, spontaneous bacterial peritonitis; SD, standard deviation; STAR, Standard Transplant Analysis and Research; SVR, sustained virologic response; TIPS, transjugular intrahepatic portosystemic shunt; UNOS, United Network for Organ Sharing.
Disclosure
The authors declare no conflicts of interest for this work.
References
1. El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60(5):1767–1775. doi:10.1002/hep.27222
2. Reddy KR, Bourlière M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology. 2015;62(1):79–86. doi:10.1002/hep.27826
3. Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67(6):1204–1212. doi:10.1016/j.jhep.2017.07.025
4. Abdelaziz AO, Nabil MM, Abdelmaksoud AH, et al. Tumor behavior of hepatocellular carcinoma after hepatitis C treatment by direct-acting antivirals: comparative analysis with non-direct-acting antivirals-treated patients. Eur J Gastroenterol Hepatol. 2019;31(1):75–79. doi:10.1097/MEG.0000000000001264
5. Cotter TG, Paul S, Sandıkçı B, et al. Improved graft survival after liver transplantation for recipients with hepatitis C virus in the direct-acting antiviral era. Liver Transplant. 2019;25(4):598–609. doi:10.1002/lt.25424
6. Belli LS, Perricone G, Adam R, et al. Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69(4):810–817. doi:10.1016/j.jhep.2018.06.010
7. Mitchell O, Gurakar A. Management of hepatitis C post-liver transplantation: a comprehensive review. J Clin Transl Hepatol. 2015;3(2):140. doi:10.14218/jcth.2015.00005
8. Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416. doi:10.1056/NEJMoa1012912
9. Kowdley KV, Lawitz E, Crespo I, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre Phase 2 trial. Lancet. 2013;381(9883):2100–2107. doi:10.1016/S0140-6736(13)60247-0
10. Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790. doi:10.1111/j.1600-6143.2006.01242.x
11. Zanetto A, Shalaby S, Vitale A, et al. Dropout rate from the liver transplant waiting list because of hepatocellular carcinoma progression in hepatitis C virus–infected patients treated with direct-acting antivirals. Liver Transplant. 2017;23(9):1103–1112. doi:10.1002/lt.24790
12. Huang AC, Mehta N, Dodge JL, et al. Direct-acting antivirals do not increase the risk of hepatocellular carcinoma recurrence after local-regional therapy or liver transplant waitlist dropout. Hepatology. 2018;68(2):449–461. doi:10.1002/hep.29855
13. Nagai S, Kitajima T, Yeddula S, et al. Effect of mandatory 6-month waiting period on waitlist and transplant outcomes in patients with hepatocellular carcinoma. Hepatology. 2020;72(6):
14. Ishaque T, Massie AB, Bowring MG, et al. Liver transplantation and waitlist mortality for HCC and non-HCC candidates following the 2015 HCC exception policy change. Am J Transplant. 2019;19(2):564–572. doi:10.1111/ajt.15144
15. Jhaveri MA, Manne V, Kowdley KV. Chronic hepatitis C in elderly patients: current evidence with direct-acting antivirals. Drugs Aging. 2018;35(2):117–122. doi:10.1007/s40266-017-0515-1
16. Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752–1762. doi:10.1002/hep.25976
17. Stepanova M, Sayiner M, de Avila L, et al. Long-term outcomes of liver transplantation in patients with hepatitis C infection are not affected by HCV positivity of a donor. BMC Gastroenterol. 2016;16(1):137. doi:10.1186/s12876-016-0551-z
18. Bowring MG, Kucirka LM, Massie AB, et al. Changes in utilization and discard of hepatitis C–infected donor livers in the recent era. Am J Transplant. 2017;17(2):519–527. doi:10.1111/ajt.13976
19. Forns X, Charlton M, Denning J, et al. Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C after liver transplantation. Hepatology. 2015;61(5):1485–1494. doi:10.1002/hep.27681
20. Cholankeril G, Li AA, March KL, et al. Improved outcomes in HCV patients following liver transplantation during the era of direct-acting antiviral agents. Clin Gastroenterol Hepatol. 2018;16(3):452–453. doi:10.1016/j.cgh.2017.08.020
21. Xiao S-Y, Lu L, Wang HL. Fibrosing cholestatic hepatitis: clinicopathologic spectrum, diagnosis and pathogenesis. Int J Clin Exp Pathol. 2008;1(5):396–402.
22. Agopian VG, Harlander-Locke M, Zarrinpar A, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416–427. Elsevier Inc. doi:10.1016/j.jamcollsurg.2014.12.025
23. Bodzin AS, Lunsford KE, Markovic D, et al. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. 2017;266:118–125. Lippincott Williams and Wilkins. doi:10.1097/SLA.0000000000001894
24. Cheung MCM, Walker AJ, Hudson BE, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65(4):741–747. doi:10.1016/j.jhep.2016.06.019
25. Foster GR, Irving WL, Cheung MCM, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64(6):1224–1231. doi:10.1016/j.jhep.2016.01.029
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

